2.1. Results
As part of the ongoing CPMC, which is a prospective research study focused on the impact that disease risk assessment has on health outcomes [
12,
13], participants are given personalized melanoma risk reports that include genetic and family history risk factors for melanoma and subsequently (at least 3 months after viewing the risk report) asked to complete optional outcome surveys that capture what they did with the information. Participants in the current study were recruited through one of three cohorts: CPMC community cohort, The Ohio State University (OSU) community cohort, or the United States Air Force Medical Service cohort (
Table 1).
Table 1.
Participant demographics.
Table 1.
Participant demographics.
| n | 718 |
|---|
| age in years, mean (range) | 52.86 (21–91) |
| male, n (%) | 245 (34.12) |
| female, n (%) | 473 (65.88) |
| Air Force Medical Service, n (%) | 118 (16.43) |
| Coriell Personalized Medicine Collaborative community, n (%) | 498 (69.36) |
| The Ohio State University community, n (%) | 102 (14.21) |
As described in more detail elsewhere [
12,
13], family history risk is collected through self-reported questionnaires, and genetic risk is evaluated through extensive literature review and by the Coriell Informed Cohort Oversight Board (ICOB). For the melanoma risk report, genetic risk evidence presented in Brown
et al. [
11] was approved by the ICOB, genotyped at Coriell’s CLIA-certified genotyping laboratory, and used in the CPMC risk report. Risk reports are available through CPMC’s online portal (an example of a melanoma risk report can be found at the following web address:
https://cpmc.coriell.org/v/Report/Demo/Melanoma/DemoNat), and participants choose which, if any, risk reports they want to view [
14].
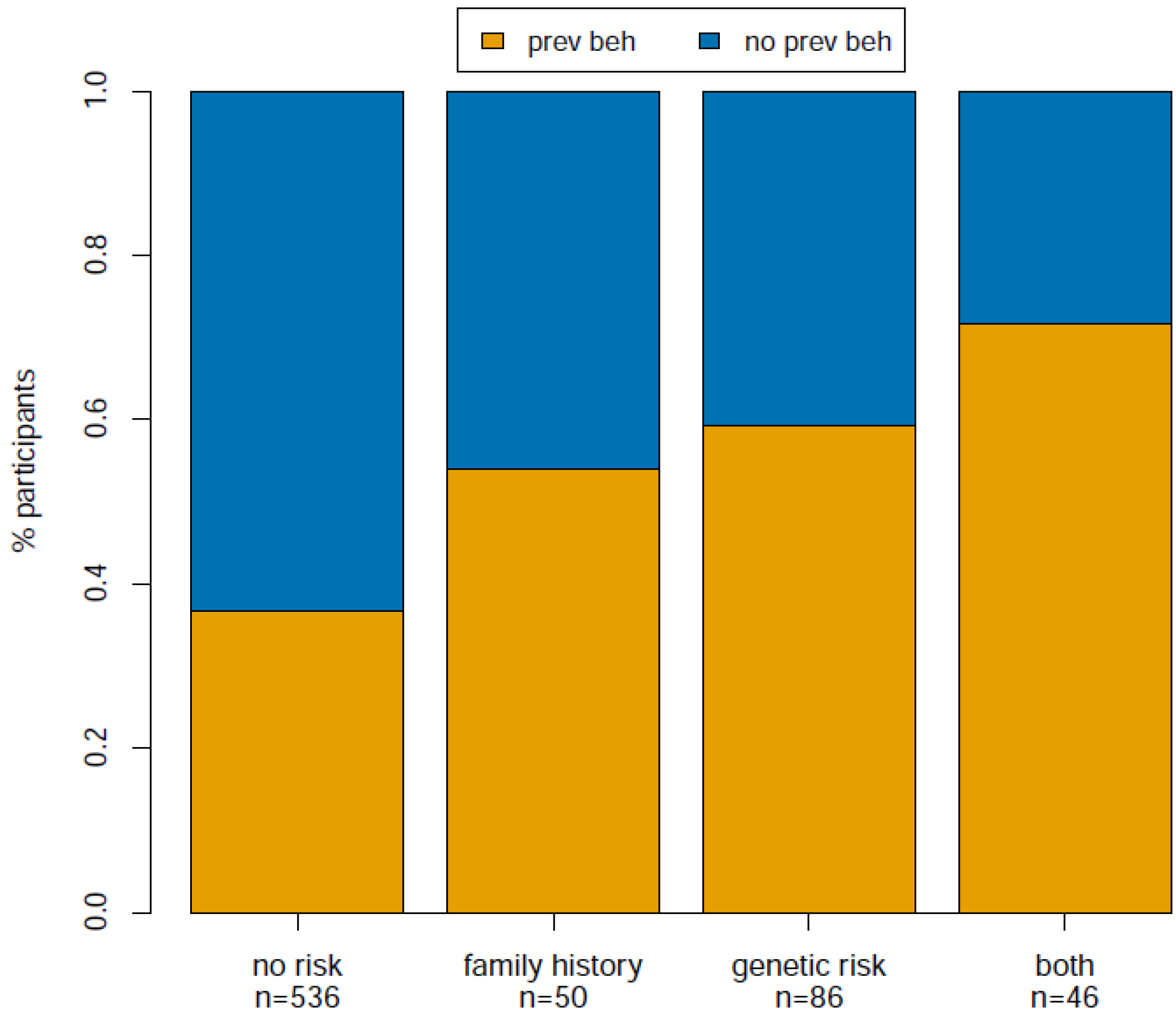
We were interested in examining whether participant understanding of their reported risk factors for melanoma was associated with behavior changes that mitigate melanoma risk. To address this question, we first asked if there was any significant difference among participants reporting a particular risk category with regard to preventive behavior change. We defined preventive behavior change as “no” or “yes”, where “yes” indicates the increase of one or more of the following protective behaviors: decreased sun exposure or increased