Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation
Abstract
:1. Introduction
1.1. Prior Work
1.2. Meteor Energy Loss Due to Ablation
1.3. Thermal Diffusion and Meteor Ablation
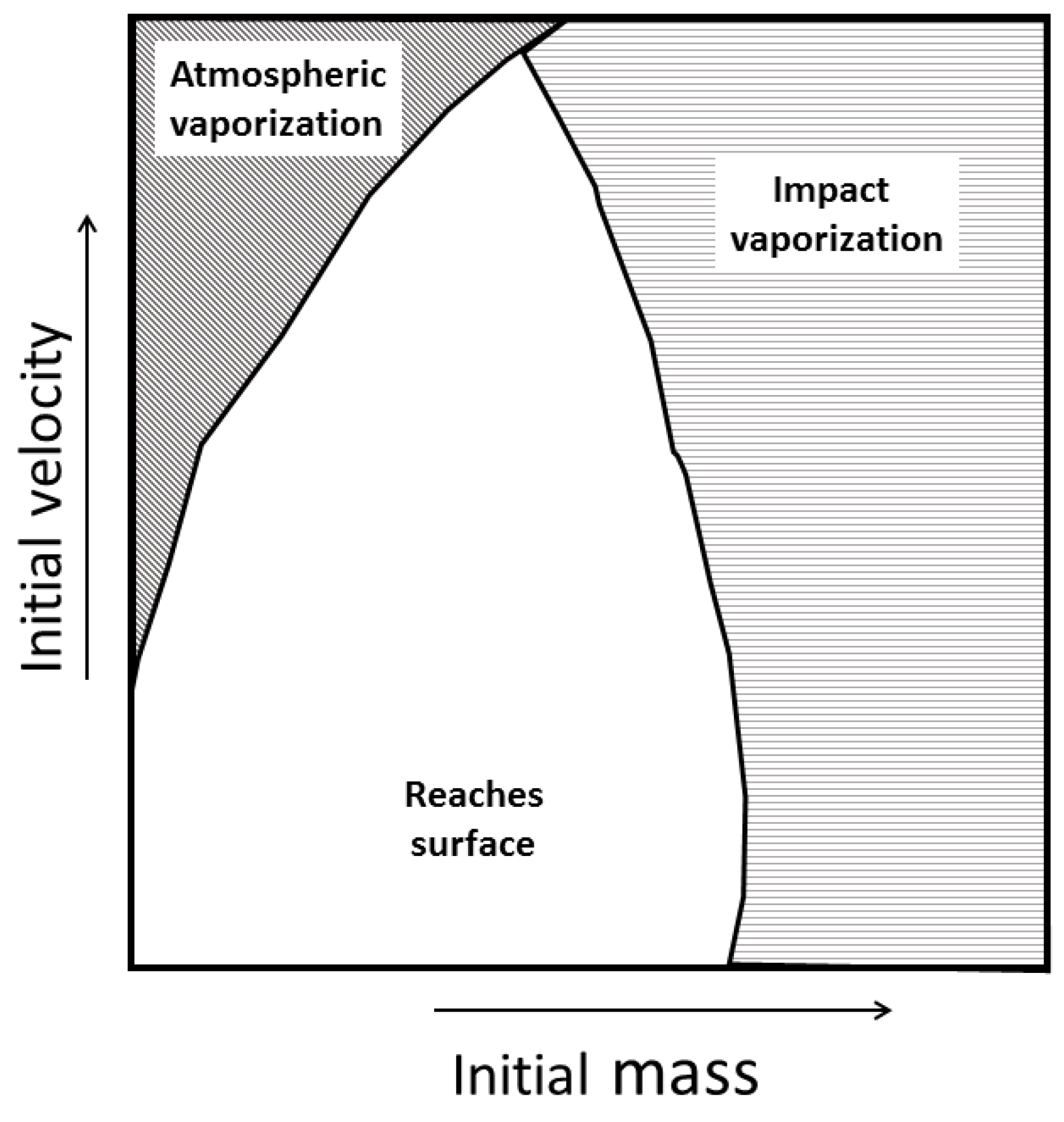
1.4. Meteoroid Source Region and Distributions on Impact
2. Methods
2.1. Ablation Model
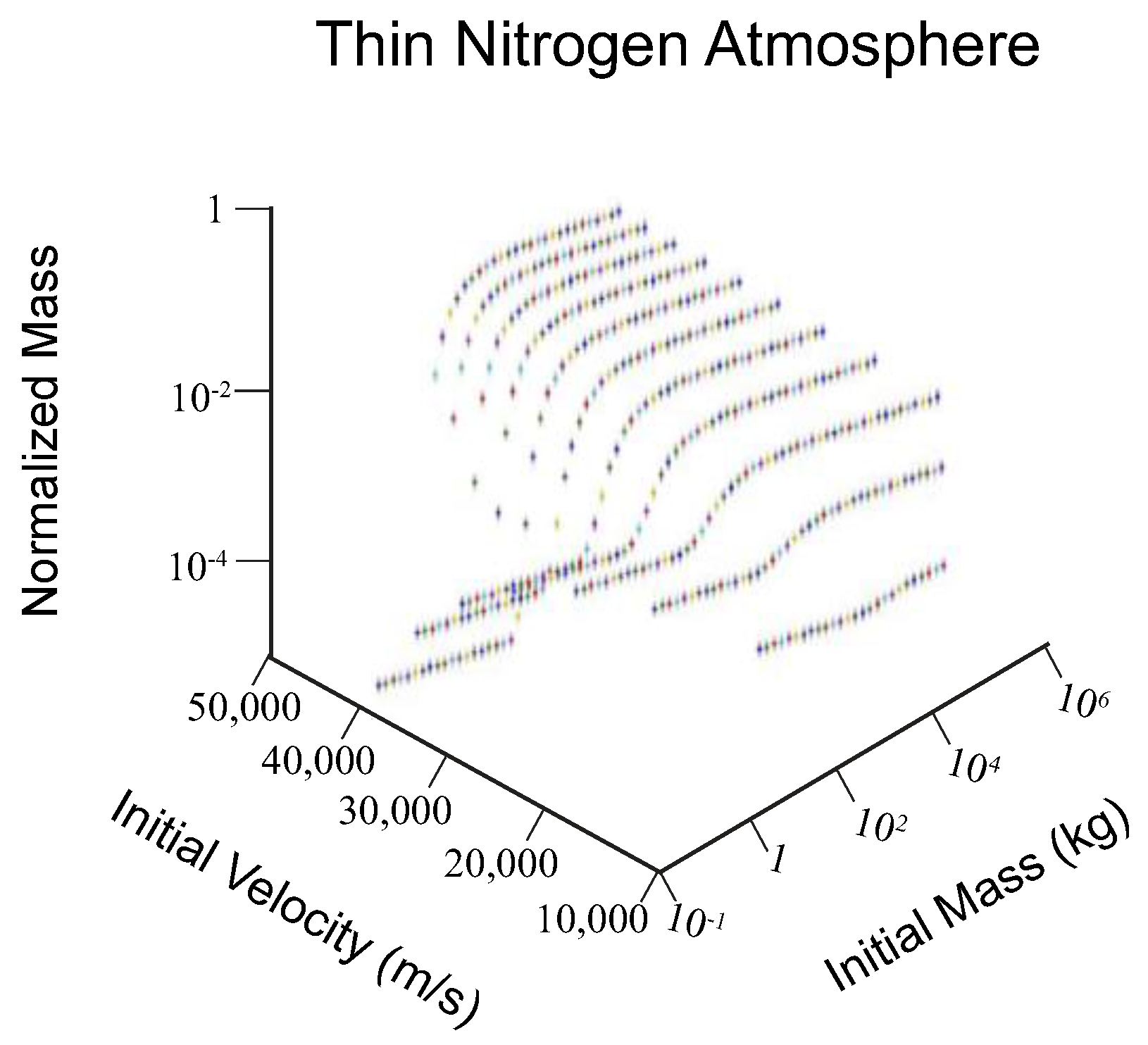
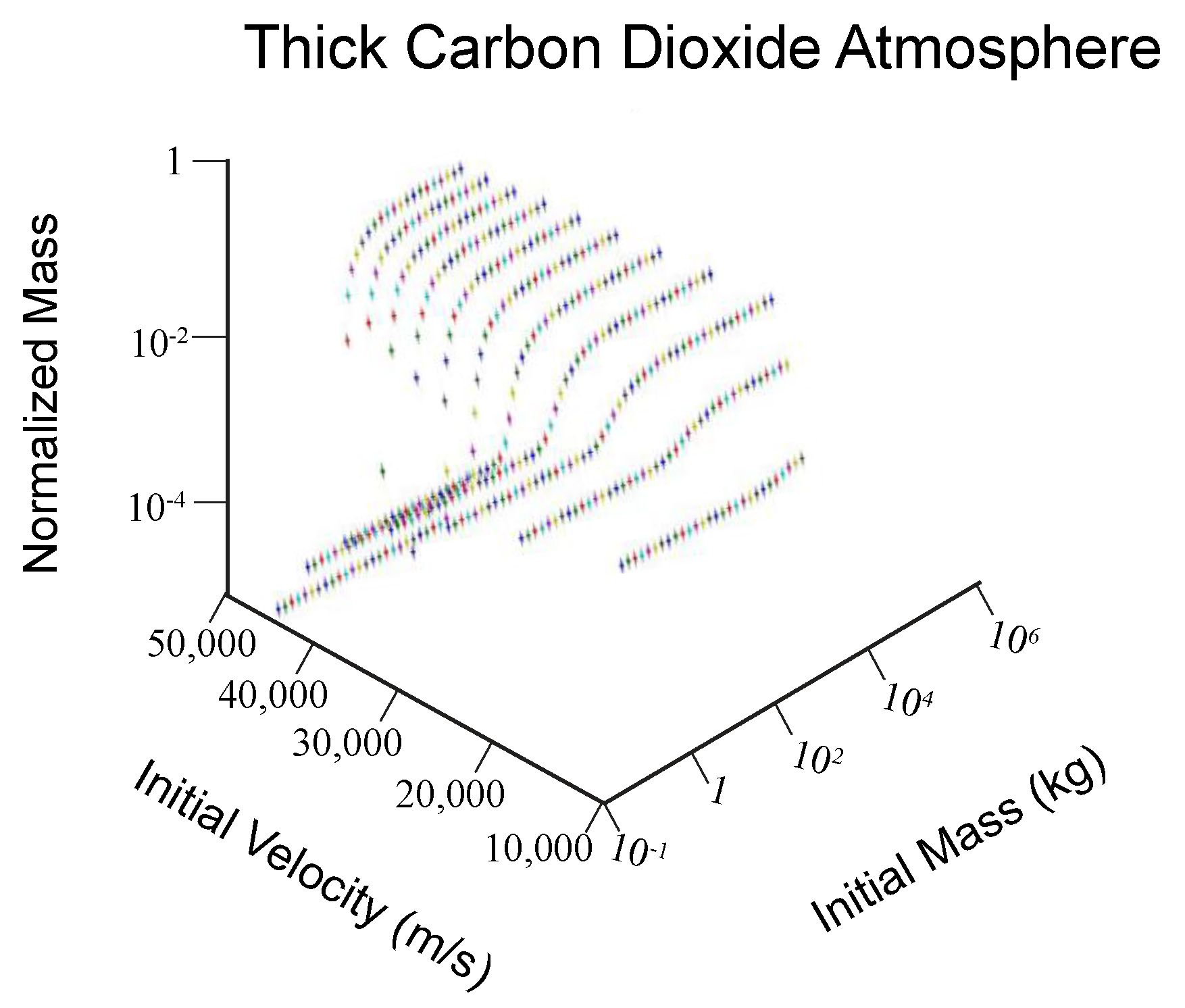
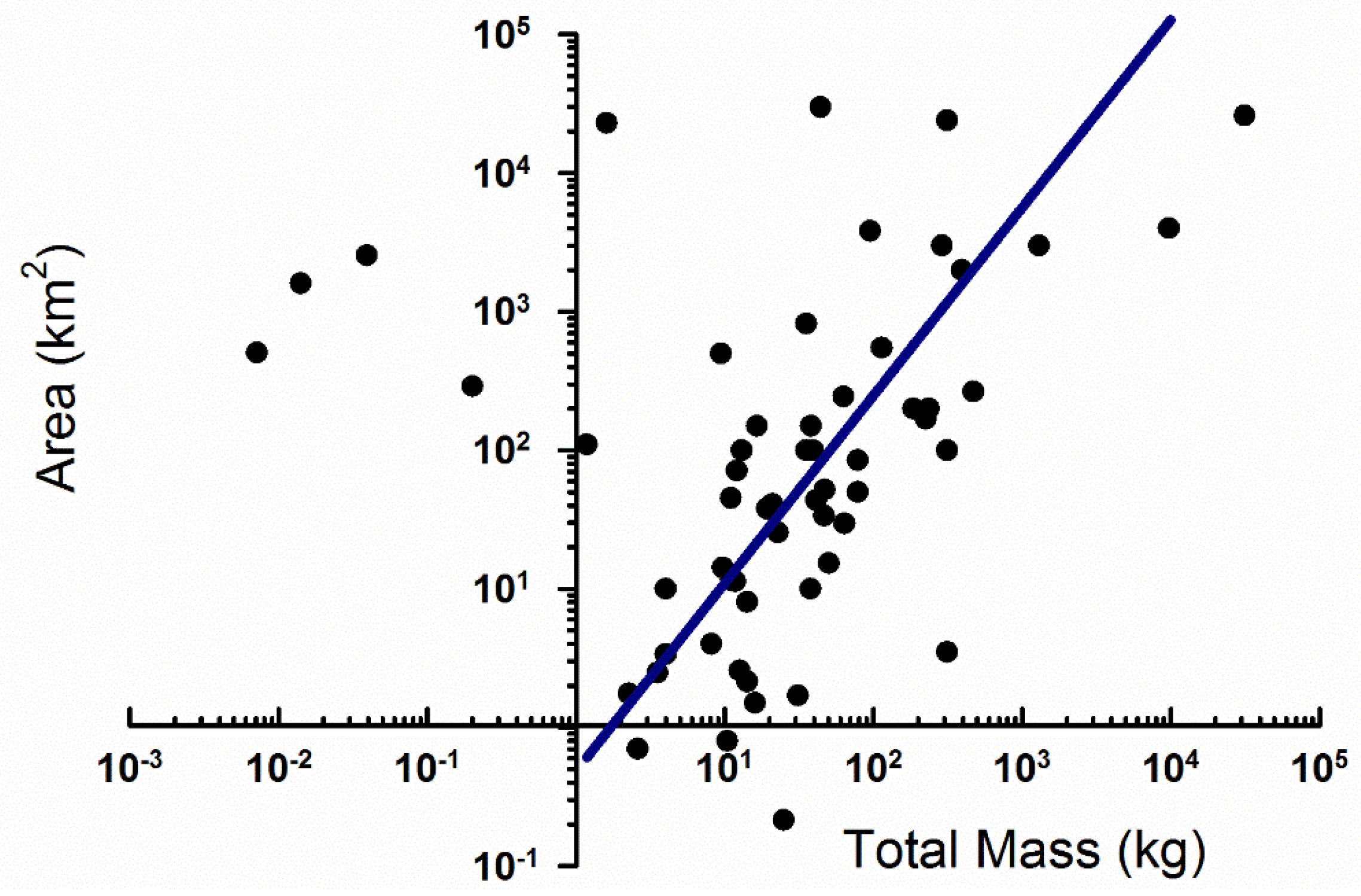
2.2. Strewn Field Analysis
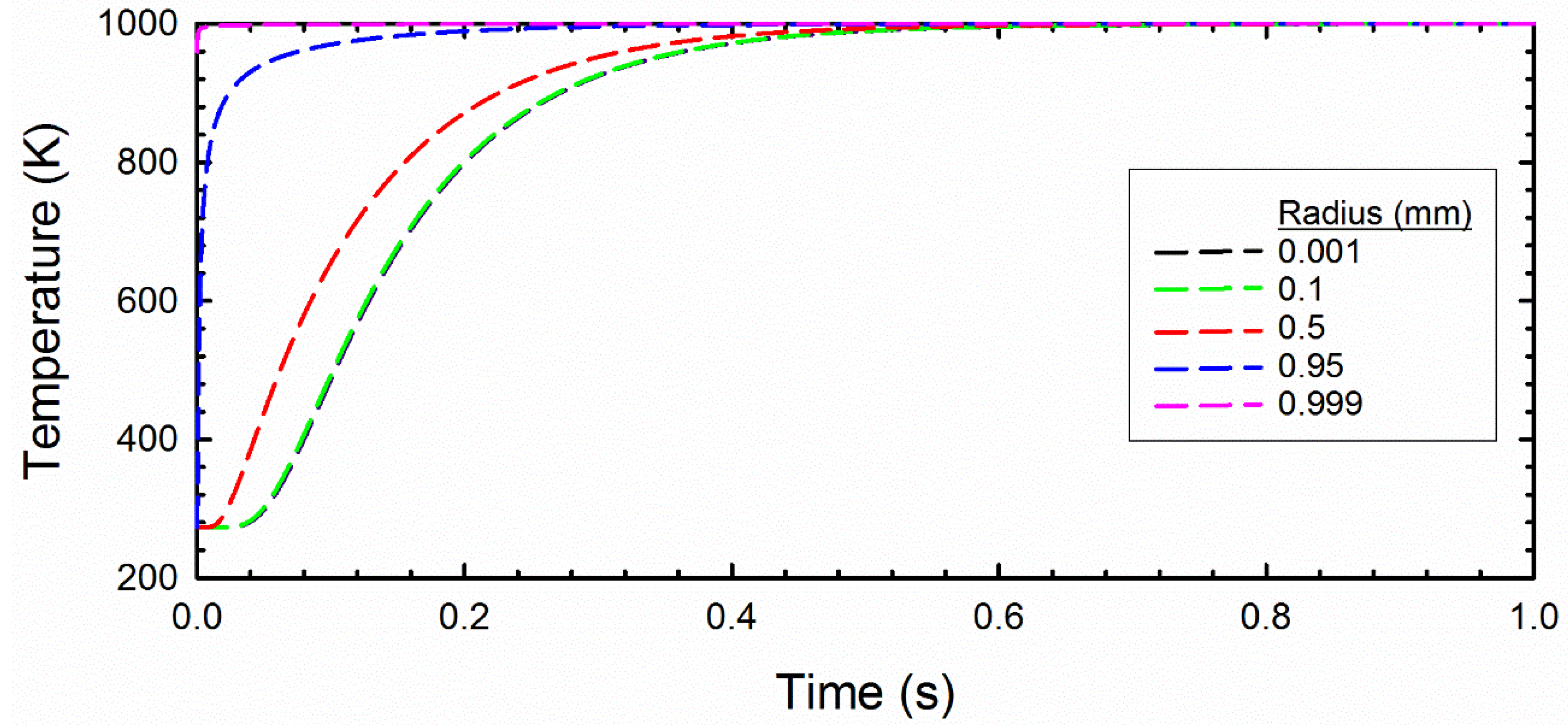
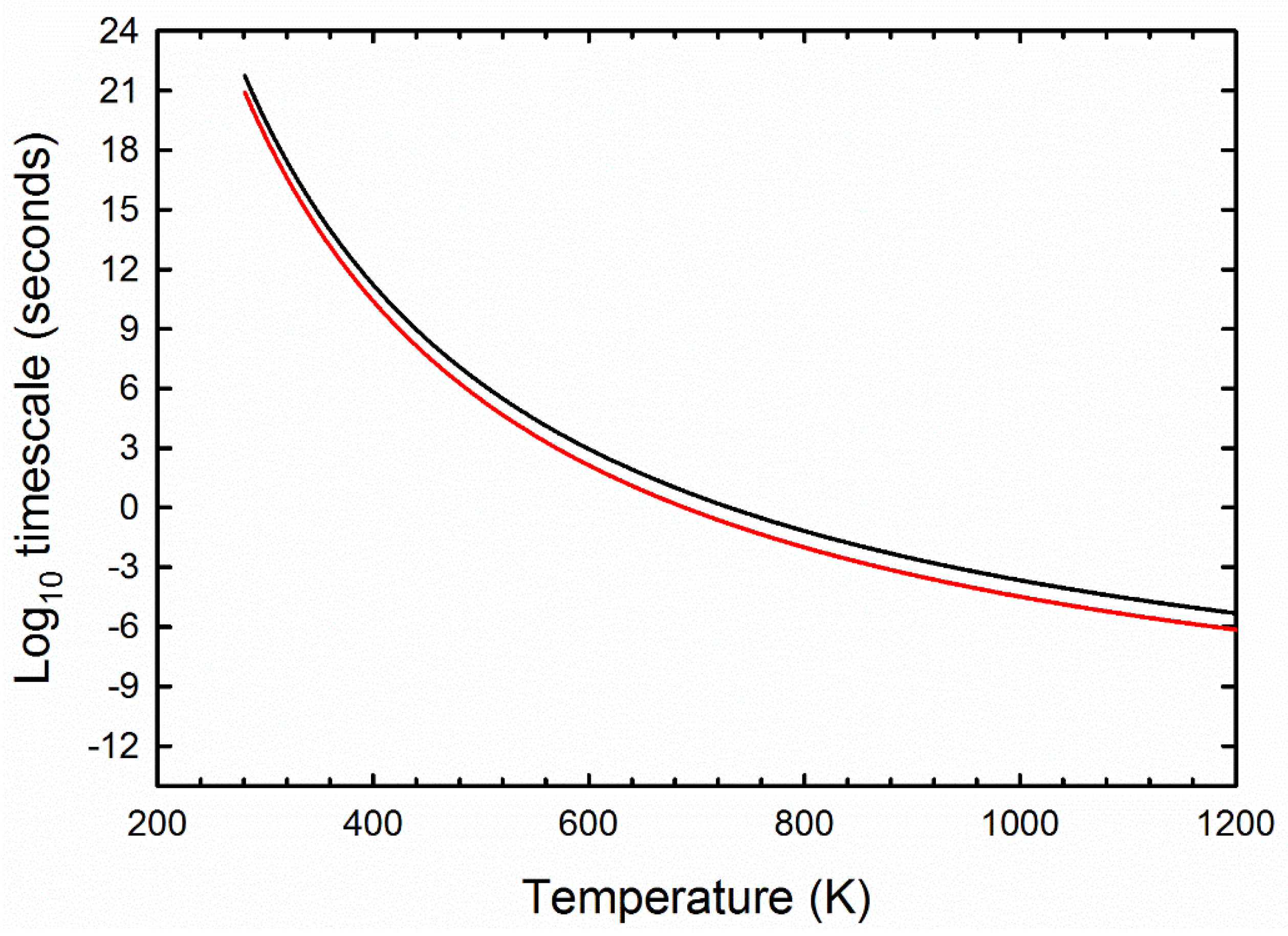
2.3. Thermal Diffusion and Chemical Kinetics Model
3. Results
3.1. Strewn Field Mass-Area Relationship
3.2. Radiation Model Results
4. Discussion
4.1. Caveats for Delivery
4.2. Comparison to Natural Samples
4.3. Calculation of Exogenous Material Delivery to the Early Earth
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Miller, S. A Production of Amino Acids under Possible Primitive Earth Conditions. Science 1953, 117, 528–529. [Google Scholar] [CrossRef] [PubMed]
- Gargaud, M.; Irvine, W.M.; Amils, R.; Cleaves, H.; Pinti, D.L.; Quintanilla, J.C.; Tirard, S. Encyclopedia of Astrobiology; Springer: Heidelberg, Germany, 2015. [Google Scholar]
- Kuhn, W.R.; Atreya, S.K. Ammonia photolysis and the greenhouse effect in the primordial atmosphere of the earth. Icarus 1979, 37, 207–213. [Google Scholar] [CrossRef]
- Buchwald, B.; Kalina, G.; Colleen, H.; Wankel, S. Constraining the role of iron in environmental nitrogen transformations: Dual stable isotope systematics of abiotic NO2− reduction by Fe(II) and its production of N2O. Geochim. Cosmochim. Acta 2016, 186, 1–12. [Google Scholar] [CrossRef]
