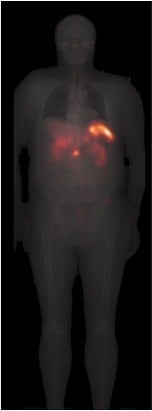
Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy
Abstract
:1. Introduction
1.1 Radiobiological Effects of Ionizing Radiation
1.2 Clinical Protocols for Radionuclide Therapy
1.3 Examples of Radionuclide Therapies Based on Dose Planning
2. Absorbed Dose Calculations
2.1. MIRD Calculation Scheme
2.2. BED Calculations
3. The Scintillation Camera
3.1. Principles
3.2. Calibration
4. Physical Factors that Affect the Activity Measurement
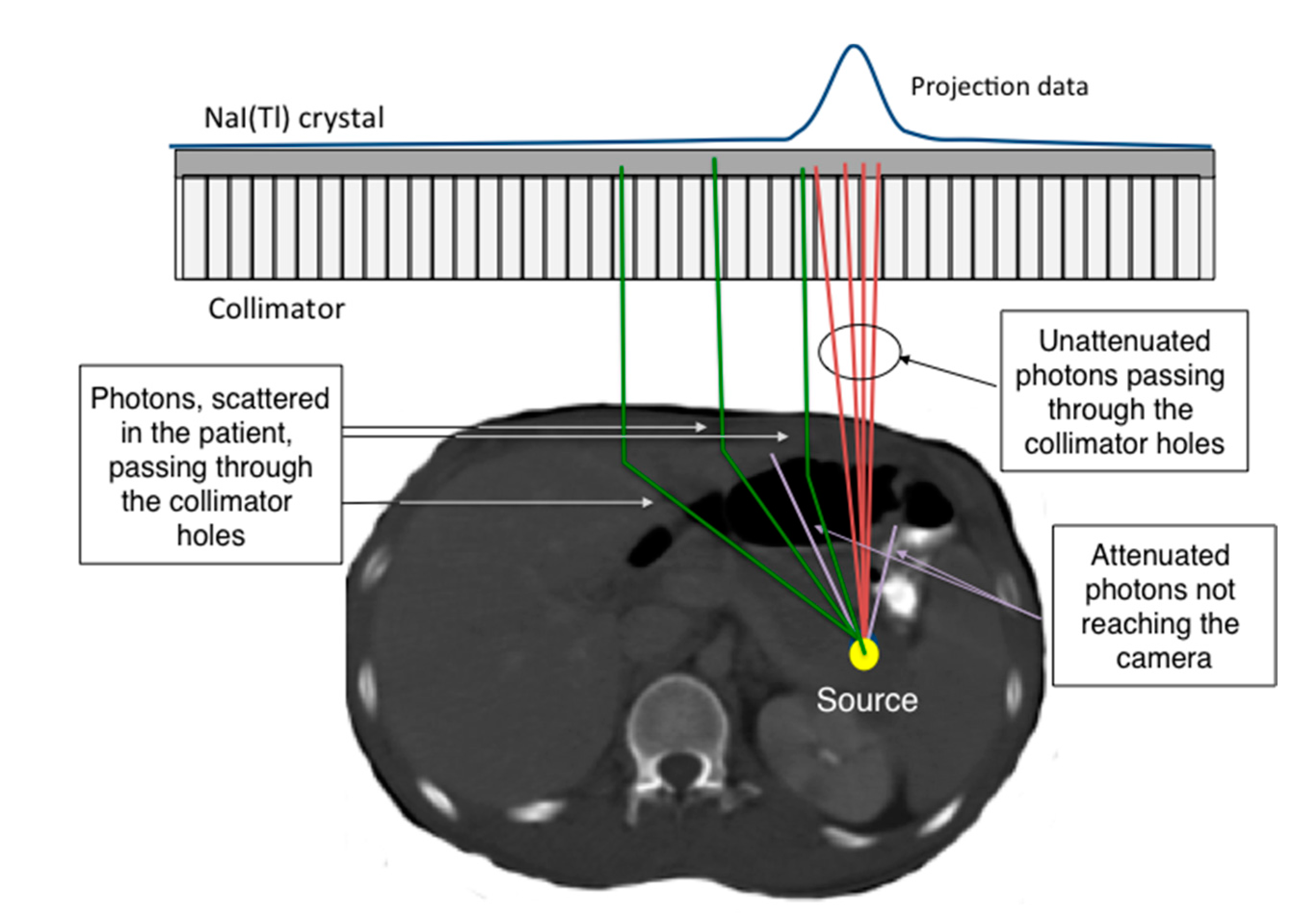
4.1. Photon Attenuation
4.2. Scatter Contribution
4.3. Collimator Resolution
5. Planar-Image Based Activity Quantification

5.1. Conjugate-View Principles
5.2. Attenuation Correction Based on X-Ray Scout
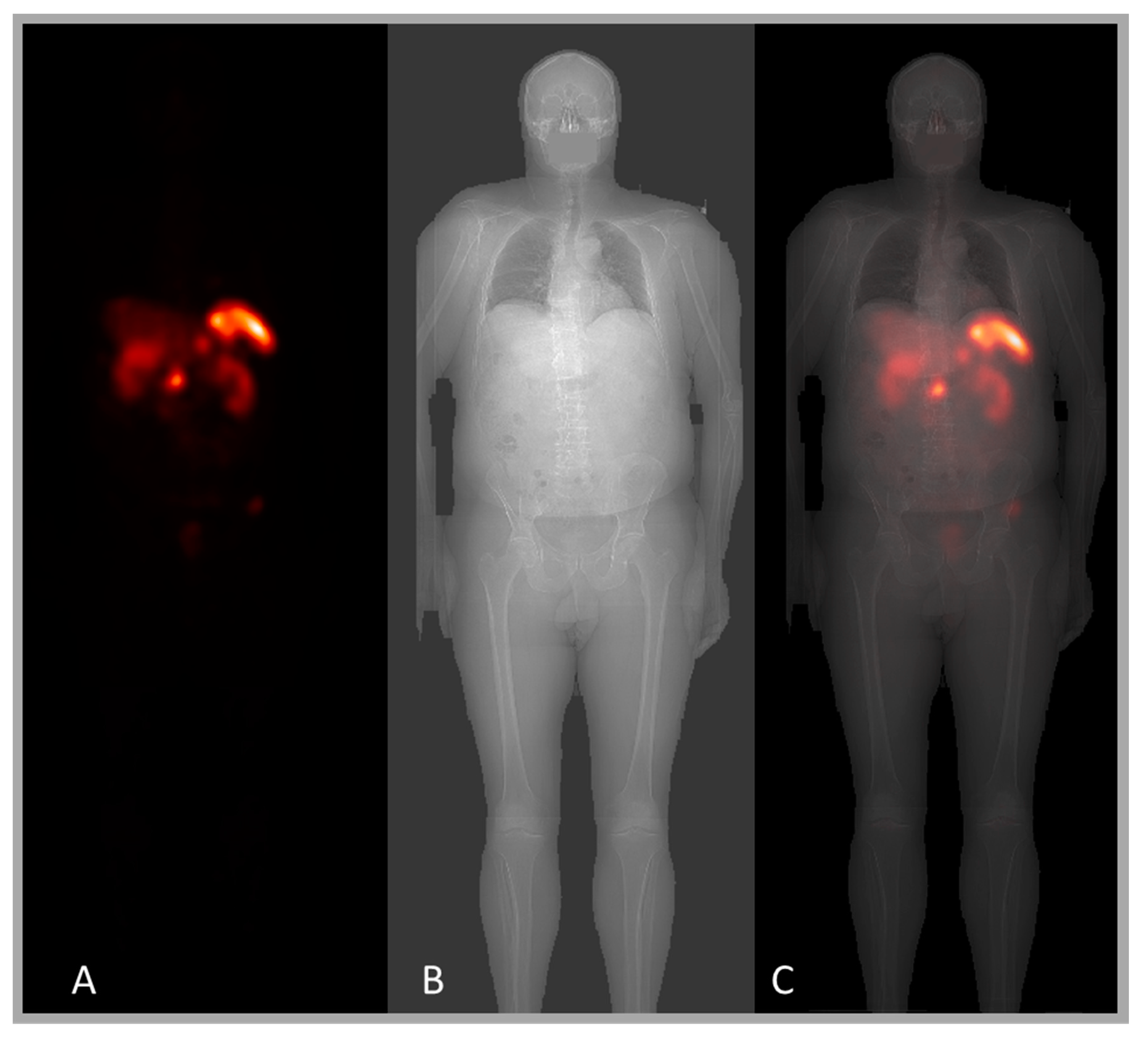
5.3. Scatter Compensation Based on X-Ray Scout
5.4. ROI Definitions Using the X-Ray Scout
6. SPECT Imaging
6.1. Acquisition of Multiple Projections
6.2. Tomographic Image Reconstruction
6.3. MAP Reconstruction Using CT Information
7. SPECT-Based Activity Quantification
7.1. Attenuation Correction Using CT Information
7.2. Scatter Correction Using CT Information
7.3. Collimator Resolution Compensation

8. Image Registration
8.1. Registration of Planar Whole-Body Images and X-Ray Scout
8.2. Registration of Sequential SPECT/CT Images
9. Combined SPECT/Whole Body Imaging
10. Time-Activity Curve
11. Voxel-Based Absorbed Dose Calculation
11.1. The Monte Carlo Method
11.2. Patient Geometry from CT Information

12. Pre-Clinical Dosimetry with μSPECT/μCT and μPET/μCT
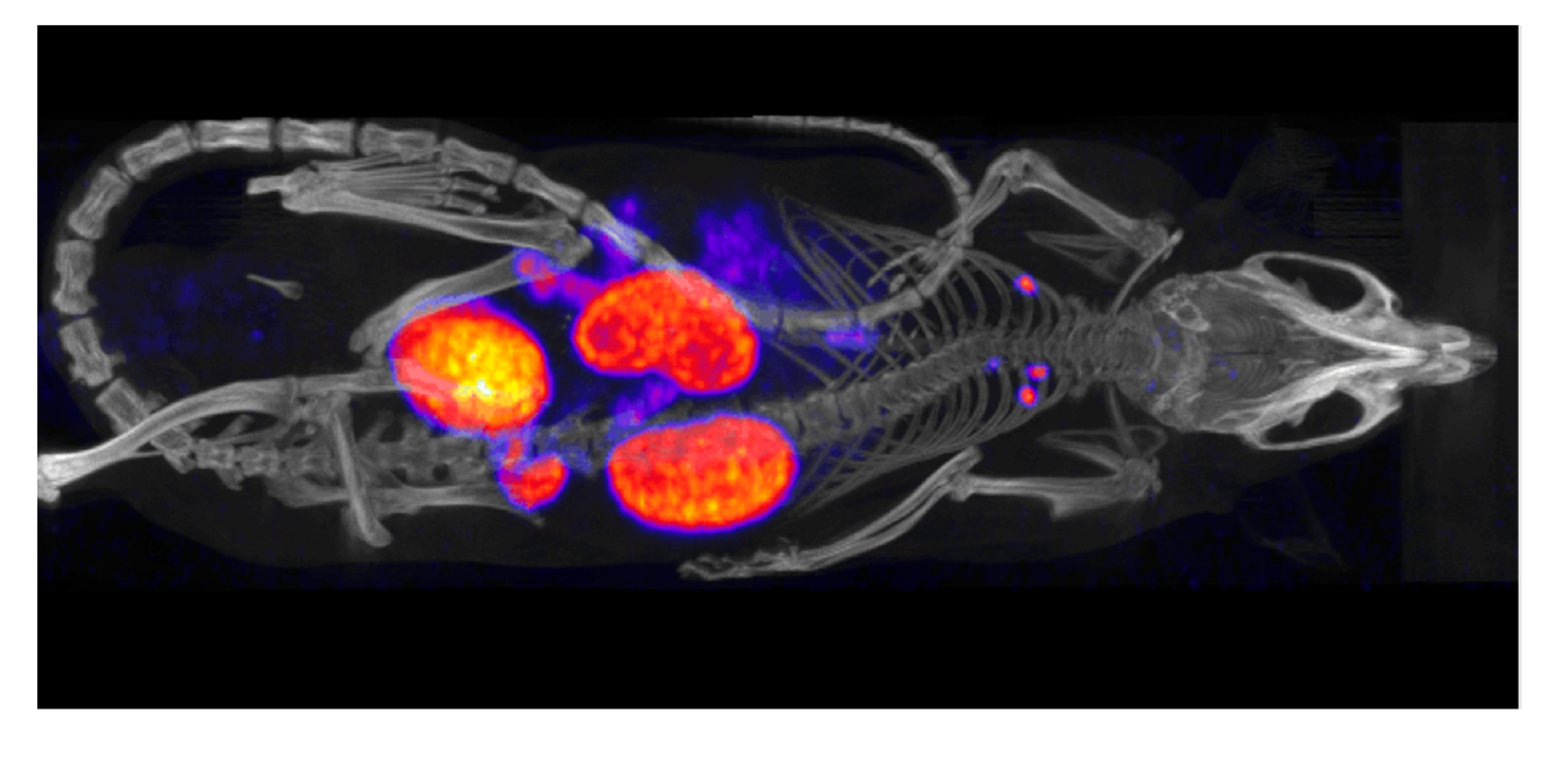
13. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ICRP. 2007 recommendations of the international commission on radiological protection (users edition). Publication 103. Ann. ICRP 2007, 37, 1–332. [Google Scholar] [PubMed]