sunscreen, protective clothing or self-skin examinations. We defined risk category as follows: no risk factors, family history risk only, genetic risk (one or two copies of the genetic risk allele) only, or both family history and genetic risk factors. We used ANOVA to test for any difference among the four risk categories with respect to preventive behavior, and indeed found a significant difference among groups (F-value = 36.93; p < 1.99 × 10−9).
To further explore the relationship between risk category and preventive behavior we used binomial logistic regression to test for association between preventive behavior and risk category after correcting for age, gender and cohort (see
Table 2). We found that all three increased risk categories (family history, genetic risk, both) are significantly more likely to result in preventive behaviors than the no risk category (
p = 0.02, 2.86 × 10
−5, 4.67 × 10
−5, respectively), and the magnitude of the effect increases such that family history is the lowest (OR = 2.04), genetic risk is intermediate (OR = 2.79), and both is the highest (OR = 4.06). This result is also illustrated in
Figure 1, which displays the raw proportion of individuals in each risk category that increased preventive behaviors after viewing their CPMC melanoma risk report. Comparisons among the three increased risk groups and comparison between participants with one and two copies of the genetic risk variant were not statistically significant (
p > 0.40). In addition, we found similar results when analyzing skin exams and sun protection as separate outcome variables (
Table A1 and
Table A2).
Table 2.
Logistic regression modeling results for preventive behavior change and melanoma risk.
Table 2.
Logistic regression modeling results for preventive behavior change and melanoma risk.
| | OR | eta | SE | z Value | p Value |
|---|
| (Intercept) | 0.20 | −1.61 | 0.36 | −4.43 | 9.25 e-06 |
| family history (vs. no risk) | 2.04 | 0.71 | 0.30 | 2.36 | 0.02 |
| genetic (vs. no risk) | 2.79 | 1.03 | 0.25 | 4.18 | 2.86 e-05 |
| both (vs. no risk) | 4.06 | 1.40 | 0.34 | 4.07 | 4.67 e-05 |
Figure 1.
Proportion of participants that adopted preventive behaviors after viewing their melanoma risk reports.
Figure 1.
Proportion of participants that adopted preventive behaviors after viewing their melanoma risk reports.
