A Hypothesis: Life Initiated from Two Genes, as Deduced from the RNA World Hypothesis and the Characteristics of Life-Like Systems
Abstract
:1. Chemical Evolution and Environments
1.1. Introduction: Chemical Evolution
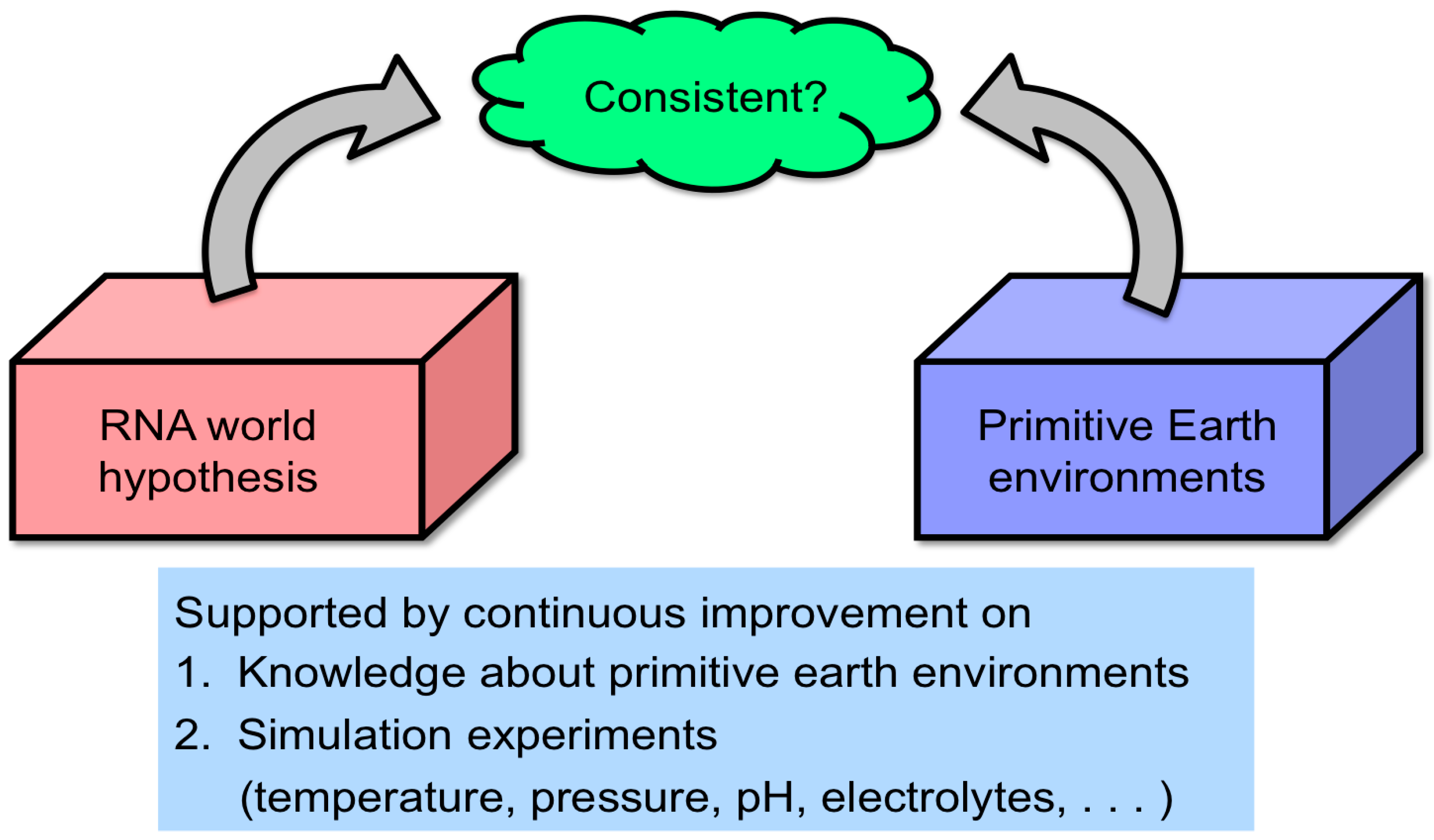
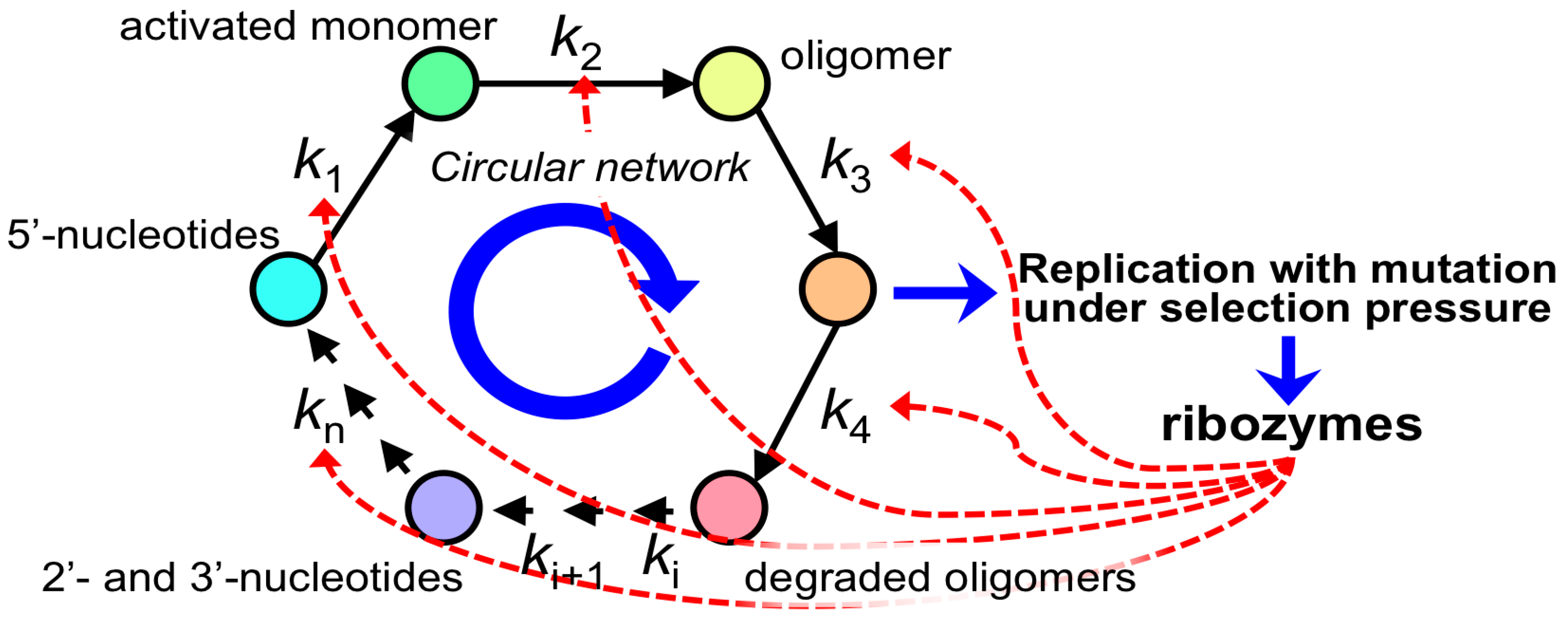
- An insufficient number of simulation experiments have been conducted to render the RNA world hypothesis compatible with extreme primitive Earth environments (Figure 2). Especially, the RNA world hypothesis has not been sufficiently evaluated from the viewpoint of the hydrothermal origin of life. This was extensively investigated to verify conditions likely for chemical evolution of RNA and related molecules by using hydrothermal flow reactors by researchers, including our group.
- There are still unknown pathways for the formation of functional RNA molecules, such as the formation of ribose and the prebiotic replication process.
- A realistic RNA-based life-like system has not been well described. It is unknown whether the first RNA-based life-like systems may include a quasi-species-type RNA system or a capsulated-type system consisting of several functional RNA molecules.
