Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities
Abstract
:1. Background
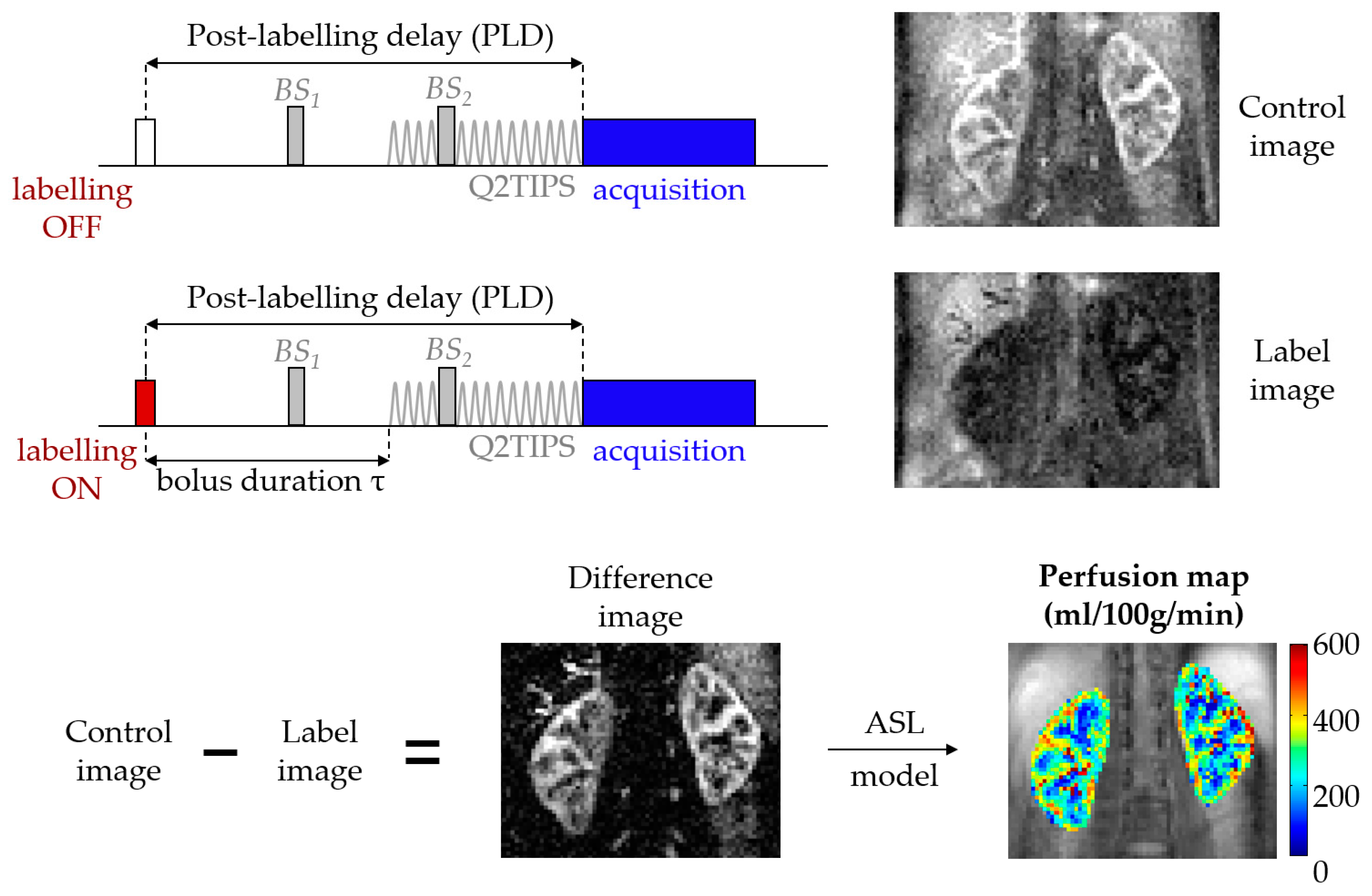
1.1. ASL in a Nutshell
1.1.1. Labeling
1.1.2. Readout
1.1.3. Modeling
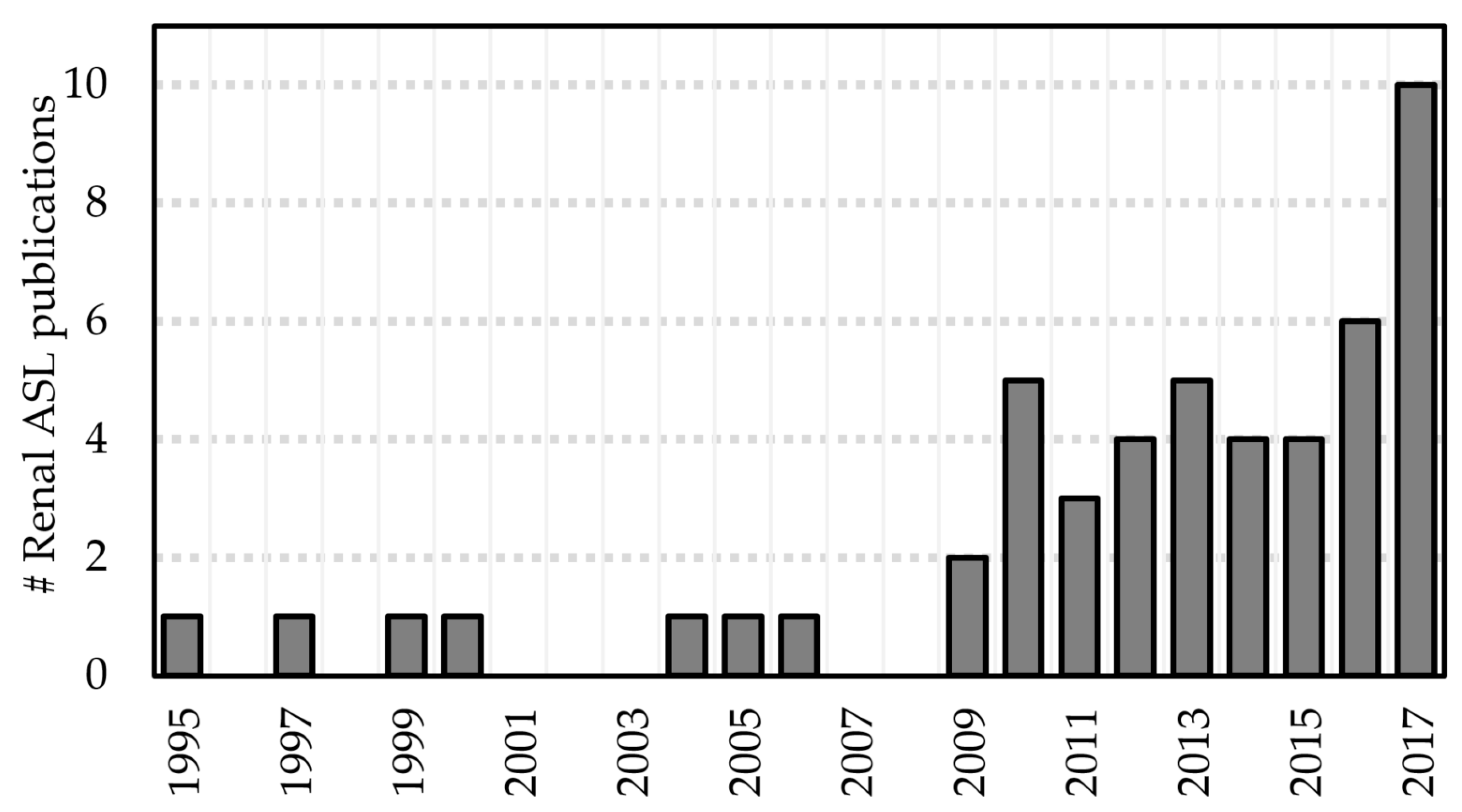
2. Recent Developments in Renal ASL
2.1. Methodological Developments
2.1.1. Validation of ASL Renal Blood Flow Measurements
2.1.2. Improving the Robustness of RBF Measurements
2.1.3. ASL at High Field
2.2. Clinical Applications
2.2.1. Monitoring Renal Allograft Function
2.2.2. Pharmacological Modulation
3. Challenges
3.1. Subject Movement
3.2. Lack of Consensus Regarding Labeling Strategy
3.3. Readout Optimization
3.4. Lack of Consensus Regarding Analysis Approach
3.4.1. Quantification Model Selection
3.4.2. Region of Interest (ROI) Definition
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aukland, K. Methods for Measuring Renal Blood Flow: Total Flow and Regional Distribution. Annu. Rev. Physiol. 1980, 42, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Knox, F.G.; Ritman, E.L.; Romero, J.C. Intrarenal Distribution of Blood Flow: Evolution of a New Approach to Measurement. Kidney Int. 1984, 25, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Pallone, T.L.; Edwards, A.; Mattson, D.L. Renal Medullary Circulation. Compr. Physiol. 2012, 2, 97–140. [Google Scholar] [CrossRef] [PubMed]
- Beierwaltes, W.H.; Harrison-Bernard, L.M.; Sullivan, J.C.; Mattson, D.L. Assessment of Renal Function; Clearance, the Renal Microcirculation, Renal Blood Flow, and Metabolic Balance. Compr. Physiol. 2013, 3, 165–200. [Google Scholar] [CrossRef] [PubMed]
- Detre, J.A.; Leigh, J.S.; Williams, D.S.; Koretsky, A.P. Perfusion imaging. Magn. Reson. Med. 1992, 23, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; Detre, J.A.; Leigh, J.S.; Koretsky, A.P. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc. Natl. Acad. Sci. USA 1992, 89, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Luh, W.M.; Wong, E.C.; Bandettini, P.A.; Hyde, J.S. QUIPSS II with thin-slice TI1 periodic saturation: A method for improving accuracy of quantitative perfusion imaging using pulsed arterial spin labeling. Magn. Reson. Med. 1999, 41, 1246–1254. [Google Scholar] [CrossRef]
- Petersen, E.T.; Zimine, I.; Ho, Y.C.; Golay, X. Non-invasive measurement of perfusion: A critical review of arterial spin labelling techniques. Br. J. Radiol. 2006, 79, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C. An introduction to ASL labeling techniques. J. Magn. Reson. Imaging 2014, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alsop, D.C.; Detre, J.A.; Golay, X.; Günther, M.; Hendrikse, J.; Hernandez-Garcia, L.; Lu, H.; Macintosh, B.J.; Parkes, L.M.; Smits, M.; et al. Recommended implementation of arterial spin-labeled Perfusion MRI for clinical applications: A consensus of the ISMRM Perfusion Study group and the European consortium for ASL in dementia. Magn. Reson. Med. 2015, 73, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.A.; Detre, J.A.; Bolinger, L.; Insko, E.K.; Lenkinski, R.E.; Pentecost, M.J.; Leigh, J.S. Renal perfusion in humans: MR imaging with spin tagging of arterial water. Radiology 1995, 196, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dixon, W.T.; Du, L.N.; Faul, D.D.; Gado, M.; Rossnick, S. Projection angiograms of blood labeled by adiabatic fast passage. Magn. Reson. Med. 1986, 3, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.C.; Buxton, R.B.; Frank, L.R. Quantitative imaging of perfusion using a single subtraction (QUIPSS and QUIPSS II). Magn. Reson. Med. 1998, 39, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Gardener, A.G.; Francis, S.T. Multislice perfusion of the kidneys using parallel imaging: Image acquisition and analysis strategies. Magn. Reson. Med. 2010, 63, 1627–1636. [Google Scholar] [CrossRef] [PubMed]