Given the significant association between risk category and preventive behaviors after correcting for age, gender, and cohort, we were interested in further exploring this relationship. We next incorporated reported anxiety levels after viewing the melanoma risk report. We used binomial logistic regression to incorporate anxiety into our model and found that after correcting for age, gender, and cohort, the significance level (
p-value = 9.64 × 10
−9) and the magnitude of effect (eta = 0.91) were more significant and stronger, respectively for anxiety than for any of the increased risk categories (see
Table 3). No interaction terms between risk category and demographic covariates were significant (
p > 0.20).
Table 3.
Logistic regression modeling results for preventive behaviors, anxiety and melanoma risk.
Table 3.
Logistic regression modeling results for preventive behaviors, anxiety and melanoma risk.
| Column1 | OR | eta | SE | z Value | p Value |
|---|
| (Intercept) | 0.07 | −2.62 | 0.41 | −6.34 | 2.36 e-10 |
| anxiety | NA | 0.91 | 0.16 | 5.74 | 9.64 e-09 |
| family history (vs. no risk) | 1.71 | 0.53 | 0.31 | 1.71 | 0.09 |
| genetic risk (vs. no risk) | 1.93 | 0.66 | 0.26 | 2.51 | 0.01 |
| both (vs. no risk) | 2.35 | 0.86 | 0.37 | 2.33 | 0.02 |
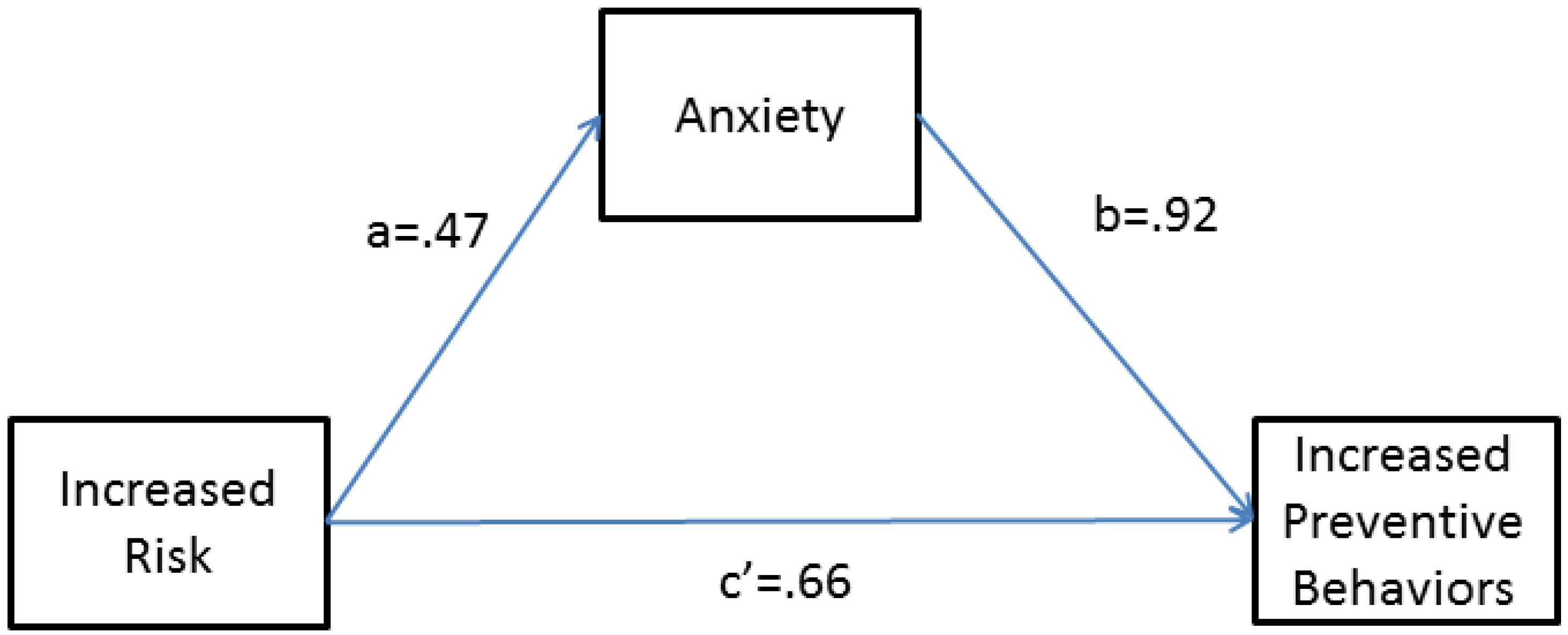
In order to better understand the relationship among preventive behavior, risk category, and participant anxiety levels (1–5 Likert-type scale) after viewing the melanoma risk report, we tested a simple mediation model [
15] after correcting for demographic covariates (age, gender, and recruitment cohort). In particular, we hypothesized that risk factors for melanoma may have influenced preventive behavior change through feelings of anxiety such that increased risk (genetic or family history) predicts increased anxiety, which in turn predicts the chance of adopting or increasing preventive behaviors. The proposed mediation model is diagrammed in
Figure A1 , and as shown, the model accounts for the impact of risk factors on anxiety, the impact of anxiety on preventive behaviors, and the impact of risk on preventive behaviors directly. We found that the effect of any increased risk category relative to no risk category on preventive behavior change is partially mediated by increased anxiety levels after viewing the melanoma risk report. More specifically, we found that risk factor significantly predicts increased anxiety (beta = 0.47, OR = 1.6,
p-value < 1.00 × 10
−4, path “a” in
Figure A1), which in turn significantly predicts the chance that a participant has adopted a preventive behavior after viewing the melanoma risk report (beta = 0.92, OR = 2.51,
p-value < 1.00 × 10
−4, path “b” in
Figure A1). Additionally, the direct effect of risk factor on preventive behavior, which quantifies the effect of risk factor on preventive behavior that is independent of anxiety, was also significant (beta = 0.66, OR = 1.93,