- It has not been determined how a simple population of functional RNA molecules became a life-like system that can be regarded as alive. That is to say, the elemental requisites (or characteristics) for defining a life-like system as alive are not completely clear.
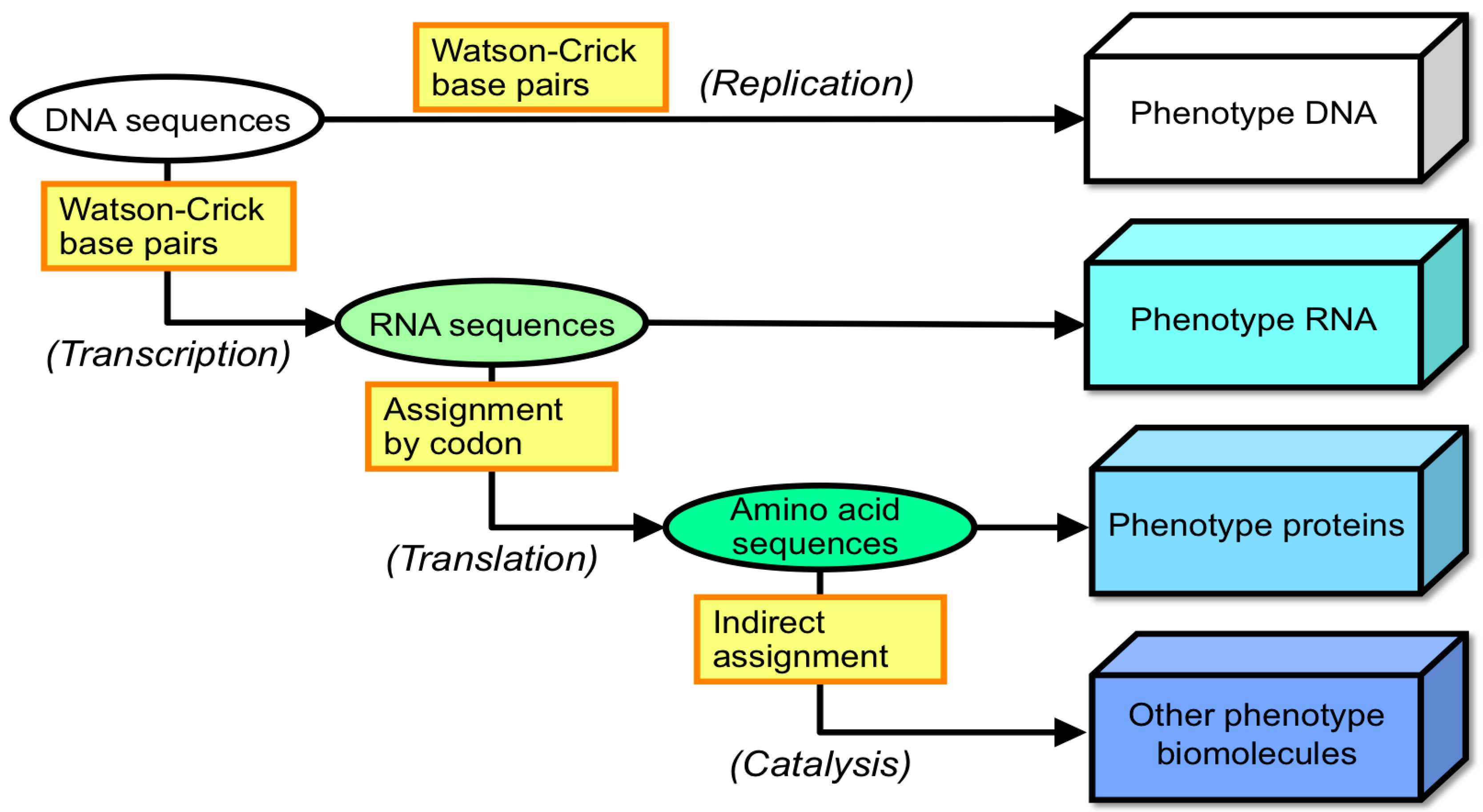
1.2. Answering the Question: Which Was First, Protein or Nucleic Acid?
1.3. Behaviors of Nucleotides and Protein-Like Molecules in Primitive Extreme Earth Environments
- The relative rates of the formation and degradation of biomolecules, such as phosphodiester bonds of RNA and amide bonds of peptides.
