Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays
Abstract
:1. Introduction
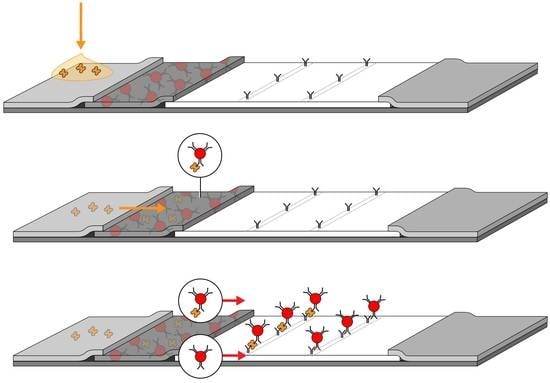
2. Conventional Lateral Flow Assay (LFA) Development
2.1. LFA Components
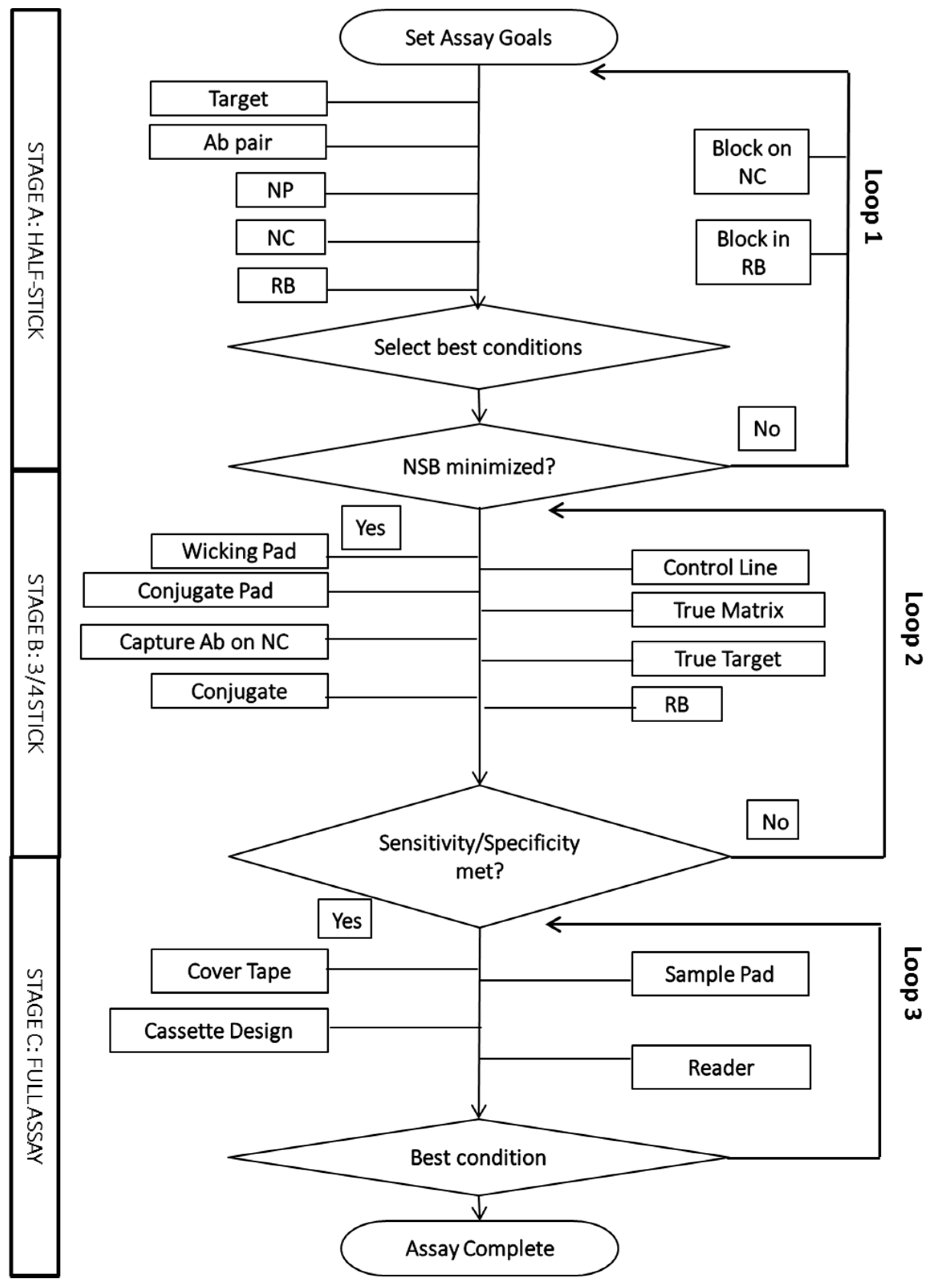
2.2. The Empirical Optimization Process
3. LFA Components and Analytical Tools for Characterization
3.1. Biological Reagents in LFAs
3.1.1. Target Molecules
3.1.2. Recognition Molecules
3.1.3. Clinical Samples
3.1.4. Analytical Tools for Biological Reagents
3.1.5. Reference Assays for Clinical Samples
3.2. LFA Materials
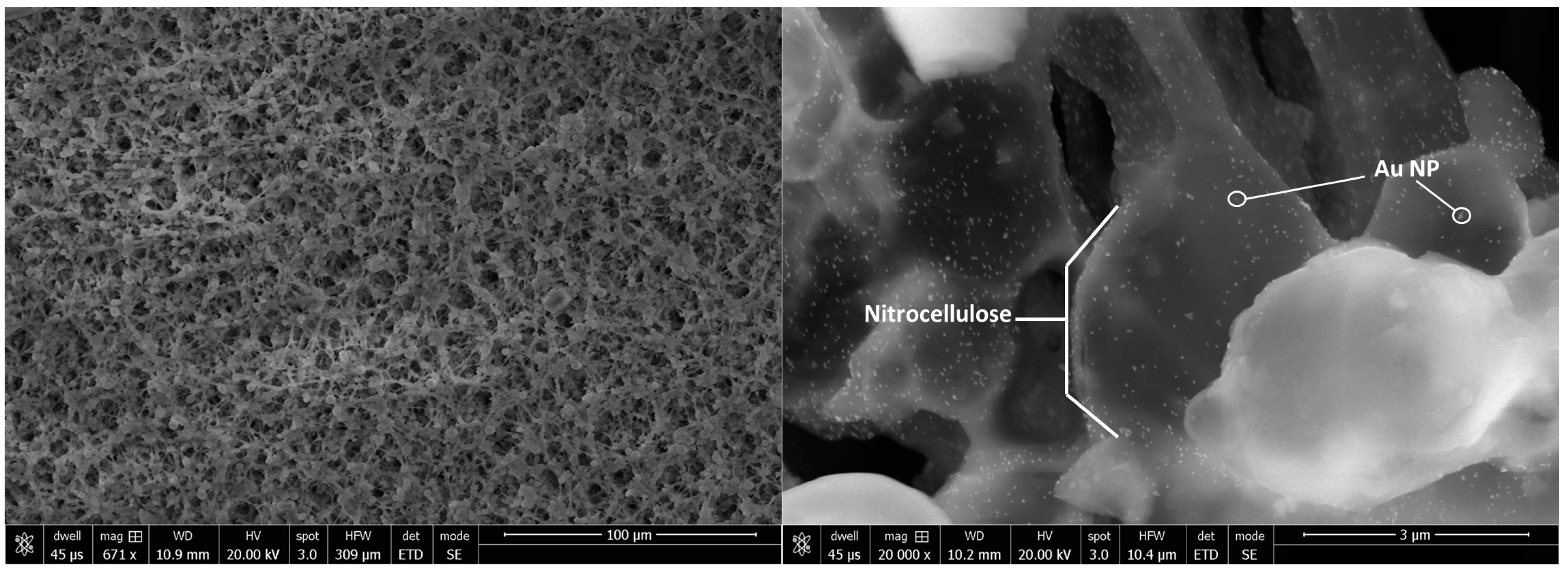
3.2.1. Membranes and Pads
3.2.2. Analytical Tools for Characterization of LFA Materials
3.3. Nanoparticles
3.3.1. Conjugated and Unconjugated Nanoparticles
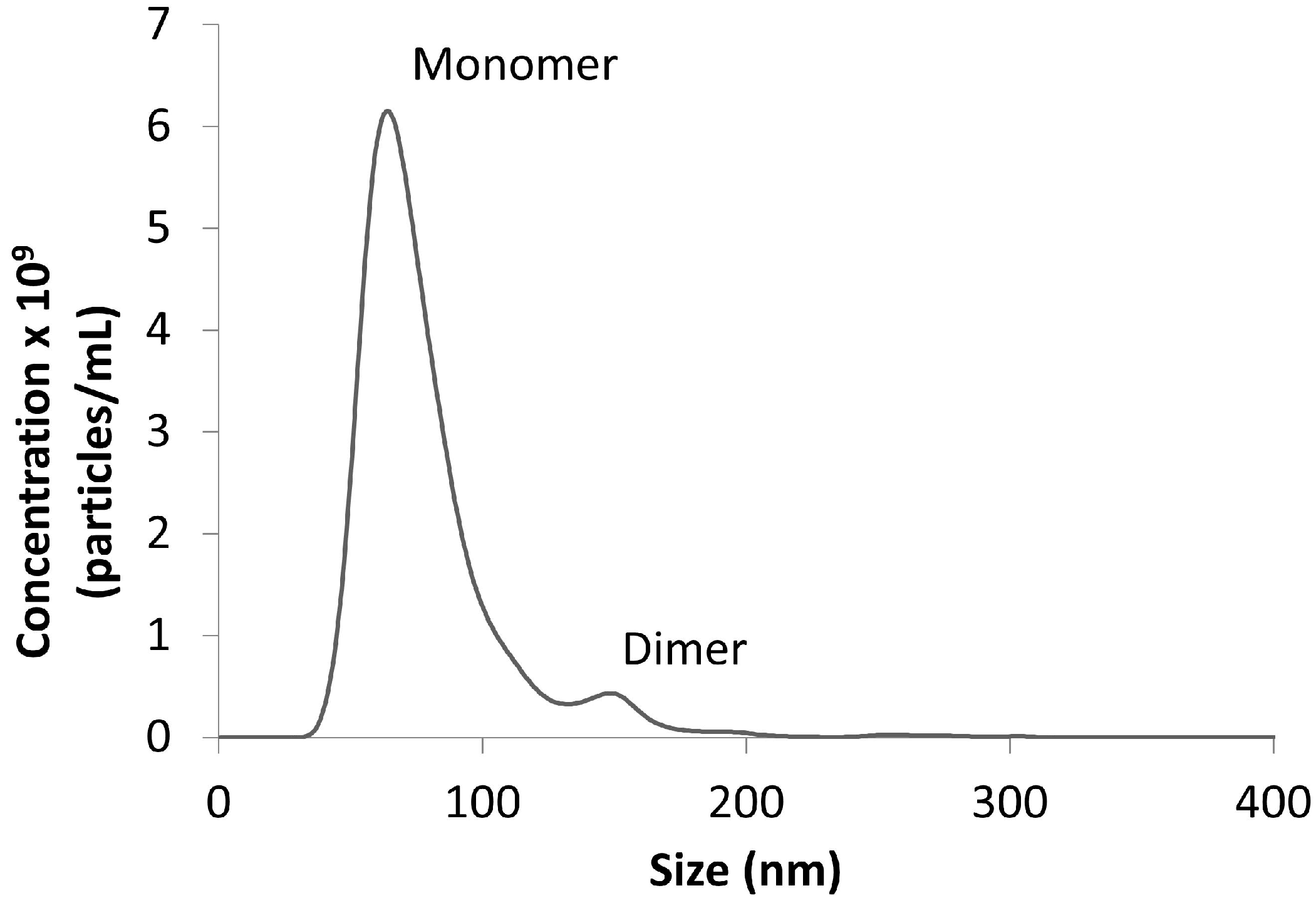
3.3.2. Analytical Tools for Characterization of Nanoparticles
3.4. The Assembled LFA
3.4.1. Visual Scoring
3.4.2. Analytical Readers
4. Advanced Analytical Optimization Tools
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. Paper-based microfluidic point-of-care diagnostic. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef] [PubMed]
- Brucker, M.C.; MacMullen, N.J. What’s new in pregnancy tests. JOGNN 1985, 14, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Mak, W.C.; Beni, V.; Turner, A.P.F. Lateral-flow technology: From visual to instrumental. Trends Analyt. Chem. 2016, 79, 297–305. [Google Scholar] [CrossRef]
- McPartlin, D.A.; O’Kennedy, R.J. Point-of-care diagnostics, a major opportunity for change in traditional diagnostic approaches: Potential and limitations. Expert Rev. Mol. Diagn. 2014, 14, 979–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Katani, R.; Li, L.; Hegde, N.; Roberts, E.L.; Kapur, V.; DebRoy, C. Rapid detection of Escherichia coli O157 and shiga toxins by lateral flow immunoassays. Toxins 2016, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Morioka, K.; Fukai, K.; Yosihda, K.; Kitano, R.; Yamazoe, R.; Yamada, M.; Nishi, T.; Kanno, T. Development and evaluation of a rapid antigen detection and serotyping lateral flow antigen detection system for foot-and-mouth disease virus. PLoS ONE 2015, 10, e0134931. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, B. Lateral Flow Immunoassay Systems: Evolution from the current state of the art to the next generation of highly sensitive, quantiative, rapid assays. In The Immunoassay Handbook, 4th ed.; Wild, D., Ed.; Elsevier: Oxford, UK, 2013; pp. 89–107. [Google Scholar]