- Furukawa, Y.; Nakazawa, H.; Sekine, T.; Kobayashi, T.; Kakegawa, T. Nucleobase and amino acid formation through impacts of meteorites on the early ocean. Earth Planet. Sci. Lett. 2015, 429, 216–222. [Google Scholar] [CrossRef]
- Johnston, D.T.; Farquhar, J.; Habicht, K.S.; Canfield, D.E. Sulphur isotopes and the search for life: Strategies for identifying sulphur metabolisms in the rock record and beyond. Geobiology 2008, 6, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Chyba, C.; Sagan, C. Endogenous production, exogenous delivery and impact-shock synthesis of organic molecules: An inventory for the origins of life. Nature 1992, 355, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Glavin, D.P.; Bada, J.L. Survival of amino acids in micrometeorites during atmospheric entry. Astrobiology 2001, 1, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Pasek, M.; Lauretta, D. Extraterrestrial Flux of Potentially Prebiotic C, N, and P to the Early Earth. Orig. Life Evol. Biosph. 2007, 38, 5–21. [Google Scholar] [CrossRef] [PubMed]
- Weisberg, M.; McCoy, T.; Krot, A. Systematics and Evaluation of Meteorite Classification. In Meteorites and the Early Solar System II; University of Arizona Press: Tucson, AZ, USA, 2009. [Google Scholar]
- Goldstein, J.; Scott, E.R.D.; Chabot, N.L. Iron Meteorites: Crystallization, Thermal History, Parent Bodies, and Origin. Chem. Erde Geochem. 2009, 69, 293–325. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Ponnamperuma, C. Nonprotein Amino Acids in the Murchison Meteorite. Proc. Natl. Acad. Sci. USA 1971, 68, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Pizzarello, S.; Shock, E. The Organic Composition of Carbonaceous Meteorites: The Evolutionary Story Ahead of Biochemistry. Cold Spring Harb. Perspect. Biol. 2010, 2, a002105. [Google Scholar] [CrossRef] [PubMed]
- Callahan, M.; Smith, K.; Cleaves, J.; Ruzicka, J.; Stern, J.; Glavin, D.; House, C.; Dworkin, J. Carbonaceous meteorites contain a wide range of extraterrestrial nucleobases. Proc. Natl. Acad. Sci. USA 2011, 108, 13995–13998. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Nooner, D.W.; Oró, J. Organic compounds in meteorites—I. Aliphatic hydrocarbons. Geochim. Cosmochim. Acta 1967, 31, 1359–1394. [Google Scholar] [CrossRef]
- Cooper, G.W.; Cronin, J.R. Linear and cyclic aliphatic carboxamides of the Murchison meteorite: Hydrolyzable derivatives of amino acids and other carboxylic acids. Geochim. Cosmochim. Acta 1995, 59, 1003–1015. [Google Scholar] [CrossRef]
- Aponte, J.C.; Dworkin, J.P.; Elsila, J.E. Assessing the origins of aliphatic amines in the Murchison meteorite from their compound-specific carbon isotopic ratios and enantiomeric composition. Geochim. Cosmochim. Acta 2014, 141, 331–345. [Google Scholar] [CrossRef]
- Elsila, J.; Aponte, J.; Blackmond, D.; Burton, A.; Dworkin, P.; Glavin, D. Meteoritic Amino Acids: Diversity in Compositions Reflects Parent Body Histories. ACS Cent. Sci. 2016, 2, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Bland, P.A.; Artemieva, N.A. The rate of small impacts on Earth. Meteor. Planet. Sci. 2006, 41, 607–631. [Google Scholar] [CrossRef]
