The Use of Imaging in the Prediction and Assessment of Cancer Treatment Toxicity
Abstract
:1. Introduction
2. Neurological Toxicity
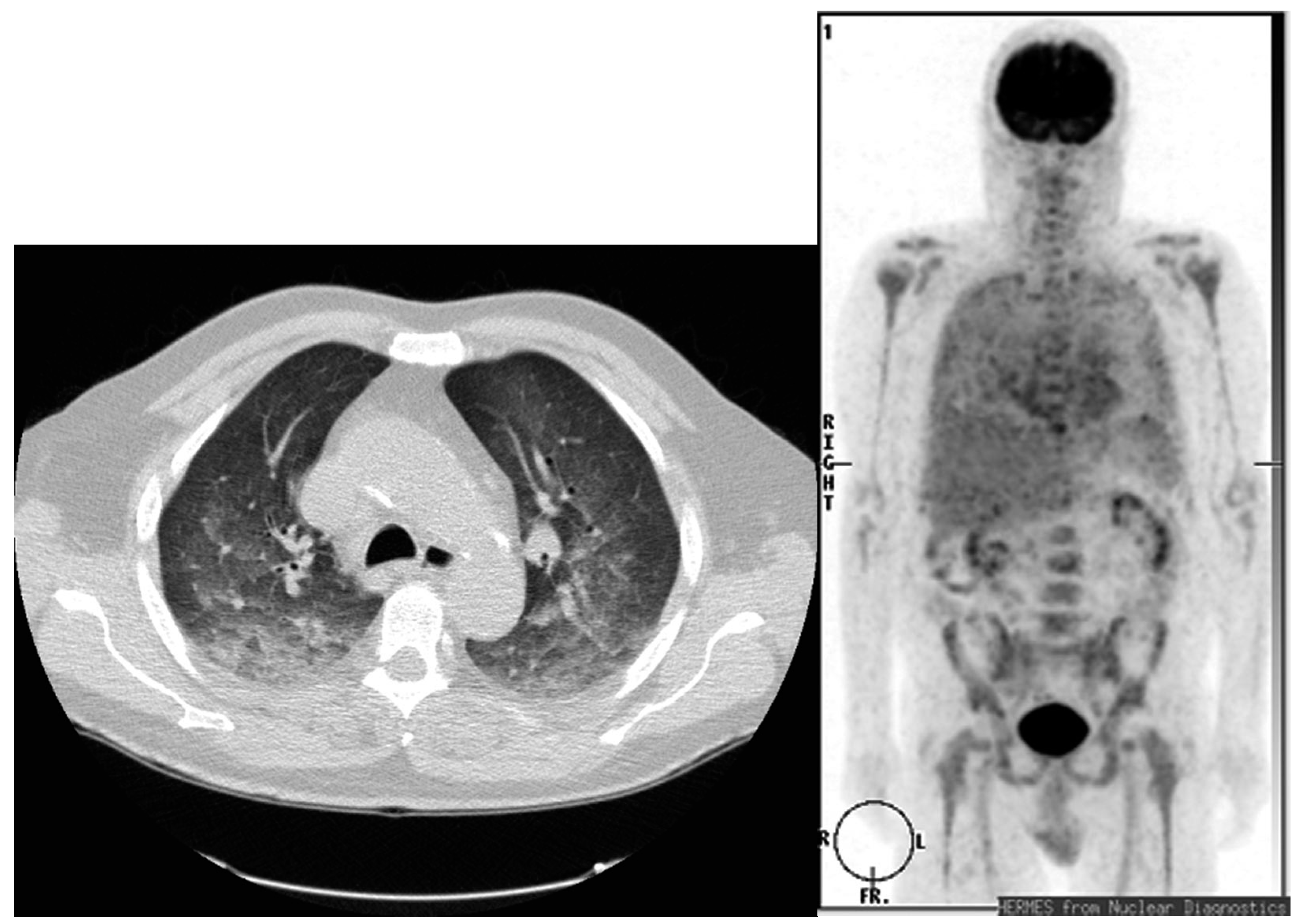
3. Pulmonary Toxicity
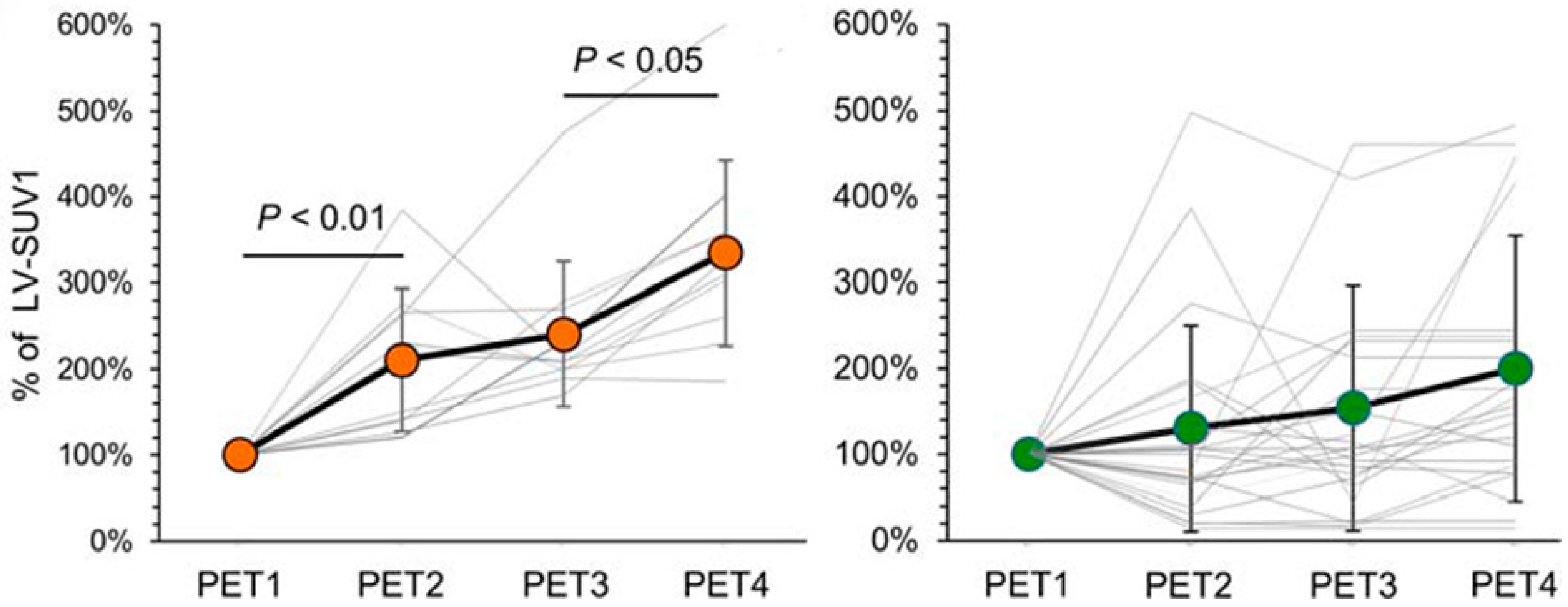
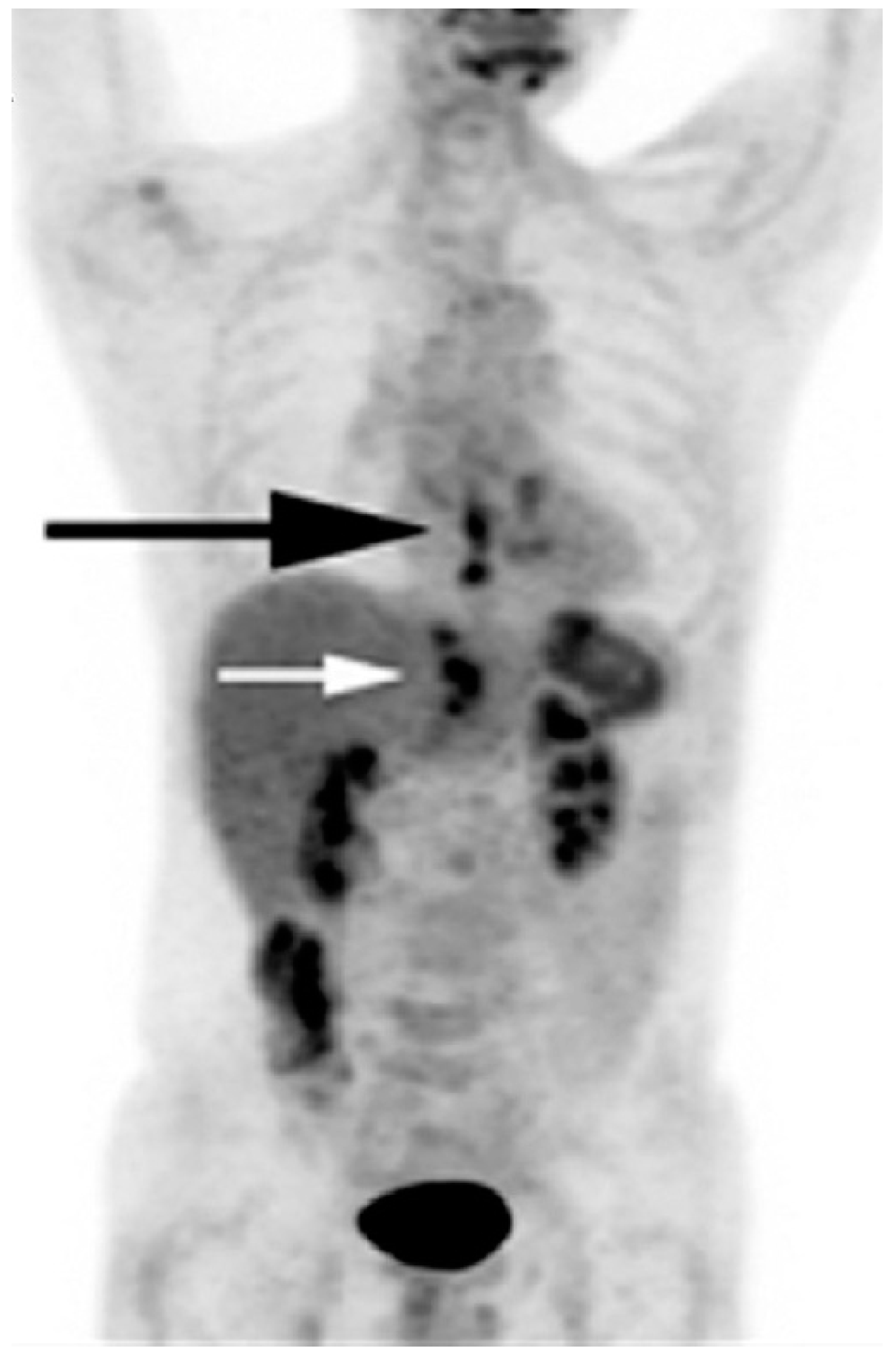
4. Cardiac Toxicity
5. Hepatic and Gastrointestinal Tract Toxicity
6. Urinary System Toxicity
7. Hematopoietic Toxicity
8. Skin Toxicity
9. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- US Department of Health and Human Services; National Institutes of Health (NIH); National Cancer Institute. Common Terminology Criteria for Adverse Events, version 4.03. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf (accessed on 28 May 2009).
- Holler Howard, S.A.; Krajewski, K.M.; Jagannathan, J.P.; Baschi-Amirfarzan, M.; Trumani, S.H.; Shinagare, A.B.; Ramaiya, N.H. A new look at toxicity in the era of precision oncology: Imaging findings, their relationship with tumor response, and effect on metastatectomy. Am. J. Roentgenol. 2016, 207, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Ricard, D.; Soussain, C.; Psimaras, D. Neurotoxicity of the CNS: Diagnosis, treatment and prevention. Rev. Neurol. 2011, 167, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.; Schmidt, R.E. Cancer therapy-associated CNS neuropathology: An update and review of the literature. Acta Neuropathol. 2006, 111, 197–212. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, J. Neurological complications of cancer chemotherapy. Curr. Opin. Oncol. 2006, 18, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, J.; Prust, M.; Kaiser, J. Chemotherapy, cognitive impairment and hipoocampal toxicity. Neuroscience 2015, 309, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Moon, C. Neurotoxicity of cancer chemotherapy. Neural Regen. Res. 2013, 8, 1606–1614. [Google Scholar] [PubMed]
- Froklage, F.E.; Reijneveld, J.C.; Heimans, J.J. Central neurotoxicity in cancer chemotherapy: Pharmacogenetic insights. Pharmacogenomics 2011, 12, 379–395. [Google Scholar] [CrossRef] [PubMed]
- Hoeffner, E.G. Central nervopus sytem complications of oncologic therapy. Hamatol. Oncol. Clin. N. Am. 2016, 30, 899–920. [Google Scholar] [CrossRef] [PubMed]
- Hodnett, P.; Coyle, J.; O’regan, K.; Maher, M.M.; Fanning, N. PRES (posterior reversible encephalopathy syndrome), a rare complication of tacrolimus therapy. Emerg. Radiol. 2009, 16, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.L.; Westcarth, L. Neurotoxicity associated with cancer therapy. J. Adv. Pract. Oncol. 2012, 3, 11–21. [Google Scholar]