- The biologically-important weak interactions by hydrogen bonding and hydrophobic interaction.
- The solubility of these molecules.
2. Life-Like Systems Deduced from the Definition of Life
2.1. Problems Regarding the Definition of Life
- The first step is to focus the characteristics of life-like systems into life on Earth.
- The characteristics that life consists of cell(s), metabolism, replication and evolution are well accepted.
- To maintain these characteristics, the fact that life possesses machinery for the replication of information and the transformation of that information to accomplish biological functions is also accepted.
2.2. Approaches for Defining Life
2.3. Comparative Analysis Using Analogies among Biosystems as a New Approach for Characterizing Life
3. Estimating the Most Primitive Life-Like System
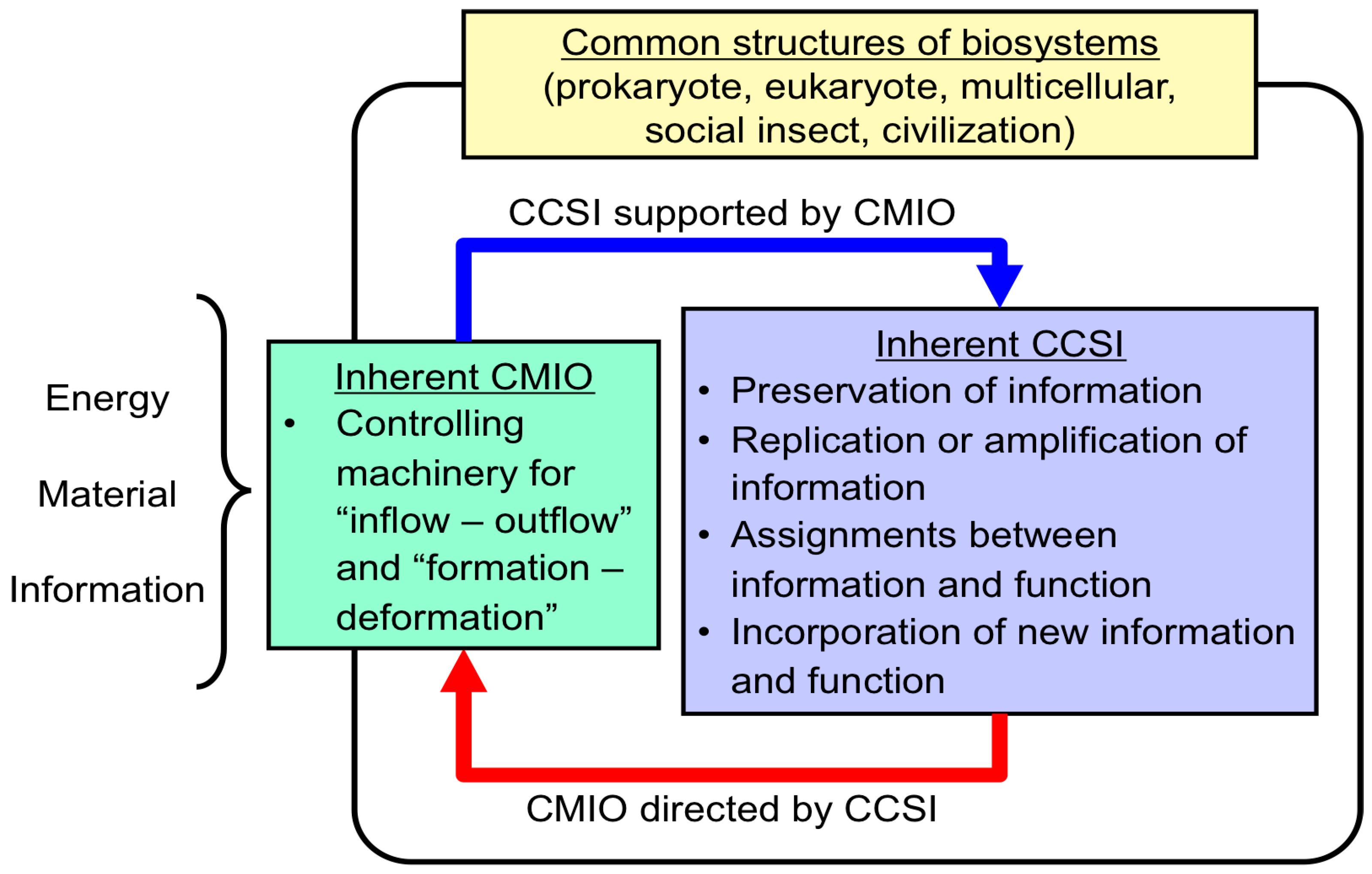
3.1. Importance of Energy and Material Supply by CMIO
3.2. A Picture of the Most Primitive Life-Like System Deduced from the Biosystems Analogy
3.3. Two Essential Genes Initiated a Primitive Life-Like System
3.4. Could RNA-Based Life-Like Systems Form Minimal Life-Like Systems?
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Crick, F. Central dogma of molecular biology. Nature 1970, 227, 561–563. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. The origin of life from the life of subjectivity. In Fundamentals of Life; Palyi, G., Zucchi, C., Eds.; Elsevier: Paris, France, 2002; pp. 563–575. [Google Scholar]
- Kawamura, K. Relativity of the roles of genes, subjectivity, and self-organization for the origin and evolution of life. In In the Shadow of Darwinism. Alternative Theories in the 20th Century; Levit, G.S., Popov, I.Y., Hossfeld, U., Olsson, L., Breidbach, O., Eds.; Fineday Press: St. Petersburg, Russia, 2003; pp. 218–238. [Google Scholar]
- Kawamura, K. Civilization as a biosystem examined by the comparative analysis of biosystems. Biosyst. 2007, 90, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, N.; Husimi, Y. A model of the virus-type strategy in the early stage of encoded molecular evolution. J. Theor. Biol. 1995, 176, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, W. The RNA world. Nature 1986, 319, 618–618. [Google Scholar] [CrossRef]
- Orgel, L.E.; Crick, F.H. Anticipating an RNA world. Some past speculations on the origin of life: Where are they today? FEBS J. 1993, 7, 238–239. [Google Scholar]
- Eigen, M. Self-organization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. RNA catalysis and the origins of life. J. Theor. Biol. 1986, 123, 127–149. [Google Scholar] [CrossRef]
- Lee, D.H.; Severin, K.; Yokobayashi, Y.; Ghadiri, M.R. Emergence of symbiosis in peptide self-replication through a hypercyclic network. Nature 1997, 390, 591–594. [Google Scholar] [PubMed]
- Ikehara, K. Possible steps to the emergence of life: The [GADV]-protein world hypothesis. Chem. Rec. 2005, 5, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Prebiotic Chemistry and the origin of the RNA world. Crit. Rev. Biochem. Mol. Biol. 2004, 39, 99–123. [Google Scholar] [PubMed]