- Cutajar, M.; Thomas, D.L.; Banks, T.; Clark, C.A.; Golay, X.; Gordon, I. Repeatability of renal arterial spin labelling MRI in healthy subjects. Magn. Reson. Mater. Phys. Biol. Med. 2012, 25, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Madhuranthakam, A.J.; Smith, M.P.; Sun, M.R.M.; Dai, W.; Rofsky, N.M.; Pedrosa, I.; Alsop, D.C. Volumetric Arterial Spin-labeled Perfusion Imaging of the Kidneys with a Three-dimensional Fast Spin Echo Acquisition. Acad. Radiol. 2016, 23, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Kosaka, N.; Fujiwara, Y.; Matsuda, T.; Yamamoto, T.; Tsuchida, T.; Tsuchiyama, K.; Oyama, N.; Kimura, H. Arterial Transit Time-corrected Renal Blood Flow Measurement with Pulsed Continuous Arterial Spin Labeling MR Imaging. Magn. Reson. Med. Sci. 2017, 16, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Martirosian, P.; Klose, U.; Mader, I.; Schick, F. FAIR true-FISP perfusion imaging of the kidneys. Magn. Reson. Med. 2004, 51, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Auerbach, E.J.; Van de Moortele, P.-F.; Ugurbil, K.; Metzger, G.J. Quantitative single breath-hold renal arterial spin labeling imaging at 7T. Magn. Reson. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Kiefer, C.; Schroth, G.; Gralla, J.; Diehm, N.; Baumgartner, I.; Husmann, M. A Feasibility Study on Model-based Evaluation of Kidney Perfusion Measured by Means of FAIR Prepared True-FISP Arterial Spin Labeling (ASL) on a 3-T MR Scanner. Acad. Radiol. 2009, 16, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B.; Frank, L.R.; Wong, E.C.; Siewert, B.; Warach, S.; Edelman, R.R. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn. Reson. Med. 1998, 40, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Siewert, B.; Bly, B.M.; Warach, S.; Edelman, R.R. STAR-HASTE: Perfusion imaging without magnetic susceptibility artifact. Magn. Reson. Med. 1997, 38, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Berr, S.S.; Hagspiel, K.D.; Mai, V.M.; Keilholz-George, S.; Spinosa, D.J.; Angle, J.F.; Matsumoto, A.H. Perfusion of the Kidney Using Extraslice Spin Tagging (EST) MRI. J. Magn. Reson. Imaging 1999, 18, 886–891. [Google Scholar] [CrossRef]
- Karger, N.; Biederer, J.; Lusse, S.; Grimm, J.; Steffens, J.-C.; Heller, M.; Gluer, C.-C. Quantitation of renal perfusion using arterial spin labeling with FAIR-UFLARE. Magn. Reson. Imaging 2000, 18, 641–647. [Google Scholar] [CrossRef]
- Boss, A.; Martirosian, P.; Graf, H.; Claussen, C.D.; Schlemmer, H.P.; Schick, F. High resolution MR perfusion imaging of the kidneys at 3 Tesla without administration of contrast media. Rofo 2005, 177, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Fenchel, M.; Martirosian, P.; Langanke, J.; Giersch, J.; Miller, S.; Stauder, N.I.; Kramer, U.; Claussen, C.D.; Schick, F. Perfusion MR imaging with FAIR true FISP spin labeling in patients with and without renal artery stenosis: Initial experience. Radiology 2006, 238, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Robson, P.M.; Madhuranthakam, A.J.; Dai, W.; Pedrosa, I.; Rofsky, N.M.; Alsop, D.C. Strategies for reducing respiratory motion artifacts in renal perfusion imaging with arterial spin labeling. Magn. Reson. Med. 2009, 61, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Ritt, M.; Janka, R.; Schneider, M.P.; Martirosian, P.; Hornegger, J.; Bautz, W.; Uder, M.; Schmieder, R.E. Measurement of kidney perfusion by magnetic resonance imaging: Comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol. Dial. Transplant. 2010, 25, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Loeffler, R.B.; Hillenbrand, C.M. Improved renal perfusion measurement with a dual navigator-gated Q2TIPS fair technique. Magn. Reson. Med. 2010, 64, 1352–1359. [Google Scholar] [CrossRef] [PubMed]