- Dietrich, J.; Klein, J.P. Imaging of cancer therapy-induced central nervous system toxicity. Neurol. Clin. 2014, 32, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Arrillage-Romany, I.C.; Dietrich, J. Imaging findings in cancer therapy-associated neurotoxicity. Semin. Neurol. 2012, 32, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Futtere, S.F.; Nemeth, A.J.; Grimm, S.A.; Ragin, A.B.; Chandler, J.P.; Muro, K.; Marymont, M.H.; Raizer, J.J. Diffusion abnormalities of the corpus callosum in patients receiving bevacisumab for malignant brain tumors: Suspected treatment toxicity. J. Neurosci. 2014, 118, 147–153. [Google Scholar]
- Wennberg, B.; Gagliardi, G.; Sundbom, L.; Svane, G.; Lind, P. Early response of lung in breast cancer irradiation: Radiologic density changes measured by CT and symptomatic radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 1196–1206. [Google Scholar] [CrossRef]
- Farr, K.P.; Kallehauge, J.F.; Moller, D.S.; Khalil, A.A.; Kramer, S.; Bluhme, H.; Morsing, A.; Grau, C. Inclusion of functional information from perfusion SPECT improves predictive value of dose-volume parameters in lung toxicity outcome after radiotherapy for non-small cell lung cancer: A prospective study. Radiother. Oncol. 2015, 117, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Ugur, O.; Caner, B.; Balbay, M.D.; Ozen, H.A.; Remzi, D.; Ulutuncer, N.; Bekdik, C. Bleomycin lung toxicity detected by technetium-99m diethyl triamine penta-acetic acid aerosol scintingraphy. Eur. J. Nucl. Med. 1993, 20, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Kao, C.H.; Wang, S.J.; Yen, S.H. Lung toxicity of chemotherapeutic agents detected by Tc-99m DTPA radioaerosol inhalation lung scintigraphy. Neoplasma 1995, 42, 133–135. [Google Scholar] [PubMed]
- Petit, S.F.; van Elmpt, W.J.; Oberije, C.J.; Vegt, E.; Dingemans, A.M.; Lambin, P.; Dekker, A.L.; de Ruysscher, D. 18F-fluorodeoxyglucose uptake patterns in lung before radiotherapy identify areas more susceptible to radiation-induced lung toxicity in non-small-cell lung cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2011, 81, 698–705. [Google Scholar] [CrossRef] [PubMed]
- Castillo, R.; Pham, N.; Ansari, S.; Meshkov, D.; Castillo, S.; Li, M.; Olanrewaju, A.; Hobbs, B.; Castillo, E.; Guerrero, T. Pre-radiotherapy FDG PET predicts radiation pneumonitis in lung cancer. Radiat. Oncol. 2014, 9, 74. [Google Scholar] [CrossRef] [PubMed]