- Bernhardt, H.S. The RNA world hypothesis: The worst theory of the early evolution of life (except for all the others). Biol. Direct 2012, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Drawbacks of the ancient RNA-based life-like system under primitive earth conditions. Biochimie 2012, 94, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Reality of the emergence of life-like systems from simple prebiotic polymers on primitive earth. In Genesis—In the Beginning: Precursors of Life, Chemical Models and Early biological Evolution; Seckbach, J., Gordon, R., Eds.; Springer: New York, NY, USA, 2012; pp. 123–144. [Google Scholar]
- Higgs, P.G.; Lehman, N. The RNA world: Molecular cooperation at the origins of life. Nat. Rev. Genet. 2014, 16, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mojzsis, S.J.; Arrhenius, G.; McKeegan, K.D.; Harrison, T.M.; Nutman, A.P.; Friend, C.R.L. Evidence for life on Earth before 3800 million years ago. Nature 1996, 384, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Watson, E.B.; Harrison, T.M. Zircon Thermometer reveals minimum melting conditions on earliest earth. Science 2005, 308, 841–844. [Google Scholar] [CrossRef] [PubMed]
- Mojzsis, S.J.; Harrison, T.M.; Pidgeon, R.T. Oxygen-isotope evidence from ancient zircons for liquid water at the Earth’s surface 4300 Myr ago. Nature 2000, 409, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Dohm, J.M.; Maruyama, S. Habitable trinity. Geosci. Front. 2015, 6, 95–101. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F.; Murchie, S.L.; Bibring, J.-B.; Meunier, A.; Fraeman, A.A.; Langevin, Y. Subsurface water and clay mineral formation during the early history of Mars. Nature 2011, 479, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Squyres, S.W.; Reynolds, R.T.; Cassen, P.M. Liquid water and active resurfacing on Europa. Nature 1983, 301, 225–226. [Google Scholar] [CrossRef]
- Kasting, J.F.; Ackerman, T.P. Climatic consequences of very high carbon dioxide levels in the earth’s early atmosphere. Science 1986, 244, 1383–1385. [Google Scholar] [CrossRef]
- Kasting, J.F. Earth’s early atmosphere. Science 1993, 259, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Sagan, C.; Mullen, G. Earth and Mars: Evolution of atmosphere and surface temperatures. Science 1972, 177, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.J.; Hall, A.J. The emergence of life from iron monosulphide bubbles at a submarine hydrothermal redox and pH front. J. Geol. Soc. 1997, 154, 377–402. [Google Scholar] [CrossRef]
- Macleod, G.; Mackeown, C.; Hall, A.J.; Russell, M.J. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig. Life Evol. Biosphere. 1994, 24, 19–41. [Google Scholar] [CrossRef]
- Grotzinger, J.P.; Kasting, J.F. New constraints on Precambrian ocean composition. J. Geol. 1993, 101, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pace, N.R. Origin of life—Facing up to the physical setting. Cell 1991, 65, 531–533. [Google Scholar] [CrossRef]