- Lanzman, R.S.; Wittsack, H.-J.; Martirosian, P.; Zgoura, P.; Bilk, P.; Kröpil, P.; Schick, F.; Voiculescu, A.; Blondin, D. Quantification of renal allograft perfusion using arterial spin labeling MRI: Initial results. Eur. Radiol. 2010, 20, 1485–1491. [Google Scholar] [CrossRef] [PubMed]
- Ellah, M.A.; Kremser, C.; Pallwein, L.; Aigner, F.; Schocke, M.; Peschel, R.; Pedross, F.; Pinggera, G.M.; Wolf, C.; Alsharkawy, M.A.M.; et al. Changes of renal blood flow after ESWL: Assessment by ASL MR imaging, contrast enhanced MR imaging, and renal resistive index. Eur. J. Radiol. 2010, 76, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-C.; Su, M.-Y.; Chang, C.-C.; Tseng, W.-Y.I.; Liu, K.-L. Renal Perfusion 3-T MR Imaging: A Comparative Study of Arterial Spin Labeling and Dynamic Contrast-enhanced Techniques. Radiology 2011, 261, 845–853. [Google Scholar] [CrossRef] [PubMed]
- Artz, N.S.; Sadowski, E.A.; Wentland, A.L.; Grist, T.M.; Seo, S.; Djamali, A.; Fain, S.B. Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn. Reson. Imaging 2011, 29, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Artz, N.S.; Sadowski, E.A.; Wentland, A.L.; Djamali, A.; Grist, T.M.; Seo, S.; Fain, S.B. Reproducibility of renal perfusion MR imaging in native and transplanted kidneys using non-contrast arterial spin labeling. J. Magn. Reson. Imaging 2011, 33, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Rossi, C.; Artunc, F.; Martirosian, P.; Schlemmer, H.-P.; Schick, F.; Boss, A. Histogram Analysis of Renal Arterial Spin Labeling Perfusion Data Reveals Differences between Volunteers and Patients with Mild Chronic Kidney Disease. Investig. Radiol. 2012, 47, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Janka, R.; Ziegler, T.; Raff, U.; Ritt, M.; Ott, C.; Veelken, R.; Uder, M.; Schmieder, R.E. Reversibility of the effects of aliskiren in the renal versus systemic circulation. Clin. J. Am. Soc. Nephrol. 2012, 7, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Y.; Yang, X.; Wang, X.; Zhang, J.; Fang, J.; Jiang, X. Hemodynamic Effects of Furosemide on Renal Perfusion as Evaluated by ASL-MRI. Acad. Radiol. 2012, 19, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Ott, C.; Janka, R.; Schmid, A.; Titze, S.; Ditting, T.; Sobotka, P.A.; Veelken, R.; Uder, M.; Schmieder, R.E. Vascular and renal hemodynamic changes after renal denervation. Clin. J. Am. Soc. Nephrol. 2013, 8, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sugimori, H.; Nakanishi, M.; Fujima, N.; Ishizaka, K.; Mito, S.; Hamaguchi, H.; Sakata, M. Evaluation of renal blood flow using multi-phase echo-planar magnetic resonance imaging and signal targeting with alternating radiofrequency (EPISTAR) in 3-T magnetic resonance imaging. Radiol. Phys. Technol. 2013, 6, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Heusch, P.; Wittsack, H.-J.; Heusner, T.; Buchbender, C.; Quang, M.N.; Martirosian, P.; Bilk, P.; Kröpil, P.; Blondin, D.; Antoch, G.; et al. Correlation of biexponential diffusion parameters with arterial spin-labeling perfusion MRI: Results in transplanted kidneys. Investig. Radiol. 