- Mac Manus, M.P.; Ding, Z.; Hogg, A.; Herschtal, A.; Binns, D.; Ball, D.L.; Hicks, R.J. Association between pulmonary uptake of fluorodeoxyglucose detected by positron emission tomography scanning after radiation therapy for non-small cell cancer and radiation pneumonitis. Int. J. Radiat. Oncol. Biol. Phys. 2011, 80, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Kalkanis, D.; Stefanovic, A.; Paes, F.; Escalon, M.P.; Serafini, A.; Lossos, I.S. 18F-fluorodeoxyglucose positron emission tomography combined with computed tomography detection of asymptomatic late pulmonary toxicity in patients with non-Hodgkin lymphoma treated with rituximab-containing chemotherapy. Leuk. Lymphoma 2009, 50, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Kazama, T.; Fraia, S.C.; Uchida, Y.; Ito, H.; Macapinlac, H.A. Pulmonary drug toxicity: FDG PET findings in patients with lymphoma. Ann. Nucl. Med. 2008, 22, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Taywade, S.K.; Kumar, R.; Bhethanabhotla, S.; Bai, C. Role G PET-CT in monitoring the cyclophosphamide induced pulmonary toxicity in patients with breast cancer-2 case reports. Nucl. Med. Mol. Imaging 2016, 50, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Von Rohr, L.; Klaeser, B.; Joerger, M.; Kluckert, T.; Cerry, T.; Gillessen, S. Increased pulmonary FDG uptake in bleomycin-associated pneumonitis. Onkologie 2007, 30, 320–323. [Google Scholar] [PubMed]
- Nishino, M.; Brais, L.K.; Brooks, N.V.; Hatabu, H.; Kulke, M.H.; Ramaiya, N.H. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: A radiographic pattern-based approach. Eur. J. Cancer 2016, 53, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Boiselle, P.M.; Morrin, M.M.; Huberman, M.S. Gemcitabine pulmonary toxicity: CT features. J. Comput. Assisted Tomogr. 2000, 24, 977–980. [Google Scholar] [CrossRef]
- Buchler, T.; Bomanji, J.; Lee, S.M. FDG-PET in bleomycin-induced pneumonitis following ABVD chemotherapy for Hodgkin’s disease—A useful tool for monitoring pulmonary toxicity and disease activity. Haematologica 2007, 92, e120–e121. [Google Scholar] [CrossRef] [PubMed]
- Post, M.C.; Grutters, J.C.; Verzijlbergen, J.F.; Biesma, D.H. PET scintigraphy of etoposide-induced pulmonary toxicity. Clin. Nucl. Med. 2007, 32, 683–684. [Google Scholar] [CrossRef] [PubMed]
- Chi, D.C.; Brogan, F.; Turenne, I.; Zelonis, S.; Schwartz, L.; Saif, M.W. Gemcitabine-induced pulmonary toxicity. Anticancer Res. 2012, 32, 4147–4149. [Google Scholar] [PubMed]
- Torrisi, J.M.; Schwartz, L.H.; Gollub, M.J.; Ginsberg, M.S.; Bosi, G.J.; Hricak, H. CT findings of chemotherapy-induced toxicity: What radiologists need to know about the clinical and radiologic manifestations of chemotherapy toxicity. Radiology 2011, 258, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Diederich, S. Chest CT for suspected pulmonary complications of oncologic therapies: How I review and report. Cancer Imaging 2016, 16, 7. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.F.; Smith, A.; Araujo, C.; Jagannathan, J.; Johnston, C.; O’Regan, K.; Shinagare, A.; Ramaiya, N. New targeted molecular therapies for cancer: Radiological response in intrathoracic malignancies and cardiopulmonary toxicity: What the radiologist needs to know. Cancer Imaging 2014, 14, 26. [Google Scholar] [PubMed]
