Beyond Screening: Can the Mini-Mental State Examination be Used as an Exclusion Tool in a Memory Clinic?
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Neuropsychological and Clinical Evaluation
2.3. Diagnosis of Dementia
2.4. Determination on Optimal Cut-off Scores of the MMSE
2.5. Statistical Analysis
3. Results
| Characteristics | Dementia-Free (n = 228) | Dementia (n = 488) | p |
|---|---|---|---|
| Age, mean (SD) | 67.6 (11.1) | 75.9 (8.3) | <0.001 |
| Education, no or primary level, n (%) | 48.7% (111) | 72.8% (355) | <0.001 |
| Gender, male, n (%) | 44.3% (101) | 45.3% (221) | 0.81 |
| Ethnicity, Chinese, n (%) | 82.5% (188) | 81.6% (398) | 0.84 |
| Non-Chinese, n (%) | 17.5% (40) | 18.4% (90) | |
| MMSE total score, mean (SD) | 23.2 (5.2) | 14.5 (5.3) | <0.001 |
| Group | AUC (95% CI) | Cut-off Score | SP | SE | No. (%) of Patients Correctly Identified as Having Dementia | No. (%) of Dementia-Free Patients Misclassified as Dementia | PLR |
|---|---|---|---|---|---|---|---|
| Whole group (n = 716) | 0.87 (0.84–0.90) | 4/5 * | 100% | 2.9% | 14 (2.0%) | 0 | - |
| 5/6 | 99.6% | 4.5% | 22 (3.1%) | 1 (0.1%) | 11.3 | ||
| 6/7 | 99.1% | 6.6% | 32 (4.5%) | 2 (0.3%) | 7.3 | ||
| 7/8 | 99.1% | 8.2% | 40 (5.6%) | 2 (0.3%) | 9.1 | ||
| 8/9 Ϯ | 99.1% | 11.9% | 58 (8.1%) | 2 (0.3%) | 13.2 | ||
| No or primary level of education (n = 474) | 0.84 (0.80–0.89) | 4/5 * | 100% | 3.0% | 11 (2.3%) | 0 | - |
| 5/6 | 99.1% | 5.2% | 18 (3.8%) | 1 (0.2%) | 5.8 | ||
| 6/7 | 98.2% | 7.7% | 27 (5.7%) | 2 (0.4%) | 4.3 | ||
| 7/8 | 98.2% | 9.9% | 34 (7.2%) | 2 (0.4%) | 5.5 | ||
| 8/9 Ϯ | 98.2% | 14.6% | 51 (10.8%) | 2 (0.4%) | 8.1 | ||
| Secondary and higher level of education (n = 242) | 0.89 (0.77–1.00) | 9/10 * | 100% | 7.1% | 9 (3.7%) | 0 | - |
| 10/11 | 99.1% | 8.7% | 11 (4.5%) | 1 (0.4%) | 9.7 | ||
| 11/12 | 99.1% | 13.5% | 17 (7.0%) | 1 (0.4%) | 15.0 | ||
| 12/13 | 99.1% | 16.7% | 21 (8.7%) | 2 (0.8%) | 18.6 | ||
| 13/14 | 99.1% | 20.6% | 26 (10.7%) | 2 (0.8%) | 22.9 | ||
| 14/15 | 98.3% | 30.2% | 35 (14.5%) | 2 (0.8%) | 17.8 | ||
| 15/16 | 98.3% | 35.7% | 44 (18.2%) | 2 (0.8%) | 21.0 | ||
| 16/17 Ϯ | 98.3% | 40.5% | 50 (20.7%) | 2 (0.8%) | 23.8 |
| Group | AUC (95% CI) | Cut-off Score | SP | SE | No. (%) of Patients Correctly Identified as Dementia-Free | No. (%) of Dementia Subjects Misclassified as Dementia-Free | NLR |
|---|---|---|---|---|---|---|---|
| Whole group (n = 716) | 0.87 (0.84–0.90) | 21/22 | 68.4% | 91.4% | 156 (21.8%) | 42 (5.9%) | 0.1 |
| 22/23 | 64.0% | 94.1% | 144 (20.1%) | 28 (3.9%) | 0.09 | ||
| 23/24 | 57.9% | 95.5% | 131 (18.3%) | 21 (2.9%) | 0.08 | ||