- Lawn, S. Point-of-care detection of lipoarabinomannan (LAM) in urine for the diagnosis of HIV-associated tuberculois: A state of the art review. BMC Infect. Dis. 2012, 12, 103. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.; Lv, Q.; Wu, C.; Hao, H.; Zheng, Y.; Jiang, Y. Development of a lateral flow immunochromatographic assay for rapid detection of Mycoplasma pneumoniae-specific IgM in human serum specimens. J. Microbiol. Methods 2016, 124, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.; Huang, L.; Wang, H.; Qu, J.; Guo, Z.; Xie, C.; Zhu, Z.; Zhang, Y.; Du, Z.; Yan, Y.; et al. Development of an up-converting phosphor technology-based 10-channel lateral flow assay for profiling antibodies against Yersinia pestis. J. Microbiol. Methods 2010, 83, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Connelly, J.T.; Rolland, J.P.; Whitesides, G.M. “Paper Machine” for molecular diagnostics. Anal. Chem. 2015, 87, 7595–7601. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Nan, T.; Cui, Y.; Guo, S.; Zhang, W.; Zhang, R.; Tan, G.; Wang, B.; Cui, L. Development of a colloidal gold-based lateral flow dipstick immunoassay for rapid qualitative and semi-quantitative analysis of artesunate and dihydroartemisinin. Malar. J. 2014, 13, 127. [Google Scholar] [CrossRef] [PubMed]
- Gasperino, D.; Grant, B.; Dantzler, J.L.; Cate, D.; Weigl, B.H. Supporting high-sensitivity lateral flow assay development through predictive modeling. In Proceedings of the 20th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Dublin, Ireland, 9–13 October 2016. [Google Scholar]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Jauset-Rubio, M.; Svobodova, M.; Mairal, T.; McNeil, C.; Keegan, N.; Saeeed, A.; Abbas, M.N.; El-Shahawii, M.S.; Bashammakh, A.S.; Alyoubi, A.O.; et al. Ultrasensitive, rapid and inexpensive detection of DNA using paper based lateral flow assay. Sci. Rep. 2016, 6, 37732. [Google Scholar] [CrossRef] [PubMed]