- Baldwin, B.; Sheaffer, Y. Ablation and breakup of large meteoroids during atmospheric entry. J. Geophys. Res. 1971, 76, 4653–4668. [Google Scholar] [CrossRef]
- Hills, J.G.; Goda, M.P. The fragmentation of small asteroids in the atmosphere. Astron. J. 1993, 105, 1114. [Google Scholar] [CrossRef]
- Chyba, C.; Thomas, P.; Zahnle, K. The 1908 Tunguska explosion: Atmospheric disruption of a stony asteroid. Nature 1993, 361, 40–44. [Google Scholar] [CrossRef]
- Shingledecker, C. Thermally induced chemistry of meteoritic complex organic mjolecules: A new heat-diffusion model for the atmospheric entry of meteorites. Astrobiology, 2014; arXiv:1412.5134. [Google Scholar]
- Zinn, J.; Judd, O.; ReVelle, O. Leonid Meteor Ablation, Energy Exchange, and Trail Morphology. Adv. Space Res. 2004, 33, 1466–1474. [Google Scholar] [CrossRef]
- Lewis, J.S. Rain of Iron and Ice: The Very Real Threat of Comet and Asteroid Bombardment; Addison-Wesley: Boston, MA, USA, 1996. [Google Scholar]
- Maurette, M.; Olinger, C.; Christophe Michel-Levy, M.; Kurat, G.; Pourchet, M.; Brandstätter, F.; Bourot-Denise, M. A collection of diverse micrometeorites recovered from 100 tonnes of Antarctic blue ice. Nature 1991, 351, 44–47. [Google Scholar] [CrossRef]
- Genge, M.J.; Grady, M. Melted micrometeorites from Antarctic ice with evidence for the separation of immiscible Fe-Ni-S liquids during entry heating. Meteor. Planet. Sci. 1998, 3, 425. [Google Scholar] [CrossRef]
- Suttle, M.D.; Genge, M.J.; Russell, S.S. Shock fabrics in fine-grained micrometeorites. Meteor. Planet. Sci. 2017, 10, 2258. [Google Scholar] [CrossRef]