| 24/25 | 52.6% | 97.1% | 121 (16.9%) | 16 (2.2%) | 0.06 | ||
| 25/26 | 45.6% | 97.7% | 104 (14.5%) | 11 (1.5%) | 0.05 | ||
| 26/27 | 34.2% | 98.6% | 76 (10.6%) | 7 (1.0%) | 0.04 | ||
| 27/28 | 22.4% | 99.2% | 50 (7.0%) | 4 (0.6%) | 0.04 | ||
| 28/29 * | 6.2% | 100% | 14 (2.0%) | 0 | 0 | ||
| No or primary level of education (n = 474) | 0.84 (0.80–0.89) | 21/22 | 46.4% | 95.9% | 51 (10.8%) | 14 (3.0%) | 0.09 |
| 22/23 | 39.3% | 98.3% | 43 (9.1%) | 6 (1.3%) | 0.04 | ||
| 23/24 | 30.4% | 99.2% | 35 (7.4%) | 3 (0.6%) | 0.03 | ||
| 24/25 | 27.7% | 99.4% | 32 (6.8%) | 2 (0.4%) | 0.02 | ||
| 25/26 | 22.3% | 99.4% | 25 (5.3%) | 2 (0.4%) | 0.03 | ||
| 26/27 | 14.3% | 99.7% | 15 (3.2%) | 1 (0.2%) | 0.02 | ||
| 27/28 * | 6.3% | 100% | 7 (1.5%) | 0 | 0 | ||
| Secondary and higher level of education (n = 242) | 0.89 (0.77–1.00) | 24/25 | 76.7% | 90.5% | 89 (36.8%) | 14 (5.8%) | 0.1 |
| 25/26 | 68.1% | 92.9% | 79 (32.6%) | 9 (3.7%) | 0.1 | ||
| 26/27 | 53.4% | 95.2% | 55 (22.7%) | 6 (2.5%) | 0.09 | ||
| 27/28 | 37.9% | 96.8% | 43 (17.8%) | 4 (1.7%) | 0.08 | ||
| 28/29 * | 21.6% | 100% | 25 (10.3%) | 0 | 0 |
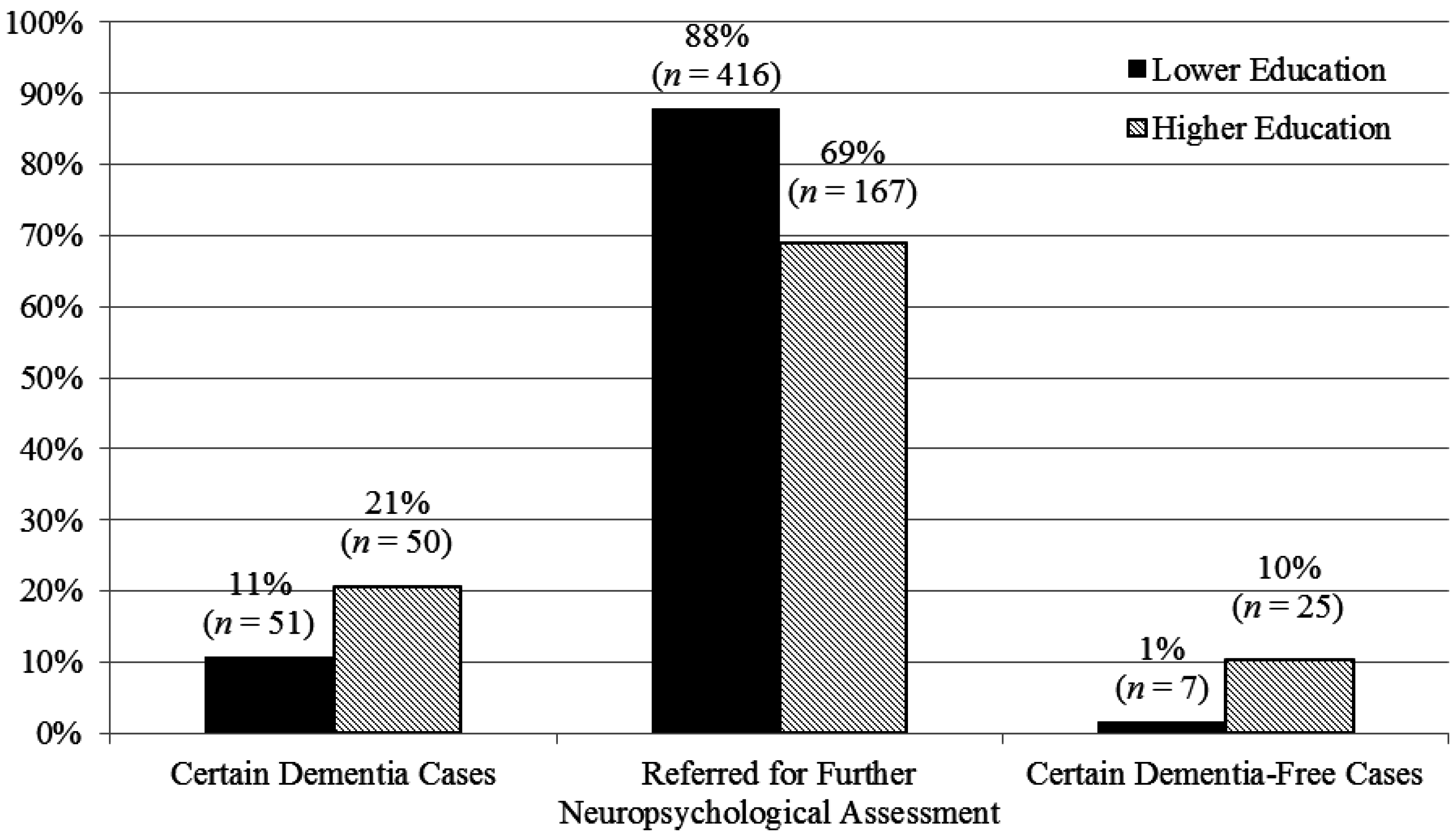
| Group | Education Stratification | Cut-off Scores | Optimal PLR | Cut-off Scores | Optimal NLR | ||
|---|---|---|---|---|---|---|---|
| No. (%) of patients not requiring neuropsychological assessment | No. (%) of dementia-free patients incorrectly categorized as dementia patients | No. (%) of dementia-free subjects not requiring neuropsychological assessment | No. (%) of dementia patients incorrectly categorized as dementia-free | ||||
| Before stratification | - | 8/9 | 58 (8.1%) | 2 (0.3%) | 28/29 | 14 (2.0%) | 0 |
| - | |||||||
| After stratification | no or primary level | 8/9 | 101 (14.1%) | 4 (0.6%) | 27/28 | 32 (4.5%) | 0 |
| Secondary and above level | 16/17 | 28/29 | - | ||||
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-Mental State”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiat. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tangalos, E.G.; Smith, G.E.; Ivnik, R.J.; Petersen, R.C.; Kokmen, E.; Kurland, L.T.; Offord, K.P.; Parisi, J.E. The Mini-Mental State Examination in general medical practice: Clinical utility and acceptance. Mayo Clin. Proc. 1996, 71, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Teresi, J.A.; Holmes, D.; Ramirez, M.; Gurland, B.J.; Lantigua, R. Performance of cognitive tests among different racial/ethnic and education groups: Findings of differential item functioning and possible test bias. Aging Ment. Health 2001, 7, 79–89. [Google Scholar]