- ICRP. 2012 international commission on radiological protection statement on tissue reactions/early and late effects of radiation in normal tissues and organs—Threshold doses for tissue reactions in a radiation protection context. Publication 118. Ann. ICRP 2012, 41. [Google Scholar] [CrossRef]
- Volkert, W.A.; Goeckleler, W.F.; Ehrhardt, G.J.; Ketring, A.R. Therapeutic radionuclides: Production and decay property considerations. J. Nucl. Med. 1991, 32, 174–185. [Google Scholar] [PubMed]
- Thomadsen B, E.W.; Mourtada, F. The physics and radiobiology of targeted radionuclide therapy. In Targeted Radionuclide Therapy; Speer, T.W., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011; pp. 71–87. [Google Scholar]
- Stokkel, M.P.; Handkiewicz Junak, D.; Lassmann, M.; Dietlein, M.; Luster, M. EANM procedure guidelines for therapy of benign thyroid disease. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2218–2228. [Google Scholar] [CrossRef] [PubMed]
- Luster, M.; Clarke, S.E.; Dietlein, M.; Lassmann, M.; Lind, P.; Oyen, W.J.; Tennvall, J.; Bombardieri, E. Guidelines for radioiodine therapy of differentiated thyroid cancer. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1941–1959. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Lam, M.; Chiesa, C.; Flux, G.; Brans, B.; Chiti, A.; Giammarile, F. EANM procedure guideline for treatment of refractory metastatic bone pain. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Franzen, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernas, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: A randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007, 8, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Chiti, A.; Lassmann, M.; Brans, B.; Flux, G. EANM procedure guidelines for 131I-meta-iodobenzylguanidine (131I-mIBG) therapy. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Tennvall, J.; Fischer, M.; Bischof Delaloye, A.; Bombardieri, E.; Bodei, L.; Giammarile, F.; Lassmann, M.; Oyen, W.; Brans, B. EANM procedure guideline for radio-immunotherapy for B-cell lymphoma with 90Y-radiolabelled ibritumomab tiuxetan (Zevalin®). Eur. J. Nucl. Med. Mol. Imaging 2007, 34, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Giammarile, F.; Bodei, L.; Chiesa, C.; Flux, G.; Forrer, F.; Kraeber-Bodere, F.; Brans, B.; Lambert, B.; Konijnenberg, M.; Borson-Chazot, F.; et al. Eanm procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 1393–1406. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Kidd, M.; Paganelli, G.; Grana, C.M.; Drozdov, I.; Cremonesi, M.; Lepensky, C.; Kwekkeboom, D.J.; Baum, R.P.; Krenning, E.P.; et al. Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: The value and limitations of clinical factors. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Zaknun, J.J.; Bodei, L.; Mueller-Brand, J.; Baum, R.P.; Pavel, M.E.; Horsch, D.; O’Dorisio, M.S.; O’Dorisio, T.M.; Howe, J.R.; Cremonesi, M.; et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 800–816. [Google Scholar] [CrossRef] [PubMed]
- Gaze, M.N.; Chang, Y.C.; Flux, G.D.; Mairs, R.J.; Saran, F.H.; Meller, S.T. Feasibility of dosimetry-based high-dose 131I-meta-iodobenzylguanidine with topotecan as a radiosensitizer in children with metastatic neuroblastoma. Cancer Biother. Radiopharm. 2005, 20, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Cremonesi, M.; Ferrari, M.; Pacifici, M.; Grana, C.M.; Bartolomei, M.; Baio, S.M.; Sansovini, M.; Paganelli, G. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-dotatoc and 177Lu-DOTATATE: The role of associated risk factors. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Loevinger, R.; Berman, M. A Revised Schema for Calculation of the absorbed dose from Biologically Distributed Radionuclides. MIRD Phamplet No. 1, Revised; Society of Nuclear Medicine: New York, NY, USA, 1976. [Google Scholar]