- Vinković, D. Thermalization of Sputtered Particles as the Source of Diffuse Radiation from High Altitude Meteors. Adv. Space Res. 2007, 39, 574–582. [Google Scholar] [CrossRef]
- Cziczo, D.J.; Thomson, D.S.; Murphy, D.M. Ablation, Flux, and Atmospheric Implications of Meteors Inferred from Stratospheric Aerosol. Science 2001, 291, 1772–1775. [Google Scholar] [CrossRef] [PubMed]
- Demeo, F.; Carry, B. The Taxonomic Distribution of Asteroids from Multi-filter All-sky Photometric Surveys. Icarus 2013, 226, 723–741. [Google Scholar] [CrossRef]
- Gladman, B.; Davis, D.; Neese, C.; Jedicke, R.; Williams, G.; Kavelaars, J.J.; Petit, J.; Scholl, H.; Holman, M.; Warrigton, B.; et al. On the Asteroid Belt’s Orbital and Size Distribution. Icarus 2009, 202, 104–118. [Google Scholar] [CrossRef]
- Catullo, V.; Zappala, V.; Farinella, P.; Paolicchi, P. Analysis of the shape distribution of astroids. Astron. Astrophy. 1984, 138, 464–468. [Google Scholar]
- Britt, D.; Guy Consolmagno, S.J.; Lebofsky, L. Main-Belt Asteroids. In Encyclopedia of the Solar System; Elsevier: New York, NY, USA, 2014; pp. 583–601. [Google Scholar]
- Brown, H. The density and mass distribution of meteoritic bodies in the neighborhood of the Earth’s orbit. J. Geophys. Res. 1960, 65, 1679. [Google Scholar] [CrossRef]
- Zhao, J.; Peichl, M.; Oquist, M.; Nilsson, M.B. Gross primary production controls the subsequent winter CO2 exchange in a boreal peatland. Glob. Chang. Biol. 2016, 12, 4028. [Google Scholar] [CrossRef] [PubMed]