- Hopp, G.; Dixon, R.; Grut, M.; Blackman, L. Longitudinal and psychometric profiles of two cognitive status tests in very old adults. J. Clin. Psychol. 1997, 53, 673–686. [Google Scholar] [CrossRef]
- Mendiondo, M.; Wesson, A.; Kryscio, R.; Schmitt, F. Modeling mini mental state examination changes in Alzheimer’s disease. Stat. Med. 2000, 19, 1607–1616. [Google Scholar] [CrossRef]
- Brown, L.M.; Schinka, J.A.; Mortimer, J.A.; Graves, A.B. 3MS normative data for elderly African Americans. J. Clin. Exp. Neuropsychol. 2003, 25, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Marcoulos, B.A.; McLain, C.A. Are our norms “normal”? A 4-year follow-up study to a biracial sample of rural elders with low education. Clin. Neuropsychol. 2003, 17, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.M.; Sachdev, P.S.; Brodaty, H.; Trollor, J.N.; Andrews, G. Effects of sociodemographic and health variables on minimental state exam scores in older Australians. Am. J. Geriatr. Psychiatry 2007, 15, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.; Pinto, J.A.; Lopes, M.A.; Litvoc, J.; Bottino, C.M. Impact of sociodemographic and health variables on Mini-Mental State Examination in a community-based sample of older people. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 260, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Bleeckr, M.L.; Bolla-Wilson, K.; Kawas, C.; Agnew, J. Age specific norms for the Mini-Mental State Exam. Neurology 1988, 38, 1565–1568. [Google Scholar] [CrossRef]
- Crum, R.; Anthony, J.C.; Basset, S.S.; Folstein, M.F. Population based norms for the Mini-Mental State Examination by age and educational level. JAMA 1993, 269, 2386–2391. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N.; Gallo, J.J. Education bias in the Mini-Mental State Examination. Int. Psychogeriatr. 2001, 13, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R.; Fanjiang, G. Mini-Mental State Examination User’s Guide; Psychological Assessment Resources, Inc.: Odessa, FL, USA, 2001. [Google Scholar]
- Ng, T.P.; Niti, M.; Chiam, P.C.; Kua, E.H. Ethnic and educational differences in cognitive test performance on Mini-Mental State Examination in Asians. Am. J. Geriatr. Psychiatry 2007, 15, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cossa, F.M.; Della, S.S.; Musicco, M.; Spinnler, H.; Ubezio, M.C. Comparison of two scoring systems of the Mini-Mental State Examination as a screening test for dementia. J. Clin. Epidemiol. 1997, 50, 961–965. [Google Scholar] [CrossRef]