p-value = 7.00 × 10
−4, path “c'’’ in
Figure A1 ). The indirect effect of risk factor on preventive behavior (a*b) is 0.44, and the total effect of risk category on preventive behavior is 1.03 (
p-value < 1.00 × 10
−4). Hayes [
15] notes that in models of dichotomous traits, the total effect is not always equal to the sum of the direct and indirect effects. Overall, the mediation analysis indicates that anxiety after viewing the melanoma risk report partially explains the association between risk factor and preventive behaviors; however, even after taking this relationship into account, risk factor is still significantly impacting preventive behavior.
Finally, we examined two additional questions included in the outcome survey related to preventive behavior motivations. More specifically, participants were asked what motivated them to make a particular reported behavior change by choosing all applicable responses from the following: “my CPMC genetic variant result for melanoma”, “my CPMC family history result for melanoma”, “I had symptoms of melanoma”, “my CPMC results for other conditions”, “my health care provider’s recommendations”, “I have/had another type of skin cancer (basal cell, squamous cell, etc.)”, or “other”. We compared the number of participants who reported only “genetic variant” (n = 65 out of 308 participants that increased preventive behaviors) with the number of participants who reported only “family history” (n = 32 out of 308 participants that increased preventive behaviors), and found significantly more participants report being motivated by their genetic result (X2 = 12.53, p-value = 4.01 × 10−4). This comparison is still significant when restricting to participants that reported genetic risk (65/86) or family history risk (25/50), respectively (X2 = 8.14, p-value=4.33 × 10−3). In addition, 23 out of 46 participants that reported both risk factors also reported being motivated by both genetic and family history risk. This proportion was significantly fewer than those who only reported being motivated by genetic risk (X2 = 7.71, p-value = 5.49 × 10−3) and not significantly different from those who only reported being motivated by family history (X2 = 0.00, p-value = 1.00).
2.2. Discussion
To our knowledge, this is the largest study (n = 718) to establish an association between reported genetic risk of melanoma and preventive behaviors, and the only study to focus on common genetic variants rather than familial cases of melanoma. We found that any increased risk for melanoma (increased genetic risk, increased family history risk, or increased genetic and family history risk) is significantly associated with increased preventive behaviors, and this association is partially mediated by anxiety. Moreover, significantly more participants that did increase preventive behaviors reported that they were motivated by their genetic risk rather than by family history risk for melanoma.