- Friend, A.; Lucht, W.; Rademacher, T.; Keribin, R.; Betts, R.; Cadule, P.; Ciais, P.; Clark, D.; Dankers, R.; Falloon, P.; et al. Carbon residence time dominates uncertainty in terrestrial vegetation responses to future climate and atmospheric CO2. Proc. Natl. Acad. Sci. USA 2014, 111, 3280–3285. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Piao, S.; Zeng, Z.; Peng, S.; Ciais, P.; Cheng, L.; Mao, J.; Poulter, B.; Shi, X.; Yao, Y.; et al. Seasonal responses of terrestrial ecosystem water-use efficiency to climate change. Glob. Chang. Biol. 2016, 6, 2165. [Google Scholar] [CrossRef] [PubMed]
- Olga, P. Meteoroid ablation models. Earth Moon Planets 2005, 95, 303. [Google Scholar]
- Pasek, M.A.; Block, K.; Pasek, V. Fulgurite morphology: A classification scheme and clues to formation. Contrib. Mineral. Petrol. 2012, 3, 477. [Google Scholar] [CrossRef]
- Yablokov, V.A.; Smel’tsova, I.L.; Faerman, V.I. Thermal stability of amino acids. Russ. J. Gen. Chem. 2013, 83, 476. [Google Scholar] [CrossRef]
- Pasek, M.A.; Hurst, M. A Fossilized Energy Distribution of Lightning. Sci. Rep. 2016, 6, 30586. [Google Scholar] [CrossRef] [PubMed]
- Pearce, B.K.; Pudritz, R.E.; Semenov, D.A.; Henning, T.K. Origin of the RNA world: The fate of nucleobases in warm little ponds. Proc. Natl. Acad. Sci. USA 2017, 114, 11327–11332. [Google Scholar] [CrossRef] [PubMed]
- Greshake, A.; Kloeck, W.; Arndt, P.; Maetz, M.; Flynn, G.J.; Bajt, S.; Bischoff, A. Heating experiments simulating atmospheric entry heating of micrometeorites: Clues to their parent body sources. Meteor. Planet. Sci. 1998, 2, 267. [Google Scholar] [CrossRef]