- Heun, R.; Papassotiropoulos, A.; Jennssen, F. The validity of psychometric instruments for detection of dementia in the elderly general population. Int. J. Geriatr. Psychiatry 1998, 13, 368–380. [Google Scholar] [CrossRef]
- Hayden, K.M.; Khachaturian, A.S.; Tschanz, J.T.; Corcoran, C.; Nortond, M.; Breitner, J.C.; Cache County Study Group. Characteristics of a two-stage screen for incident dementia. J. Clin. Epidemiol. 2003, 56, 1038–1045. [Google Scholar] [CrossRef]
- Feng, L.; Chong, M.S.; Lim, W.S.; Ng, T.P. The modified Mini-Mental State Examination Test: Normative data for Singapore Chinese older adults and its performance in detecting early cognitive impairment. Singapore Med. J. 2012, 53, 458–462. [Google Scholar] [PubMed]
- Randolph, C. Repeatable Battery for the Assessment of Neuropsychological Status (RBANS); San Antonio: Harcourt, TX, USA, 1998. [Google Scholar]
- Mack, W.J.; Freed, D.M.; Williams, B.W.; Hendersen, V.W. Boston Naming Test: Shortened versions for use in Alzheimer’s disease. J. Gerontol. 1992, 47, 154–158. [Google Scholar] [CrossRef]
- D’Elia, L.F.; Satz, P.; Uchiyama, C.L.; White, T. Color Trails Test; Psychological Assessment Resources, Inc.: Lutz. FL, USA, 1996. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th ed.; American Psychiatric Association: Arlington, WV, USA, 2000. [Google Scholar]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- McGee, S. Simplifying likelihood ratios. J. Gen. Intern. Med. 2002, 17, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Morris, J.C. Reply: The impact of dementia prevalence on the utility of the AD8. Brain 2012, 135, e204. [Google Scholar] [CrossRef]
- Hogervorst, E.; Combrinck, M.; Lapuerta, P.; Rue, J.; Swales, K.; Budge, M. The Hopkins Verbal Learning Test and screening for dementia. Dement. Geriatr. Cogn. Disord. 2002, 13, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Xiao, S.; Rahardjo, T.B.; Hogervorst, E.J. Tofu intake is associated with poor cognitive performance among community-dwelling elderly in China. J. Alzheimer’s Dis. 2015, 43, 669–675. [Google Scholar]
- .Xu, X.; Rahardjo, T.B.; Xiao, S.F.; Hogervorst, E. The Hopkins Verbal Learning Test and detection of MCI and mild dementia: A literature review. J. Alzheimer’s Dis. Parkinsonism 2014, 4. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Schramm, U.; Berger, G.; Muller, R.; Kratzsch, T.; Peters, J.; Frölich, L. Psychometric properties of Clock Drawing Test and MMSE or Short Performance Test (SKT) in dementia screening in a memory clinic population. Int. J. Geriatr. Psychiatry 2002, 17, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Milian, M.; Leiherr, A.M.; Straten, G.; Müller, S.; Leyhe, T.; Eschweiler, G.W. The Mini-Cog versus the Mini-Mental State Examination and the Clock Drawing Test in daily clinical practice: Screening value in a German Memory Clinic. Int. Psychogeriatr. 