- Rossi, S.E.; Erasmus, J.J.; McAdams, H.P.; Sporn, T.A.; Goodman, P.C. Pulmonary drug toxicity: Radiologic and pathologic manifestations. Radiographics 2000, 20, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Hamo, C.E.; Bloom, M.W.; Cardinale, D.; Ky, B.; Nohria, A.; Baer, L.; Skopicki, H.; Lenihan, D.J.; Gheorghiade, M.; Lyon, A.R.; et al. Cancer therapy-related cardiac dysfunction and heart failure: Part 2: Prevention, treatment, guidelines, and future directions. Circ. Heart Fail. 2016, 9, e002843. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Ethier, J.L.; Lee, D.S.; Thavendiranathan, P.; Amir, E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 53, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Rosa, G.M.; Gigli, L.; Tagliasacchi, M.I.; Di Iorio, C.; Carbone, F.; Nencioni, A.; Montecucco, F.; Brunelli, C. Update on cardiotoxicity of anti-cancer treatments. Eur. J. Clin. Investig. 2016, 46, 264–284. [Google Scholar] [CrossRef] [PubMed]
- Caro Coddon, J.; Rosillo Rodriguez, S.O.; Lopez Fernandez, T. Cardiotoxicity from the cardiologist’s perspective. Future Cardiol. 2015, 11, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Jurcut, R.; Wildiers, H.; Ganame, J.; D’hooge, J.; Parridaens, R.; Voight, J.U. Detection and monitoring of cardiotoxicity-what does modern cardiology offer? Support. Care Cancer 2008, 16, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Steinherz, L.J.; Steinherz, P.G.; Tan, C.T.; Heller, G.; Murphy, M.L. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA 1991, 266, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Markman, T.M.; Markman, M. Cardiotoxicity of antineoplastic agents: What is the present and future role for imaging? Curr. Oncol. Rep. 2014, 16, 396. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.; Scherrer-Crosbie, M.; Coelho-Filho, O.; Francis, S.A.; Neilan, T.G. Imaging methods for detection of chemotherapy-associated cardiotoxicity and dysfunction. Expert Rev. Cardiovasc. Ther. 2014, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Monsuez, J.J. Detection and prevention of cardiac complications of cancer chemotherapy. Ach. Cardiovasc. Dis. 2012, 105, 593–604. [Google Scholar] [CrossRef] [PubMed]