- Butler, S.A.; Khanlian, S.A.; Cole, L.A. Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices. Clin. Chem. 2001, 47, 2131–2136. [Google Scholar] [PubMed]
- Blacksell, S.D. Commercial dengue rapid diagnostic tests for point-of-care application: Recent evaluations and future needs? J. Biomed. Biotechnol. 2012, 2012, 151967. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, A.; Takayama, K.; Nomura, N.; Yamamoto, N.; Sakoda, Y.; Kobayashi, Y.; Kida, H.; Shibasaski, F. Multi-colored immunochromatography using nanobeads for rapid and sensitive typing of seasonal influenza viruses. J. Virol. Methods 2014, 209, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Houde, D.J.; Berkowitz, S.A. Biophysical Characterization of Proteins in Developing Biopharmaceuticals; Elsevier: New York, NY, USA, 2014. [Google Scholar]
- Hnasko, R. ELISA: Methods and Protocols; Humana Press: New York, NY, USA, 2015. [Google Scholar]
- Dunbar, S.A.; Hoffmeyer, M.R. Microsphere-based multiplex immunoassays: Development and applications using Luminex XMAP technology. In The Immunoassay Handbook, 4th ed.; Wild, D., Ed.; Elsevier: Oxford, UK, 2013; pp. 157–174. [Google Scholar]
- Kerkhof, K.; Sluydts, V.; Willen, L.; Kim, S.; Canier, L.; Heng, S.; Tsuboi, T.; Sochantha, T.; Sovannaroth, S.; Ménard, D.; et al. Serological markers to measure recent changes in malaria at population level in Cambodia. Malar. J. 2016, 15, 529. [Google Scholar] [CrossRef] [PubMed]
- Abdiche, Y.; Malashock, D.; Pinkerton, A.; Pons, J. Determining kinetics and affinities of protein interactions using a parallel real-time label-free biosensor, the Octet. Anal. Biochem. 2008, 377, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Ye, H. Simultaneous determination of protein aggregation, dedradation, and absolute molecular weight by size exclusion chromatography-multiangle laser light scattering. Anal. Biochem. 2006, 356, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Minton, A.P. Recent applications of light scattering measurement in the biological and biopharmaceutical sciences. Anal. Biochem. 2016, 501, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Rissin, D. M.; Fournier, D.R.; Piech, T.; Patel, P.P.; Wilson, D.H.; Duffy, D.C. Single molecule enzyme-linked immunosorbent assays: Theoretical considerations. J. Immunol. Methods 2012, 378, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Armbruster, D.A.; Overcash, D.R.; Reyes, J. Clinical chemistry laboratory automation in the 21st Century-Amat Victoria curam (Victory loves careful preparation). Clin. Biochem. Rev. 2014, 35, 143–153. [Google Scholar] [PubMed]