2012, 24, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Uhlmann, R.F.; Larson, E.B. Effect of education on the Mini-Mental State Examination as a screening test for dementia. J. Am. Geriatr. Soc. 1991, 39, 876–880. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.C.; Teng, E.L.; Lin, K.N.; Hsu, T.C.; Guo, N.W.; Chou, P.; Hu, H.H.; Cheng, W.N.; Chiang, B.N. Performance on a dementia screening test in relation to demographic variables: Study of 5297 community residents in Taiwan. Arch. Neurol. 1994, 51, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Mungas, D.; Marshall, S.C.; Weldon, M.; Haan, M.; Reed, B.R. Age and education correction of Mini-Mental State Examination for English and Spanish-speaking elderly. Neurology 1996, 46, 700–706. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Humphreys, J.D.; Smith, G.E.I.R.; Ivnik, R.J.; Graff-Radford, N.R.; Petersen, R.C.; Lucas, J.A. Detecting dementia with the Mini-Mental State Examination in highly educated individuals. Arch. Neurol. 2008, 65, 963–967. [Google Scholar] [CrossRef]
- Catindig, J.A.; Venketasubramanian, N.; Ikram, M.K.; Chen, C. Epidemiology of dementia in Asia: Insights on prevalence, trends and novel risk factors. J. Neurol. Sci. 2012, 321, 6–11. [Google Scholar] [CrossRef]
- Graham, J.E.; Rockwood, K.; Beattie, B.L.; McDowell, I.; Eastwood, R.; Gauthier, S. Standardization of the diagnosis of dementia in the Canadian study of health and aging. Neuroepidemiology 1996, 15, 246–256. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Chong, E.; Hilal, S.; Ikram, M.K.; Venketasubramanian, N.; Chen, C. Beyond Screening: Can the Mini-Mental State Examination be Used as an Exclusion Tool in a Memory Clinic? Diagnostics 2015, 5, 475-486. https://doi.org/10.3390/diagnostics5040475
Xu X, Chong E, Hilal S, Ikram MK, Venketasubramanian N, Chen C. Beyond Screening: Can the Mini-Mental State Examination be Used as an Exclusion Tool in a Memory Clinic? Diagnostics. 2015; 5(4):475-486. https://doi.org/10.3390/diagnostics5040475
Chicago/Turabian StyleXu, Xin, Eddie Chong, Saima Hilal, Mohammad Kamran Ikram, Narayanaswamy Venketasubramanian, and Christopher Chen. 2015. "Beyond Screening: Can the Mini-Mental State Examination be Used as an Exclusion Tool in a Memory Clinic?" Diagnostics 5, no. 4: 475-486. https://doi.org/10.3390/diagnostics5040475