2013, 48, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Yang, L.; Su, T.; Yang, X.; Chen, B.; Zhang, J.; Wang, X.; Jiang, X. Quantitative assessment of acute kidney injury by noninvasive arterial spin labeling perfusion MRI: A pilot study. Sci. China Life Sci. 2013, 56, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Wang, D.J.J.; Duong, T.Q. Balanced steady state free precession for arterial spin labeling MRI: Initial experience for blood flow mapping in human brain, retina, and kidney. Magn. Reson. Imaging 2013, 31, 1044–1050. [Google Scholar] [CrossRef] [PubMed]
- Heusch, P.; Wittsack, H.J.; Blondin, D.; Ljimani, A.; Nguyen-Quang, M.; Martirosian, P.; Zenginli, H.; Bilk, P.; Kröpil, P.; Heusner, T.A.; et al. Functional evaluation of transplanted kidneys using arterial spin labeling MRI. J. Magn. Reson. Imaging 2014, 40, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.A.; McComb, C.; Foster, J.E.; Taylor, A.H.M.; Patel, R.K.; Morris, S.T.W.; Jardine, A.G.; Schneider, M.P.; Roditi, G.H.; Delles, C.; et al. Inter-study reproducibility of arterial spin labelling magnetic resonance imaging for measurement of renal perfusion in healthy volunteers at 3 Tesla. BMC Nephrol. 2014, 15. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Koktzoglou, I.; Prasad, P.V. Renal perfusion imaging with two-dimensional navigator gated arterial spin labeling. Magn. Reson. Med. 2014, 71, 570–579. [Google Scholar] [CrossRef] [PubMed]
- Cutajar, M.; Thomas, D.L.; Hales, P.W.; Banks, T.; Clark, C.A.; Gordon, I. Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: Quantification and reproducibility. Eur. Radiol. 2014, 24, 1300–1308. [Google Scholar] [CrossRef] [PubMed]
- Rapacchi, S.; Smith, R.X.; Wang, Y.; Yan, L.; Sigalov, V.; Krasileva, K.E.; Karpouzas, G.; Plotnik, A.; Sayre, J.; Hernandez, E.; et al. Towards the identification of multi-parametric quantitative MRI biomarkers in lupus nephritis. Magn. Reson. Imaging 2015, 33, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Hueper, K.; Gueler, F.; Bräsen, J.H.; Gutberlet, M.; Jang, M.-S.; Lehner, F.; Richter, N.; Hanke, N.; Peperhove, M.; Martirosian, P.; et al. Functional MRI detects perfusion impairment in renal allografts with delayed graft function. Am. J. Physiol. 2015, 308. [Google Scholar] [CrossRef] [PubMed]
- Breidthardt, T.; Cox, E.F.; Squire, I.; Odudu, A.; Omar, N.F.; Eldehni, M.T.; Francis, S.T.; Mcintyre, C.W. The pathophysiology of the chronic cardiorenal syndrome: A magnetic resonance imaging study. Eur. Radiol. 2015, 25, 1684–1691. [Google Scholar] [CrossRef] [PubMed]
- Cutajar, M.; Hilton, R.; Olsburgh, J.; Marks, S.D.; Thomas, D.L.; Banks, T.; Clark, C.A.; Gordon, I. Renal blood flow using arterial spin labelling MRI and calculated filtration fraction in healthy adult kidney donors Pre-nephrectomy and post-nephrectomy. Eur. Radiol. 2015, 25, 2390–2396. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Wen, C.-L.; Chen, L.-H.; Xie, S.-S.; Cheng, Y.; Fu, Y.-X.; Oesingmann, N.; de Oliveira, A.; Zuo, P.-L.; Yin, J.-Z.; et al. Evaluation of renal allografts function early after transplantation using intravoxel incoherent motion and arterial spin labeling MRI. Magn. Reson. Imaging 2016, 34, 908–914. [Google Scholar] [CrossRef] [PubMed]
- Hammon, M.; Janka, R.; Siegl, C.; Seuss, H.; Grosso, R.; Martirosian, P.; Schmieder, R.E.; Uder, M.; Kistner, I. Reproducibility of Kidney Perfusion Measurements With Arterial Spin Labeling at 1.5 Tesla MRI Combined With Semiautomatic Segmentation for Differential Cortical and Medullary Assessment. Medicine (Baltimore) 2016, 95, e3083. [Google Scholar] [CrossRef] [PubMed]