- Corliss, J.B.; Baross, J.A.; Hoffman, S.E. An hypothesis concerning the relationship between submarine hot springs and the origin of life on Earth. Oceanol. Acta 1981, 4, 59–69. [Google Scholar]
- Forterre, P. A hot topic: the origin of hyperthermopiles. Cell 1996, 85, 789–792. [Google Scholar] [CrossRef]
- Galtier, N.; Tourasse, N.; Gouy, M. A nonhyperthermophilic common ancestor to extant life forms. Science 1999, 283, 220–221. [Google Scholar] [CrossRef] [PubMed]
- Boussau, B.; Blanquart, S.; Necsulea, A.; Lartillot, N.; Gouy, M. Parallel adaptations to high temperatures in the Archaean eon. Nature 2008, 456, 942–945. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Behaviour of RNA under hydrothermal conditions and the origins of life. Int. J. Astrobiol. 2005, 3, 301–309. [Google Scholar] [CrossRef]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nat. Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Imai, E.; Honda, H.; Hatori, K.; Brack, A.; Matsuno, K. Elongation of oligopeptides in a simulated submarine hydrothermal system. Science 1999, 283, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Mielke, R.; Russell, M.J.; Wilson, P.R.; McGlynn, S.E.; Coleman, M.; Kidd, R.; Kanik, I. Design, fabrication, and test of a hydrothermal reactor for origin-of-life experiments. Astrobiol. 2010, 10, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Monitoring of hydrothermal reactions in 3 ms using fused-silica capillary tubing. Chem. Lett. 1999, 28, 125–126. [Google Scholar] [CrossRef]
- Kawamura, K. Monitoring hydrothermal reactions on the millisecond time scale using a micro-tube flow reactor and kinetics of ATP hydrolysis for the RNA world hypothesis. Bull. Chem. Soc. Jpn. 2000, 73, 1805–1811. [Google Scholar] [CrossRef]
- Kawamura, K. In situ UV-VIS detection of hydrothermal reactions using fused-silica capillary tubing within 0.08–3.2 s at high temperatures. Anal. Sci. 2002, 18, 715–716. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Nagayoshi, H.; Yao, T. In situ analysis of proteins at high temperatures mediated by capillary-flow hydrothermal UV-Vis spectrophotometer with a water-soluble chromogenic reagent. Anal. Chim. Acta 2010, 667, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Takeya, H.; Kushibe, T.; Koizumi, Y. Mineral-enhanced hydrothermal oligopeptide formation at the second time scale. Astrobiology 2011, 11, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Kinetics and activation parameter analyses of hydrolysis and interconversion of 2′,5′- and 3′,5′-linked dinucleoside monophosphate at extremely high temperatures. Biochim. Biophys. Acta 2003, 1620, 199–210. [Google Scholar] [CrossRef]
- Kawamura, K. Kinetic analysis of cleavage of ribose phosphodiester bond within guanine and cytosine rich oligonucleotides and dinucleotides at 65–200 °C and its implications on the chemical evolution of RNA. Bull. Chem. Soc. Jpn. 2003, 76, 153–162. [Google Scholar] [CrossRef]