- Bolch, W.E.; Eckerman, K.F.; Sgouros, G.; Thomas, S.R. MIRD pamphlet No. 21: A generalized schema for radiopharmaceutical dosimetry—Standardization of nomenclature. J. Nucl. Med. 2009, 50, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Howell, R.W. The MIRD schema—From organ to cellular dimensions. J. Nucl. Med. 1994, 35, 531–533. [Google Scholar] [PubMed]
- Stabin, M.G.; Sparks, R.B.; Crowe, E. OLINDA/EXM: The second-generation personal computer software for internal dose assessment in nuclear medicine. J. Nucl. Med. 2005, 46, 1023–1027. [Google Scholar] [PubMed]
- Barendsen, G.W. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1981–1997. [Google Scholar] [CrossRef]
- Fowler, J.F. The linear-quadratic formula and progress in fractionated radiotherapy. Br. J. Radiol. 1989, 62, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Lea, D.E.; Catcheside, D.G. The mechanism of the induction by radiation of chromosome aberrations in tradescantia. J. Genet. 1942, 44, 216–245. [Google Scholar] [CrossRef]
- Barone, R.; Borson-Chazot, F.; Valkema, R.; Walrand, S.; Chauvin, F.; Gogou, L.; Kvols, L.K.; Krenning, E.P.; Jamar, F.; Pauwels, S. Patient-specific dosimetry in predicting renal toxicity with 90Y-dotatoc: Relevance of kidney volume and dose rate in finding a dose-effect relationship. J. Nucl. Med. 2005, 46 (Suppl. 1), 99S–106S. [Google Scholar] [PubMed]
- Wessels, B.W.; Konijnenberg, M.W.; Dale, R.G.; Breitz, H.B.; Cremonesi, M.; Meredith, R.F.; Green, A.J.; Bouchet, L.G.; Brill, A.B.; Bolch, W.E.; et al. MIRD pamphlet No. 20: The effect of model assumptions on kidney dosimetry and response—Implications for radionuclide therapy. J. Nucl. Med. 2008, 49, 1884–1899. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Sciuto, R.; Rea, S.; Carpanese, L.; Pizzi, G.; Soriani, A.; Iaccarino, G.; Benassi, M.; Ettorre, G.M.; Maini, C.L. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y-SIR spheres: Radiobiologic considerations. J. Nucl. Med. 2010, 51, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Strigari, L.; Konijnenberg, M.; Chiesa, C.; Bardies, M.; Du, Y.; Gleisner, K.S.; Lassmann, M.; Flux, G. The evidence base for the use of internal dosimetry in the clinical practice of molecular radiotherapy. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1976–1988. [Google Scholar] [CrossRef] [PubMed]
- Lhommel, R.; van Elmbt, L.; Goffette, P.; van den Eynde, M.; Jamar, F.; Pauwels, S.; Walrand, S. Feasibility of 90Y TOF PET-based dosimetry in liver metastasis therapy using SIR-Spheres. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1654–1662. [Google Scholar] [CrossRef] [PubMed]
- Sorenson, J.A. Methods for Quantitation of Radioactivity in vivo by External Counting Measurements; University of Wisconsin: Madison, WI, USA, 1971. [Google Scholar]
- Fleming, J.S. A technique for the absolute measurement of activity using a gamma camera and computer. Phys. Med. Biol. 1979, 24, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Minarik, D.; Sjogreen, K.; Ljungberg, M. A new method to obtain transmission images for planar whole-body activity quantification. Cancer Biother. Radiopharm. 2005, 20, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Sjögreen Gleisner, K.; Ljungberg, M. Patient-specific whole-body attenuation correction maps from a CT system for conjugate-view based activity quantification: Method development and evaluation. Cancer Biother. Radiopharm. 2012, 27, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Sjogreen, K.; Ljungberg, M.; Strand, S.E. An activity quantification method based on registration of CT and whole-body scintillation camera images, with application to 131I. J. Nucl. Med. 2002, 43, 972–982. [Google Scholar] [PubMed]
- Sjögreen-Gleisner, K. Scatter correction by deconvolution of planar whole-body scintillation-camera images using an image-based estimate of the signal-to-noise ratio. Eur. J. Nucl. Med. 2012, 39, S304–S353. [Google Scholar] [CrossRef]