- Kurat, G.; Koeberl, C.; Presper, T.; Brandstaetter, F. Petrology and geochemistry of Antarctic micrometeorites. Geochim. Cosmochim. Acta 1994, 18, 3879. [Google Scholar] [CrossRef]
- Parnell, J.; Bowden, S.A.; Muirhead, D.; Blamey, N.; Westall, F.; Demets, R.; Brack, A. Preservation of organic matter in the STONE 6 artificial meteorite experiment. Icarus 2011, 1, 390. [Google Scholar] [CrossRef]
- Hahn, J.H.; Zenobi, R.; Bada, J.L.; Zare, R.N. Application of two-step laser mass spectrometry to cosmogeochemistry: Direct analysis of meteorites. Science 1988, 239, 1523–1525. [Google Scholar] [CrossRef] [PubMed]
- Zenobi, R.; Philippoz, J.; Zare, R.N.; Wing, M.R.; Bada, J.L.; Marti, K. Article: Organic compounds in the Forest Vale, H4 ordinary chondrite. Geochim. Cosmochim. Acta 1992, 56, 2899–2905. [Google Scholar] [CrossRef]
- Brinton, K.L.F.; Engrand, C.; Glavin, D.P.; Bada, J.L.; Maurette, M. A search for extraterrestrial amino acids in carbonaceous Antarctic micrometeorites. Orig. Life Evol. Biosph. 1998, 28, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Marchi, S. Widespread mixing and burial of Earth’s Hadean crust by asteroid impacts. Nature 2014, 511, 578–582. [Google Scholar] [CrossRef] [PubMed]
- Huss, G.R. Meteorite mass distributions and differences between Antarctic and non-Antarctic meteorites. Geochim. Cosmochim. Acta 1991, 55, 105–111. [Google Scholar] [CrossRef]

| Mass (kg) | kg/year | Intervals between Years |
|---|---|---|
| 0.10 | 110,000.00 | 0.00001 |
| 0.50 | 52,000.00 | 0.00002 |
| 1.00 | 37,000.00 | 0.00003 |
| 5.00 | 12,000.00 | 0.00008 |
| 100.00 | 800.00 | 0.00130 |