- Niles, D.J.; Artz, N.S.; Djamali, A.; Sadowski, E.A.; Grist, T.M.; Fain, S.B. Longitudinal Assessment of Renal Perfusion and Oxygenation in Transplant Donor-Recipient Pairs Using Arterial Spin Labeling and Blood Oxygen Level-Dependent Magnetic Resonance Imaging. Investig. Radiol. 2016, 51, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Getzin, T.; May, M.; Schmidbauer, M.; Gutberlet, M.; Martirosian, P.; Oertel, R.; Wacker, F.; Schindler, C.; Hueper, K. Usability of Functional MRI in Clinical Studies for Fast and Reliable Assessment of Renal Perfusion and Quantification of Hemodynamic Effects on the Kidney. J. Clin. Pharmacol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Cox, E.F.; Buchanan, C.E.; Bradley, C.R.; Prestwich, B.; Mahmoud, H.; Taal, M.; Selby, N.M.; Francis, S.T. Multiparametric renal magnetic resonance imaging: Validation, interventions, and alterations in chronic kidney disease. Front. Physiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ruan, D.; Liu, W.; Stenger, V.A.; Pohmann, R.; Fernández-Seara, M.A.; Nair, T.; Jung, S.; Luo, J.; Motai, Y.; et al. Respiratory motion prediction and prospective correction for free-breathing arterial spin-labeled perfusion MRI of the kidneys. Med. Phys. 2017, 44, 962–973. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Li, Z.; Zuo, P.; Pfeuffer, J.; Li, Y.; Liu, F.; Liu, R. Diagnostic Value of Renal Perfusion in Patients With Chronic Kidney Disease Using 3D Arterial Spin Labeling. J. Magn. Reson. Imaging 2017, 46, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gutiérrez, J.M.; Garcia-Fernandez, N.; Slon Roblero, M.F.; Páramo, J.A.; Escalada, F.J.; Wang, D.J.; Benito, A.; Fernández-Seara, M.A. Arterial spin labeling MRI is able to detect early hemodynamic changes in diabetic nephropathy. J. Magn. Reson. Imaging 2017, 46, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Conlin, C.C.; Oesingmann, N.; Bolster, B.; Huang, Y.; Lee, V.S.; Zhang, J.L. Renal plasma flow (RPF) measured with multiple-inversion-time arterial spin labeling (ASL) and tracer kinetic analysis: Validation against a dynamic contrast-enhancement method. Magn. Reson. Imaging 2017, 37, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Shim, W.H.; Yoon, S.K.; Oh, J.Y.; Kim, J.K.; Jung, H.; Matsuda, T.; Kim, D. Measurement of arterial transit time and renal blood flow using pseudocontinuous ASL MRI with multiple post-labeling delays: Feasibility, reproducibility, and variation. J. Magn. Reson. Imaging 2017, 46, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-P.; Tan, H.; Thacker, J.M.; Li, W.; Zhou, Y.; Kohn, O.; Sprague, S.M.; Prasad, P.V. Evaluation of Renal Blood Flow in Chronic Kidney Disease Using Arterial Spin Labeling Perfusion MRI. Kidney Int. Rep. 2017, 2, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.A.; McComb, C.; Patel, R.K.; Stevens, K.K.; Schneider, M.P.; Radjenovic, A.; Morris, S.T.W.; Roditi, G.H.; Delles, C.; Mark, P.B. Non-Contrast Renal Magnetic Resonance Imaging to Assess Perfusion and Corticomedullary Differentiation in Health and Chronic Kidney Disease. Nephron 2016, 133, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Skeoch, S.; Hubbard Cristinacce, P.L.; Dobbs, M.; Naish, J.; Woodhouse, N.; Ho, M.; Waterton, J.C.; Parker, G.J.M.; Bruce, I.N. Evaluation of non-contrast MRI biomarkers in lupus nephritis. Clin. Exp. Rheumatol. 2017, 35, 954–958. [Google Scholar] [PubMed]