- Ljungberg, M.; Strand, S.E. A Monte Carlo program for the simulation of scintillation camera characteristics. Comput. Methods Programs Biomed. 1989, 29, 257–272. [Google Scholar] [CrossRef]
- Bruyant, P.P. Analytic and iterative reconstruction algorithms in SPECT. J. Nucl. Med. 2002, 43, 1343–1358. [Google Scholar] [PubMed]
- Hudson, H.M.; Larkin, R.S. Accelerated image reconstruction using ordered subsets of projection data. IEEE Trans. Nucl. Sci. 1994, 13, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Nuyts, J.; Dupont, P.; Stroobants, S.; Benninck, R.; Mortelmans, L.; Suetens, P. Simultaneous maximum a posteriori reconstruction of attenuation and activity distributions from emission sinograms. IEEE Trans. Med. Imaging 1999, 18, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Horne, M.; Maneval, D.; Amartey, J.; Brechner, R. Non-uniform attenuation and scatter correction in SPECT. IEEE Trans. Nucl. Sci. 1988, NS-35, 767–771. [Google Scholar] [CrossRef]
- Bailey, D.L.; Hutton, B.F.; Walker, P.J. Improved SPECT using simultaneous emission and transmission tomography. J. Nucl. Med. 1987, 28, 844–851. [Google Scholar] [PubMed]
- Jaszczak, R.J.; Greer, K.L.; Floyd, C.E.; Harris, C.C.; Coleman, R.E. Improved SPECT quantification using compensation for scattered photons. J. Nucl. Med. 1984, 25, 893–900. [Google Scholar] [PubMed]
- Ogawa, K.; Harata, Y.; Ichihara, T.; Kubo, A.; Hashimoto, S. A practical method for position-dependent compton-scatter correction in single photon emission CT. IEEE Trans. Med. Imaging 1991, 10, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Frey, E.C.; Tsui, B.M.W. A new method for modeling the spatially-variant, object-dependent scatter response function in SPECT. In Proceedings of the Conference Records of the IEEE Medical Imaging Conference, Anaheim, CA, USA, 2–9 November 1996; pp. 1082–1086.