- Galderisi, M.; Marra, F.; Esposito, R.; Lomoriello, V.S.; Pardo, M.; de Divtiis, O. Cancer therapy and cardiotoxicity: The need of serial Doppler echocardiography. Cardiovasc. Ultrasound 2007, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Poulin, F.; Thavendiranathan, P. Cardiotoxicity due to chemotherapy: Role of cardiac imaging. Curr. Cardiol. Rep. 2015, 17, 564. [Google Scholar] [CrossRef] [PubMed]
- Yeh, E.T.; Bickford, C.L. Cardiovascular complications of cancer therapy: Incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009, 53, 2231–2247. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.G.; MvKenzie, W.B.; Alexander, J.; Sanger, P.; D’Souza, A.; Manatunga, A.; Schwartz, P.E.; Berger, H.J.; Setaro, J.; Surkin, L.; et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven year experience using serial radionuclide angiocardiograhy. Am. J. Med. 1987, 82, 1109–1118. [Google Scholar] [CrossRef]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002, 20, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Aiken, M.J.; Suhag, V.; Garcia, C.A.; Acio, E.; Moreau, S.; Priebat, D.A.; Chennupat, S.P.; van Nostrand, D. Doxorubicin-induced cardiac toxicity and cardiac rest gated blood pool imaging. Clin. Nucl. Med. 2009, 34, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Zellars, R.; Bravo, P.E.; Tryggestad, E.; Hopfer, K.; Myers, L.; Tahari, A.; Asrari, F.; Ziessman, H.; Garrett-Mayer, E. SPECT analysis of cardiac perfusion changes after whole-breast/chest wall radiation therapy with or without active breathing coordinator: Results of a randomized phase 3 trial. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Bauckneht, M.; Ferrarazzo, G.; Fiz, F.; Morbelli, S.; Sarocchi, M.; Pastorino, F.; Ghidella, A.; Pomposelli, E.; Miglino, M.; Ameri, P.; et al. Doxorubicin effect on myocardial metabolism as a pre-requisite for subsequent development of cardiac toxicity: A translational 18F-FDG PET/CT observation. J. Nucl. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Hundley, W.G. Understanding cardiovascular injury after treatment for cancer: An overview of current uses and future directions of cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2013, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Meyersohn, N.M.; Pursnani, A.; Neilan, T.G. Detection of cardiac toxicity due to cancer treatment: Role of cardiac MRI. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 396. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Wintersperger, B.J.; Flamm, S.D.; Marwick, T.H. Cardiac MRI in the assessment of cardiac injury and toxicity from cancer chemotherapy: A systematic review. Circ. Cardiovasc. Imaging 2013, 6, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Konski, A.; Li, T.; Christensen, M.; Cheng, J.D.; Yu, J.Q.; Crawford, K.; Haluszka, O.; Tokar, J.; Scott, W.; Meropoi, N.J.; et al. Symptomatic cardiac toxicity is predicted by dosimetric and patient factors rather than changes in 18F-FDG PET determination of myocardial activity after chemoradiotherapy for esophageal cancer. Radiother. Oncol. 2012, 104, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Inglese, E.; Leva, L.; Matheoud, R.; Sacchetti, G.; Secco, C.; Gandolfo, P.; Brambilia, M.; Sambuceli, G. Spatial and temporal heterogeneity of regional myocardial uptake in patients without heart disease under fasting conditions on repeated whole-body 18F-FDG PET/CT. J. Nucl. Med. 2007, 48, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Losik, S.B.; Studentsova, Y.; Margouleff, D. Chemotherapy-induced pericarditis on F-18 FDG positron emission tomography scan. Clin. Nucl. Med. 2003, 28, 913–915. [Google Scholar] [CrossRef] [PubMed]
- Floyd, J.; Mirza, I.; Sachs, B.; Perry, M.C. Hepatotoxicity of chemotherapy. Semin. Oncol. 2006, 33, 50–67. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.J. The effects of cancer chemotherapy on liver imaging. Eur. Radiol. 2009, 19, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Mortele, K.J.; Ros, P.R. Imaging of diffuse liver disease. Semin. Liver Dis. 2000, 21, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.J.; Anthony, M.-P.; Khong, P.L. Hepatic radiation injury in distal esophageal carcinoma: A case report. Clin. Nucl. Med. 2012, 37, 709–711. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, M.F.; Salanci, B.V.; Ugur, O. Intra-arterial radionuclide therapies for liver tumors. Semin. Nucl. Med. 2016, 46, 324–339. [Google Scholar] [CrossRef] [PubMed]
- Bester, L.; Meteling, B.; Boshell, D.; Chua, T.C.; Morris, D.L. Transarterial chemoembolisation and radioembolisation for the treatment of primary liver cancer and secondary liver cancer: A review of the literature. J. Med. Imaging Radiat. Oncol. 2014, 58, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H. Targeted radionuclide therapy: An evolution toward precision cancer treatment. Am. J. Roentgenol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Riaz, A.; Awais, R.; Salem, R. Side effects of yttrium-90 radioembolization. Front. Oncol. 2014, 4, 198. [Google Scholar] [CrossRef] [PubMed]