- Van Dam, G.J.; de Dood, C.J.; Lewis, M.; Deelder, A. M.; van Lieshout, L.; Tanke, H.J.; van Rooyen, L.H.; Corstjens, P.L. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp. Parasitol. 2013, 135, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.; D'Andrea, A. Lateral flow assay with near-infrared dye for multiplex detection. Clin. Chem. 2013, 59, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Simerville, J.A.; Maxted, W.C.; Pahira, J.J. Urinalysis: A Comprehensive Review. Am. Fam. Physician 2005, 71, 1153–1162. [Google Scholar] [PubMed]
- Tortora, G.J.; Derrickson, B. Principles of Anatomy and Physiology, 14th ed.; John Wiley Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Tate, J.; Ward, G. Interferences in Immunoassay. Clin. Biochem. Rev. 2004, 25, 105–120. [Google Scholar] [PubMed]
- Lange, L.; Heide, M.L.; Hobolth, L.; Olson, L.W. Serological Detection of Plasmodiophora brassicae by Dot Immunobinding and Visualization of the Serological Reaction by Scanning Electron Microscopy. Phytopathology 1988, 79, 1066–1071. [Google Scholar] [CrossRef]
- EMD Millipore. Rapid Lateral Flow Test Strips: Considerations for Product Development; EMD Millipore Corporation: Billerica, MA, USA, 2013. [Google Scholar]
- Zhao, Y.; Wang, H.; Zhang, P.; Sun, C.; Wang, X.; Wang, X.; Yang, R.; Wang, C.; Zhou, L. Rapid multiplex detection of 10 foodborne pathogens with an up-converting phosphor technology-based 10-channel lateral flow assay. Sci. Rep. 2016. [Google Scholar] [CrossRef] [PubMed]
- Linares, E.M.; Kubota, L.T.; Michaelis, J.; Thalhammer, S. Enhancement of the detection limit for lateral flow immunoassays: Evaluation and comparison of bioconjugates. J. Immunol. Methods 2012, 375, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.B.; Tian, B.; Zhang, Z.P.; Wang, X.Y.; Fleming, J.; Bi, L.J.; Yang, R. F.; Zhang, X.E. Detection of Bacillus anthracis spores by super-paramagnetic lateral-flow immunoassays based on “Road Closure”. Biosens. Bioelectron. 2015, 67, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M. A. Gold nanoparticles: Optical properties and implementations in cancer diagnostics and photothermal therapy. J. Adv. Res. 2010, 1, 13–18. [Google Scholar] [CrossRef]