- Tsui, B.M.W.; Hu, H.B.; Gullberg, G.T. Implementation of simultaneous attenuation and detector response correction in SPECT. IEEE Trans. Nucl. Sci. 1988, NS-35, 778–783. [Google Scholar] [CrossRef]
- El-Fakhri, G.N.; Buvat, I.; Pelegrini, M.; Benali, H.; Almeida, P.; Bendriem, B.; Todd, P.A.; di-Paola, R. Respective roles of scatter, attenuation, depth-dependent collimator response and finite spatial resolution in cardiac single-photon emission tomography quantitation: A Monte Carlo study. Eur. J. Nucl. Med. 1999, 26, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Sjogreen, K.; Ljungberg, M.; Wingardh, K.; Erlandsson, K.; Strand, S.E. Registration of emission and transmission whole-body scintillation-camera images. J. Nucl. Med. 2001, 42, 1563–1570. [Google Scholar] [PubMed]
- Sjogreen-Gleisner, K.; Rueckert, D.; Ljungberg, M. Registration of serial SPECT/CT images for three-dimensional dosimetry in radionuclide therapy. Phys. Med. Biol. 2009, 54, 6181–6200. [Google Scholar] [CrossRef] [PubMed]
- Garkavij, M.; Nickel, M.; Sjogreen-Gleisner, K.; Ljungberg, M.; Ohlsson, T.; Wingardh, K.; Strand, S.E.; Tennvall, J. 177Lu-[DOTA0,Tyr3] octreotate therapy in patients with disseminated neuroendocrine tumors: Analysis of dosimetry with impact on future therapeutic strategy. Cancer 2010, 116, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Andreo, P. Monte Carlo techniques in medical radiation physics. Phys. Med. Biol. 1991, 36, 861–920. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, M.; Sjogreen, K.; Liu, X.; Frey, E.; Dewaraja, Y.; Strand, S.E. A 3-dimensional absorbed dose calculation method based on quantitative SPECT for radionuclide therapy: Evaluation for 131I using Monte Carlo simulation. J. Nucl. Med. 2002, 43, 1101–1109. [Google Scholar] [PubMed]
- Ljungberg, M.; Frey, E.; Sjogreen, K.; Liu, X.; Dewaraja, Y.; Strand, S.E. 3D absorbed dose calculations based on SPECT: Evaluation for 111-In/90-Y therapy using monte carlo simulations. Cancer Biother. Radiopharm. 2003, 18, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Dewaraja, Y.K.; Schipper, M.J.; Roberson, P.L.; Wilderman, S.J.; Amro, H.; Regan, D.D.; Koral, K.F.; Kaminski, M.S.; Avram, A.M. 131I-tositumomab radioimmunotherapy: Initial tumor dose-response results using 3-dimensional dosimetry including radiobiologic modeling. J. Nucl. Med. 2010, 51, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ljungberg, M.; Gleisner, K.S. Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy. Diagnostics 2015, 5, 296-317. https://doi.org/10.3390/diagnostics5030296
Ljungberg M, Gleisner KS. Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy. Diagnostics. 2015; 5(3):296-317. https://doi.org/10.3390/diagnostics5030296
Chicago/Turabian StyleLjungberg, Michael, and Katarina Sjögreen Gleisner. 2015. "Hybrid Imaging for Patient-Specific Dosimetry in Radionuclide Therapy" Diagnostics 5, no. 3: 296-317. https://doi.org/10.3390/diagnostics5030296