- Atassi, B.; Bangash, A.K.; Bahrani, A.; Pizzi, G.; Lewandowski, R.J.; Ryu, R.K.; Sato, K.T.; Gates, V.L.; Mulcahy, M.F.; Kulik, L.; et al. Multimodality imaging following 90Y radioembolization: A comprehensive review and pictorial essay. RadioGraphics 2008, 28, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, Q.; Sun, A.; Becker, N.; Higgins, J.; Marshall, A.; Le, L.W.; Vines, D.C.; McCloskey, P.; Ford, V.; Clarke, K.; et al. Predicting radiation esophagitis using 18F-FDG PET during chemoradiotherapy for locally advanced non-small cell lung cancer. J. Thorac. Oncol. 2016, 11, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Niedzielski, J.S.; Yang, J.; Liao, Z.; Gomez, D.R.; Stingo, F.; Mohan, R.; Martel, M.A.; Briere, T.M.; Court, L.E. 18F-fluorodeoxyglucose positron emission tomography can quantify and predict esophageal injury during radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 670–678. [Google Scholar] [CrossRef] [PubMed]
- Kelvin, F.M.; Gramm, H.F.; Gluck, W.L.; Lokich, J.J. Radiologic manifestation of small-bowel toxicity due to floxuridine therapy. Am. J. Roentgenol. 1996, 146, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Asnaciaos, A.; Naveau, S.; Perlemuter, G. Gastrointestinal toxicities of novel agents in cancer therapy. Eur. J. Cancer 2009, 45 (Suppl. 1), 332–342. [Google Scholar] [CrossRef]
- Galm, O.; Fabry, U.; Adam, G.; Osieka, R. Pneumatosis intestinalis following cytoxic or immunosuppressive treatment. Digestion 2001, 64, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Saif, M.W.; Elfiky, A.; Salem, R.R. Gastrointestinal perforation due to bevacizumab in colorectal cancer. Ann. Surg. Oncol. 2007, 14, 1860–1869. [Google Scholar] [CrossRef] [PubMed]
- McDonald, G.B.; Tirumali, N. Intestinal and liver toxicity of antineoplastic drugs. West. J. Med. 1984, 140, 250–259. [Google Scholar] [PubMed]
- Wade, D.S.; Nava, H.R.; Douglass, H.O., Jr. Neutropenic enterocolitis. Clinical diagnosis and treatment. Cancer 1992, 69, 17–23. [Google Scholar] [CrossRef]
- De Jonge, M.J.; Verweij, J. Renal toxicities of chemotherapy. Semin. Oncol. 2006, 33, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Rolleman, E.J.; Melis, M.; Valkema, R.; Boerman, O.C.; Krenning, E.P.; de Jong, M. Kidney protection during peptide receptor radionuclide therapy with somatostatin analogues. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Giesel, F.L.; Leotta, K.; Eder, M.; Hoppe-Tich, T.; Youssoufian, H.; Kopka, K.; Babich, J.W.; Haberkorn, U. PMPA for nephroprotection in PSMA-targeted radionuclide therapy of prostate cancer. J. Nucl. Med. 2015, 56, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Elicin, O.; Callaway, S.; Prior, J.O.; Bourhis, J.; Ozsahin, M.; Herrera, F.G. 18F-FDG-PET standard uptake value as a metabolic predictor of bone marrow response to radiation: Impact on acute and late hematological toxicity in cervical cancer patients treated with chemoradiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Rose, B.S.; Liang, Y.; Lau, S.K.; Jensen, L.G.; Yashar, C.M.; Hoh, C.K.; Mell, L.K. Correlation between radiation dose to 18F-FDG–PET defined active bone marrow subregions and acute hematologic toxicity in cervical cancer patients treated with chemoradiotherapy. Int. Radiat. Oncol. Biol. Phys. 2012, 83, 1185–1191. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; Arcadipane, F.; Ragona, R.; Lesca, A.; Gallio, E.; Mistrangelo, M.; Cassoni, P.; Arena, V.; Bustreo, S.; Faletti, R.; et al. Dose to specific subregions of pelvic bone marrow defined with FDG-PET as a predictor of hematologic nadirs during concomitant chemoradiation in anal cancer patients. Med. Oncol. 2016, 33, 72. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Yan, L.M.; Guo, K.Y.; Wang, Y.J.; Zou, F.; Gu, W.W.; Tang, H.; Li, Y.L.; Wu, S.J. The diagnostic value of 18F-FDG-PET/CT in hematopoietic radiation toxicity: A Tibet minipig model. J. Radiat. Res. 2012, 53, 537–544. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S.M.; Bhatia, S.K.; Sun, W.; Jacobson, G.M.; Menda, Y.; Ponto, L.L.; Smith, B.J.; Gross, B.A.; Bayouth, J.E.; Sunderland, J.J.; et al. Using 18F-fluorothymidine imaged with positron emission tomography to quantify and reduce hematologic toxicity due to chemoradiation therapy for pelvic cancer patients. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, H.R.; Prasad, V.; Schuchardt, C.; Baum, R.P. Is there correlation between peptide receptor radionuclide therapy-associated hematological toxicity and spleen dose? Recent Results Cancer Res. 2013, 194, 561–566. [Google Scholar] [PubMed]
- Kumar, K.; Singh, H.; Gupta, R.K.; Bal, C.; Kumar, R. Erlotinib-induced cutaneous toxicity: Findings on 18F-FDG PET/CT imaging. Clin. Nucl. Med. 2015, 40, e251–e252. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Rodriguez, I.; Garcia-Castano, A.; Quirce, R.; Jimenez-Bonilla, J.; Banzo, I. Erythema nodosum-like panniculitis as a false positive 18F-FDG PET/CT in advanced melanoma treated with dabrafenib and trametinib. Clin. Nucl. Med. 2017, 42, 44–46. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadvar, H. The Use of Imaging in the Prediction and Assessment of Cancer Treatment Toxicity. Diagnostics 2017, 7, 43. https://doi.org/10.3390/diagnostics7030043
Jadvar H. The Use of Imaging in the Prediction and Assessment of Cancer Treatment Toxicity. Diagnostics. 2017; 7(3):43. https://doi.org/10.3390/diagnostics7030043
Chicago/Turabian StyleJadvar, Hossein. 2017. "The Use of Imaging in the Prediction and Assessment of Cancer Treatment Toxicity" Diagnostics 7, no. 3: 43. https://doi.org/10.3390/diagnostics7030043