- Tassa, C.; Duffner, J.L.; Lewis, T.A.; Weissleder, R.; Schreiber, S.L.; Koehler, A.N.; Shaw, S.Y. Binding Affinity and Kinetic Analysis of Targeted Small Molecule-Modified Nanoparticles. Bioconjug. Chem. 2010, 21, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Bell, N. C.; Minelli, C.; Shard, A. G. Quantitation of IgG protein adsorption to gold nanoparticles using particle size measurement. Anal. Methods 2013, 5, 4591–4601. [Google Scholar] [CrossRef]
- James, A.E.; Driskell, J.D. Monitoring gold nanoparticle conjugation and analysis of biomolecular binding with nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS). Analyst 2013, 138, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Filipe, V.; Hawe, A.; Jiskoot, W. Critical evaluation of nanoparticle tracking analysis (NTA) by Nanosight for the measurement of nanoparticles and protein aggregates. Pharm. Res. 2010, 27, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Rice, S.B.; Chan, C.; Brown, S.C.; Eschbach, P.; Han, L.; Ensor, D.S.; Stefaniak, A.B.; Bonevich, J.; Vladár, A.E.; Walker, A.R.H.; et al. Particle size distributions by transmission electron microscopy: An interlaboratory comparison case study. Metrologia 2013, 50, 663–678. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gupta, A.; Verma, N.C.; Nandi, C. K. Kinetics of protein adsorption on gold nanoparticle with variable protein structure and nanoparticle size. J. Chem. Phys. 2015, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Worsley, G.J.; Kumarswami, N.; Minelli, C.; Noble, J.E. Characterisation of antibody conjugated particles and their influence on diagnostic assay response. Anal. Methods 2015, 7, 9596–9603. [Google Scholar] [CrossRef]