- Tobé, S.; Heams, T.; Vergne, J.; Hervé, G.; Maurel, M.-C. The catalytic mechanism of hairpin ribozyme studied by hydrostatic pressure. Nucl. Acid. Res. 2005, 33, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Karrour, H.; Vergne, J.; Hervé, G.; Maurel, M.-C. High-pressure analysis of a hammerhead ribozyme from Chrysanthemum chlorotic mottle viroid reveals two different populations of self-cleaving molecule. FEBS J. 2011, 278, 3739–3747. [Google Scholar]
- Kawamura, K.; Yukioka, M. Kinetics of the racemization of amino acids at 225–275 °C using a real-time monitoring method of hydrothermal reactions. Thermochim. Acta 2001, 375, 9–16. [Google Scholar] [CrossRef]
- Kawamura, K.; Nishi, T.; Sakiyama, T. Consecutive elongation of alanine oligopeptides at the second time range under hydrothermal condition using a micro flow reactor system. J. Am. Chem. Soc. 2005, 127, 522–523. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K.; Silva, L.D.; Ogawa, M.; Konagaya, N.; Maurel, M.-C. Verification of chemical evolution of RNA under hydrothermal environments on the primitive Earth. In BIO Web of Conferences 4, 00011; EDP Sciences: London, UK, 2015. [Google Scholar]
- Kawamura, K. A new probe for the indirect measurement of the conformation and interaction of biopolymers at extremely high temperatures using a capillary flow hydrothermal reactor system for UV-visible spectrophotometry. Anal. Chim. Acta 2005, 543, 236–241. [Google Scholar] [CrossRef]
- Kawamura, K.; Nagayoshi, H. Behavior of DNA under hydrothermal conditions with MgCl2 additive using an in situ UV-visible spectrophotometer. Thermochim. Acta 2007, 466, 63–68. [Google Scholar] [CrossRef]
- Luisi, P.L. About various definitions of life. Origins Life Evol. Biosph. 1998, 28, 613–622. [Google Scholar] [CrossRef]
- Palyi, G.; Zucchi, C.; Caglioti, L. Fundamentals of Life; Elsevier: Paris, France, 2002; pp. 15–55. [Google Scholar]
- Luisi, P.L. The Emergence of Life: From Chemical Origins to Synthetic Biology; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Ng, S.-F.; Lin, R.C.Y.; Laybutt, D.R.; Barres, R.; Owens, J.A.; Morris, M.J. Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 2010, 467, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Global environmental protection on the basis of the biosystematic view of civilization—importance of information flow, and roles of energy and material flow. In Proceedings of The 3rd Environment Asia International Conference on “Towards International Collaboration for an Environmentally Sustainable World”, Bangkok, Thailand, 17–19 June 2015; Thai Society of Higher Education Institutes on Environment: Bangkok, Thailand, 2015; pp. 385–395. [Google Scholar]
- Kawamura, K. A biosystematic view of civilizations: Western Europe and Japan: before and after the industrial revolution. Comp. Civiliz. Rev. 2015, 73, 77–100. [Google Scholar]
- Kawamura, K. Global environmental protection on the basis of a biosystem view of civilization, the role of education and invention. J. Hum. Environ. Stud. 2014, 12, 65–83. [Google Scholar]
- Kawamura, K. Reconsideration of the definition of life. Viva Orig. 2014, 42, 26–31. [Google Scholar]