- Artz, N.S.; Wentland, A.L.; Sadowski, E.A.; Djamali, A.; Grist, T.M.; Seo, S.; Fain, S.B. Comparing Kidney Perfusion Using Noncontrast Arterial Spin Labeling MRI and Microsphere Methods in an Interventional Swine Model. Investig. Radiol. 2011, 46, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Warmuth, C.; Nagel, S.; Hegemann, O.; Wlodarczyk, W.; Lüdemann, L. Accuracy of blood flow values determined by arterial spin labeling: A validation study in isolated porcine kidneys. J. Magn. Reson. Imaging 2007, 26, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Winter, J.D.; St. Lawrence, K.S.; Cheng, H.L. Quantification of renal perfusion: Comparison of arterial spin labeling and dynamic contrast-enhanced MRI. J. Magn. Reson. Imaging 2011, 34, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, F.; Zöllner, F.G.; Hoeger, S.; Klotz, S.; Tsagogiorgas, C.; Krämer, B.K.; Schad, L.R. Quantitative Renal Perfusion Measurements in a Rat Model of Acute Kidney Injury at 3T: Testing Inter- and Intramethodical Significance of ASL and DCE-MRI. PLoS ONE 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Bokkers, R.P.H.; Van Der Worp, H.B.; Mali, W.P.T.M.; Hendrikse, J. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology 2009, 73, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Tsujikawa, T.; Kimura, H.; Matsuda, T.; Fujiwara, Y.; Isozaki, M.; Kikuta, K.I.; Okazawa, H. Arterial transit time mapping obtained by pulsed continuous 3D ASL imaging with multiple post-label delay acquisitions: Comparative study with PET-CBF in patients with chronic occlusive cerebrovascular disease. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Robson, P.M.; Shankaranarayanan, A.; Alsop, D.C. Reduced resolution transit delay prescan for quantitative continuous arterial spin labeling perfusion imaging. Magn. Reson. Med. 2012, 67, 1252–1265. [Google Scholar] [CrossRef] [PubMed]
- De Bazelaire, C.; Rofsky, N.M.; Duhamel, G.; Michaelson, M.D.; George, D.; Alsop, D.C. Arterial spin labeling blood flow magnetic resonance imaging for the characterization of metastatic renal cell carcinoma. Acad. Radiol. 2005, 12, 347–357. [Google Scholar] [CrossRef] [PubMed]
- De Bazelaire, C.; Alsop, D.C.; George, D.; Pedrosa, I.; Wang, Y.; Michaelson, M.D.; Rofsky, N.M. Magnetic Resonance Imaging—Measured Blood Flow Change after Antiangiogenic Therapy with PTK787/ZK 222584 Correlates with Clinical Outcome in Metastatic Renal Cell Carcinoma. Clin. Cancer Res. 2008, 14, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Lanzman, R.S.; Robson, P.M.; Sun, M.R.; Patel, A.D.; Mentore, K.; Wagner, A.A.; Genega, E.M.; Rofsky, N.M.; Alsop, D.C.; Pedrosa, I. Arterial Spin-labeling MR Imaging of Renal Masses: Correlation with Histopathologic Findings. Radiology 2012, 265, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Kapur, P.; Yuan, Q.; Xi, Y.; Carvo, I.; Signoretti, S.; Dimitrov, I.; Cadeddu, J.A.; Margulis, V.; Muradyan, N.; et al. Tumor Vascularity in Renal Masses: Correlation of Arterial Spin-Labeled and Dynamic Contrast-Enhanced Magnetic Resonance Imaging Assessments. Clin. Genitourin. Cancer 2016, 14, e25–e36. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Kapur, P.; Zhang, Y.; Xi, Y.; Carvo, I.; Signoretti, S.; Dimitrov, I.E.; Cadeddu, J.A.; Margulis, V.; Brugarolas, J.; et al. Intratumor Heterogeneity of Perfusion and Diffusion in Clear-Cell Renal Cell Carcinoma: Correlation With Tumor Cellularity. Clin. Genitourin. Cancer 2016, 14, e585–e594. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, J.R.; Anderson, S. The Aging Kidney: Physiological Changes. Adv. Chronic Kidney Dis. 2010, 17, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.H.; Cox, E.F.; Francis, S.T.; Lobo, D.N. A Randomized, Controlled, Double-Blind Crossover Study on the Effects of 2-L Infusions of 0.9% Saline and Plasma-Lyte® 148 on Renal Blood Flow Velocity and Renal Cortical Tissue Perfusion in Healthy Volunteers. Ann. Surg. 2012, 256, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chavhan, G.B.; Babyn, P.S.; Vasanawala, S.S. Abdominal MR imaging in children: Motion compensation, sequence optimization, and protocol organization. Radiographics 2013, 33, 703–719. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, Y.; Xue, W.; Zuo, P.; Oesingmann, N.; Gan, Q.; Huang, Z.; Wu, M.; Hu, F.; Kuang, M.; et al. Arterial spin labelling MRI for detecting pseudocapsule defects and predicting renal capsule invasion in renal cell carcinoma. Clin. Radiol. 2017, 72, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Maldjian, J.A.; Pollock, J.M.; Burdette, J.H.; Yang, L.Y.; Deibler, A.R.; Kraft, R.A. A fast, effective filtering method for improving clinical pulsed arterial spin labeling {MRI}. J. Magn. Reson. Imaging 2009, 29, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Shirzadi, Z.; Crane, D.E.; Robertson, A.D.; Maralani, P.J.; Aviv, R.I.; Chappell, M.A.; Goldstein, B.I.; Black, S.E.; MacIntosh, B.J. Automated removal of spurious intermediate cerebral blood flow volumes improves image quality among older patients: A clinical arterial spin labeling investigation. J. Magn. Reson. Imaging 2015, 42, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Tanenbaum, A.B.; Snyder, A.Z.; Brier, M.R.; Ances, B.M. A method for reducing the effects of motion contamination in arterial spin labeling magnetic resonance imaging. J. Cereb. Blood Flow Metab. 2015, 35, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Tipirneni, A.; Johnson, P.; Loeffler, R.B.; Hillenbrand, C.M. Evaluation of respiratory liver and kidney movements for MRI navigator gating. J. Magn. Reson. Imaging 2011, 33, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Pham, D.; Gill, S.; Bressel, M.; Dang, K.; Devereux, T.; Kron, T.; Foroudi, F. An analysis of respiratory induced kidney motion on four-dimensional computed tomography and its implications for stereotactic kidney radiotherapy. Radiat. Oncol. 2013, 8. [Google Scholar] [CrossRef] [PubMed]
- Buxton, R.B. Quantifying CBF with arterial spin labeling. J. Magn. Reson. Imaging 2005, 22, 723–726. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sadowski, E.A.; Artz, N.S.; Seo, S.; Djamali, A.; Grist, T.M.; Fain, S.B. Measurement and comparison of T1 relaxation times in native and transplanted kidney cortex and medulla. J. Magn. Reson. Imaging 2011, 33, 1241–1247. [Google Scholar] [CrossRef] [PubMed]

| Technique | Labeling | Temporal Bolus Width | T1 Relaxation | Label Efficiency | SNR | Robustness |
|---|---|---|---|---|---|---|
| PASL (FAIR) | Spatial | Unknown * | More | More | Less | More |
| pCASL | Temporal | Labeling duration | Less | Less | More | Less |
| Readout | Nominal SNR | Spatial Resolution | Robustness to Motion | Background Suppression | Post-Labeling Delay | Typical Sequences |
|---|---|---|---|---|---|---|
| 2D (single or multislice) | ✓ | ✓✓✓ | ✓✓ | Slice-dependent | Slice-dependent | EPI [17], bSSFP [18] |
| 3D (segmented) | ✓✓✓ | ✓✓ | ✓ | Strongest, constant across slices | Constant across slices | GRASE [15], RARE [19] |
| 3D (single-shot) | ✓✓ | ✓ | ✓✓✓ |
| Reference | n | RBF (mL/100/min) * | p-Value (t-Test) | |