| 500.00 | 170.00 | 0.01 |
| 1000.00 | 91.00 | 0.01 |
| 5000.00 | 21.00 | 0.05 |
| 10,000.00 | 11.00 | 0.09 |
| 50,000.00 | 2.40 | 0.42 |
| 100,000.00 | 1.30 | 0.77 |
| 500,000.00 | 0.29 | 3.46 |
| 1,000,000.00 | 0.15 | 6.58 |
| 5,000,000.00 | 0.02 | 41.32 |
| Parameter | Symbol | Value |
|---|---|---|
| Atmosphere Surface Density | ρatm,Present day | 40.1 moles/m3 |
| Scale Height Present Day | H | 7 km |
| Thin N2 Atmosphere Surface Density | ρatm,N2 | 20 moles/m3 |
| Scale Height | hN2 | 7.5 km |
| Thick CO2 Atmosphere Surface Density | ρatm,C02 | 120 moles/m3 |
| Scale Height | hCO2 | 5 km |
| Starting Altitude | Z | 200,000 km |
| Drag Coefficient | cd | 0.47 |
| Angle of Entry | θ | 45° |
| Density | ρ | 3.5 g/cc |
| Ablation Coefficient | ca | 0.014 |
| Ch/2Q | N/A | 6.25 × 10−9 s2/m2 |
| Initial Velocity (m/s) | Minimum Initial Mass (kg) |
|---|---|
| 10,000 | 0.05 |
| 14,000 | 0.1 |
| 18,000 | 0.2 |
| 22,000 | 0.72 |
| 26,000 | 2.64 |
| 30,000 | 13.5 |
| 34,000 | 132 |
| 38,000 | 13,000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, C.; Perez, A.; Thompson, G.; Pasek, M.A. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life 2018, 8, 13. https://doi.org/10.3390/life8020013
Mehta C, Perez A, Thompson G, Pasek MA. Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation. Life. 2018; 8(2):13. https://doi.org/10.3390/life8020013
Chicago/Turabian StyleMehta, Chris, Anthony Perez, Glenn Thompson, and Matthew A. Pasek. 2018. "Caveats to Exogenous Organic Delivery from Ablation, Dilution, and Thermal Degradation" Life 8, no. 2: 13. https://doi.org/10.3390/life8020013