The US Preventive Services Task Force and others (e.g., [
16]) already emphasize the importance of family history in assessing disease risk in the primary care setting, and our results suggest that genetic testing for melanoma risk will complement the current standard of care in motivating preventive behaviors. Furthermore, there are situations in which family history is either not known (e.g., adopted individuals), incompletely known, or incorrectly known that may be particularly suitable for supplementary genetic testing.
It is worth noting that in many respects melanoma represents a “best case scenario” for incorporating genetic risk for complex disease into preventive clinical care. Indeed, studies of behavior change related to other conditions have not been as encouraging [
17]. First, there are several lifestyle changes that mitigate the risk of melanoma that are accessible and affordable to everyone (e.g., avoiding direct sunlight and wearing protective clothing outside). Second, skin exams are non-invasive clinical screening tools for melanoma. Third, early detection of melanoma has profound impact on prognosis [
18]. Therefore, genetic risk assessment may not be appropriate for other conditions where risk mitigation is undefined or inaccessible, clinical screening is invasive and/or poses risk to patients, or prognosis is not improved by early detection. Moreover, in the CPMC melanoma risk assessment, the reported risk factors are not deterministic, but rather contribute to increased risk for the disease relative to individuals without the risk factors. The maximum genetic and family history relative risk a participant can have is 3 and 2.2, respectively. We acknowledge the possibility that participants may be “over-interpreting” their risk; however, we did not evaluate this in the current study. In cases where interventions based on genetic risk factors pose additional risks to patients, appropriate understanding of genetic risk factors will be critical.
To explore any potentially negative impact to participants’ state of mind, we evaluated levels of anxiety after viewing the melanoma risk report. We found that only 2 (0.3%) participants reported high, and 29 (4%) participants reported moderate levels of anxiety. The vast majority (74%) of participants that did report moderate or high levels of anxiety reported having at least one risk factor for melanoma, which suggests that genomic testing may contribute to a heightened level of anxiety which may cause individuals to take actions to further address and investigate the implications of disease.
One hundred participants (14%) reported sharing their CPMC risk report for melanoma with a health care provider. Health care providers for 17 of these individuals conducted additional tests, including 11 biopsies. Six of these biopsies were normal; however, three were pre-cancerous and two were malignant (basal cell carcinoma). Therefore, five participants who pursued additional clinical care as a result of their CPMC melanoma risk reports may have benefitted from earlier detection. Based on these numbers, we also have no reason to suspect any over-utilization of health care resources as a result of the CPMC melanoma risk report.
This study is not without limitations. Results may not generalize to the US population at large. In particular, self-selection bias may be introduced since participants that have chosen to join the CPMC study may be more interested in knowing their genetic risk for reported diseases, less concerned about increased genetic risk for a given disease, and more motivated to mitigate disease risk compared to the general population. The study relies on self-reported data and is, therefore, also subject to reporting bias. Due to the need for outcome survey completion, preventive behavior for those who did not view the available risk report and those who did not complete an outcome survey could not be analyzed. In addition, we did not address risk report comprehension or retention in the current study. Outcome survey completion was 36% despite a fifteen to twenty minute survey completion time. Participant recruitment, risk report viewing, and outcome survey completion occurs on a rolling basis, and we were therefore unable to control the seasonality associated with our data collection.
Results were reported for just one melanoma genetic risk variant among many possible risk alleles. Since the CPMC did not initially capture other important risk factors for melanoma, such as ultraviolet light exposure due to sun, lamps or beds, immune suppression status, presence of moles, fair skin, freckling and light hair, participants did not receive risk estimates for these factors. Moreover, the family history component of the risk report only differentiates between participants with no reported family history and participants with one or more family members with one or multiple melanomas. The outcome survey also did not capture the magnitude of change in preventive behaviors.
The clinical validity and utility of genomic risk assessment in the presence of family history must be further analyzed with respect to cost and clinical effectiveness to further validate or refute the potential benefits of testing reported in this study. In addition, further research is needed to evaluate what if any additional training and educational resources will be required to support health care providers that incorporate genetic risk testing in their clinical care. Finally, it will be critical to identify interventions that achieve sustainable preventive behaviors to reduce the rising prevalence of melanoma cancer.