|---|---|---|---|---|
| ASL | DCE | |||
| Winter et al. [66] | 6 rabbits | 328 ± 59 | 298 ± 60 | >0.05 |
| Wu et al. [32] | 19 humans | 227 ± 30 | 272 ± 60 | <0.001 |
| Zimmer et al. [67] | 6 rats | HK: 416 ± 124 | HK: 542 ± 85 | <0.01 |
| AKI: 316 ± 102 | AKI: 407 ± 119 | <0.01 | ||
| Cutajar et al. [46] | 16 humans | 263 ± 41 | 287 ± 70 | 0.43 |
| Conlin et al. [59] | 7 humans | 151 ± 37 mL/min | 152 ± 41 mL/min | N/A |
| Reference | Labeling | PLD (s) (n) | Multi-PLD Fit | Mean RBF (mL/100 g/min) * | Δt * | Τ * | Quantification Highlights |
|---|---|---|---|---|---|---|---|
| [15] | FAIR | 0.1:0.2:2.7 (14) | Yes | 196 and 204 (two scans) | 143 ± 45 ms | N/A | 1st multi-PLD study. Repeatable ASL parameters. |
| [39] | EPISTAR | 0.25:0.1:1.85 (17) | No | 287 ± 49 | N/A | N/A | Single-PLD quantification at highest signal PLD (peak time = 1330 ± 148 ms). |
| [46] | FAIR | 0.1:0.2:2.7 (14) | Yes | 263 ± 41 | 0.3 ± 0.7 s | 1.2 ± 0.2 | ASL and DCE agree. ASL more repeatable. |
| [50] | FAIR | 0.1:0.2:2.7 (14) | Yes | Pre/post-nephrectomy: 186 ± 36/184 ± 37 | N/A | N/A | First study to assess RBF in healthy living kidney donors, pre and post-donation. |
| [17] | pCASL | 0.5:0.5:1.5 (3) | Yes | Young/older: 157 ± 38/117 ± 24 | Young/older (ms): 961 ± 260/1228 ± 227 | pCASL-defined (2.0) | Higher RBF/shorter Δt in young subjects. |
| [19] | FAIR | 0.3:0.3:2.1 (7) | Yes | 309 ± 31 | 110 ± 26 ms | 702 ± 69 ms | RBF from multi-PLD and single-PLD study similar. |
| [57] | FAIR | 1.2:0.2:2 (5) | No | Healthy subjects/Patients: 191 ± 9/102 ± 11 at PLD = 1.8 s | 700 ms (assumed) | N/A | RBF increased at higher PLDs. |
| [59] | FAIR | 0.15 + 0.2:0.1:1.6 (16) | Yes | Healthy subjects/Patients (mL/min): 151 ± 37/158 ± 103 | N/A | N/A | RBF derived from slope of ASL difference signal. |
| [60] | pCASL | 0.5:0.5:2.0 (4) | Yes | 215 ± 65 | 1141 ± 262 ms | pCASL-defined (2.0) | Cortical RBF repeatable. Poor reproducibility of cortical Δt, medullary RBF/Δt. |
| Motion Correction Technique | Prospective | Retrospective | Extra Setup Time | Extra Scan Time | Patient-Friendly | Easily Available | Time-Consuming Post-Processing | |
|---|---|---|---|---|---|---|---|---|
| Breath-holding | Traditional | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Synchronized breathing | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | |
| Respiratory-triggering (bellows) | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | |
| MR-navigators | ✓ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | |
| Snapshot Imaging | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | |
| Background-suppression | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | |
| Signal averaging | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ | |
| Data rejection | Visual sorting | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Automatic approaches | ✗ | ✓ | ✗ | ✗ | ✓ | ✗ | ✗ | |
| Image registration | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nery, F.; Gordon, I.; Thomas, D.L. Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities. Diagnostics 2018, 8, 2. https://doi.org/10.3390/diagnostics8010002
Nery F, Gordon I, Thomas DL. Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities. Diagnostics. 2018; 8(1):2. https://doi.org/10.3390/diagnostics8010002
Chicago/Turabian StyleNery, Fabio, Isky Gordon, and David L. Thomas. 2018. "Non-Invasive Renal Perfusion Imaging Using Arterial Spin Labeling MRI: Challenges and Opportunities" Diagnostics 8, no. 1: 2. https://doi.org/10.3390/diagnostics8010002