- Sharma, V.; Park, K.; Srinivasarao, M. Colloidal dispersion of gold nanorods: Historical background, optical properties, seed-mediated synthesis, shape separation and self-assembly. Mat. Sci. Eng. 2009, 65, 1–38. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, Z.; Boulware, D.R.; Pritt, B.S.; Sloan, L.M.; Gonzalez, I.J.; Bell, D.; Rees-Channer, R.R.; Chiodini, P.; Chan, W.C.; et al. Thermal contrast amplification reader yielding 8 Fold analytical improvement for disease detection with lateral flow assays. Anal. Chem. 2016, 88, 11774–11782. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, A.; Luong, J.H. Emerging technologies for next generation point-of-care testing. Trends Biotech. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Eltzov, E.; Guttel, S.; Kei, A.L.Y.; Sinawan, P.D.; Ionescu, R.E.; Marks, R.S. Lateral flow immunoassays-from paper strip to smartphone technology. Electroanalysis 2015, 27, 2116–2130. [Google Scholar] [CrossRef]
- Rantanen, J.; Khinast, J. The future of pharmaceutical manufacturing sciences. J. Pharm. Sci. 2015, 104, 3612–3638. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.A.; Patel, V.; Shih, J.; Macaraeg, C.; Wu, Y.; Thway, T.; Ma, M.; Lee, J.W.; DeSilva, B. Application of multi-factorial design of experiments to successfully optimize immunoassays for robust measurements of therapeutic proteins. J. Pharm. Biomed. Anal. 2009, 49, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Bau, H.H. A mathematical model of lateral flow bioreactions applied to sandwich assays. Anal. Biochem. 2003, 322, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ragavendar, M.S.; Anmol, C.M. A mathematical model to predict the optimal test line location and sample volume for lateral flow imunoassays. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2012, 2012, 2408–2411. [Google Scholar] [PubMed]
- Berli, C.L. A.; Kler, P.A. A quantiative model for lateral flow assay. Microfluid. Nanofluid. 2016, 20, 104. [Google Scholar] [CrossRef]
- Jiang, X.; Fan, Z.H. Fabrication and operation of paper-based analytical devices. Annu. Rev. Anal. Chem. 2016, 9, 203–222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Macdonald, J. Multiplexed lateral flow biosensors: Technological advances for radically improving point-of-care diagnoses. Biosens. Bioelectron. 2016, 83, 177–192. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Robinson, R.; Houghtaling, J.; Fridley, G.; Ramsey, S. A.; Fu, E. Investigation of reagent delivery formats in a multivalent malaria sandwich immunoassay and implications for assay performance. Anal. Chem. 2016, 88, 2311–2320. [Google Scholar] [CrossRef] [PubMed]
- Wong, R.; Tse, H. Lateral Flow Immunoassays; Humana Press: New York, NY, USA, 2009. [Google Scholar]

| Analytical Tool | Applications | Refs |
|---|---|---|
| UV-Vis Spectroscopy | Biological reagent concentration | [19] |
| Gel Electrophoresis | Biological reagent purity | [19] |
| ELISA | Screening antibody pairs; testing antibody sensitivity/specificity; reference assay | [20] |
| Luminex | Testing antibody sensitivity/specificity; multiplex systems; reference assay | [21,22] |
| Optical Biosensors (SPR, BLI) | Screening antibody pairs; antibody sensitivity/specificity; kinetic rates; reagent activity | [23] |
| SEC-MALS | Biological reagent purity/aggregation state | [24] |
| DLS | Biological reagent aggregation state; stability | [25] |
| CD, DSC, intrinsic protein fluorescence | Biological reagent stability | [19] |
| Digital ELISA | Reference assay | [26] |
| Automated clinical analyzers | Analysis of clinical samples; reference assay | [27] |
| Analytical Tool | Applications | References |
|---|---|---|
| UV-Vis | Protein adsorption, NP particle concentration/aggregation | [38] |
| Optical biosensors | Activity | [39] |
| DLS | Protein adsorption, NP aggregation | [40,41] |
| Nanoparticle tracking analysis | Protein adsorption, NP concentration/aggregation state | [40,41,42] |
| TEM | Shape/size | [43] |
| Zeta potential | Protein adsorption, NP stability | [44] |
| DCS | Protein adsorption | [45] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, H.V.; Dantzler, J.L.; Weigl, B.H. Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays. Diagnostics 2017, 7, 29. https://doi.org/10.3390/diagnostics7020029
Hsieh HV, Dantzler JL, Weigl BH. Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays. Diagnostics. 2017; 7(2):29. https://doi.org/10.3390/diagnostics7020029
Chicago/Turabian StyleHsieh, Helen V., Jeffrey L. Dantzler, and Bernhard H. Weigl. 2017. "Analytical Tools to Improve Optimization Procedures for Lateral Flow Assays" Diagnostics 7, no. 2: 29. https://doi.org/10.3390/diagnostics7020029