- Kawamura, K. Global environmental protection on the basis of a biosystem view of civilization: implications on inflow and outflow of energy, material, and information. J. Hum. Environ. Stud. 2015, 13, 53–69. [Google Scholar]
- La Scola, B.; Audic, S.; Robert, C.; Jungang, L.; de Lamballerie, X.; Drancourt, M.; Birtles, R.; Claverie, J.M.; Raoult, D. A giant virus in amoebae. Science 2003, 299, 2033–2033. [Google Scholar] [CrossRef] [PubMed]
- Johnston, W.K.; Unrau, P.J.; Lawrence, M.S.; Glasner, M.E.; Bartel, D.P. RNA-catalyzed RNA polymerization: Accurate and general RNA-templated primer extension. Science 2001, 292, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Joyce, G.F. Forty years of in vitro evolution. Angew. Chem. Int. Ed. 2007, 46, 6420–6436. [Google Scholar] [CrossRef] [PubMed]
- Radzicka, A.; Wolfenden, R. A proficient enzyme. Science 1995, 267, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, K. Temperature limit for the emergence of life-like system deduced from the prebiotic chemical kinetics under the hydrothermal conditions. In Proceedings of the Twelfth International Conference on the Simulation and Synthesis of Living Systems, Odense, Denmark, Artificial Life 12. 19–23 August 2010; Fellermann, H., Dörr, M., Hanczyc, M.M., Lauren, L.L., Maurer, S., Merkle, D., Monnard, P.-A., Støy, K., Rasmussen, S., Eds.; pp. 37–44.
- Eigen, M.; McCaskill, J.; Schuster, P. Molecular quasi-species. J. Phys. Chem. 1988, 92, 6881–6891. [Google Scholar] [CrossRef]
- Attwater, J.; Wochner, A.; Holliger, P. In-ice evolution of RNA polymerase ribozyme activity. Nat. Chem. 2013, 5, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.R.; Orgel, L.E.; Tf, W.U. The limits of template-directed synthesis with nucleoside-5’-phosphoro (2-methyl) imidazolides. Orig. Life Evol. Biosph. 1993, 23, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Unrau, P.J.; Bartel, D.P. RNA-catalysed nucleotide synthesis. Nature 1998, 395, 260–263. [Google Scholar] [PubMed]
- Lau, M.W.L.; Cadieux, K.E.C.; Unrau, P.J. Isolation of fast purine nucleotide synthase ribozymes. J. Am. Chem. Soc. 2004, 126, 15686–15693. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, N.; Ellington, A.D. Ribozyme catalysis of metabolism in the RNA world. Chem. Biodivers. 2007, 4, 633–655. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Higgs, P.G. Origin of self-replicating biopolymers: autocatalytic feedback can jump-start the RNA world. J. Mol. Evol. 2009, 69, 541–554. [Google Scholar] [CrossRef] [PubMed]
- Shay, J.A.; Huynh, C.; Higgs, P.G. The origin and spread of a cooperative replicase in a prebiotic chemical system. J. Theor. Biol. 2015, 364, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Zaher, H.S.; Unrau, P.J. Selection of an improved RNA polymerase ribozyme with superior extension and fidelity. RNA 2007, 13, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Luisi, P.L.; Ferri, F.; Stano, P. Approaches to semi-synthetic minimal cells: A review. Naturwissenschaften 2006, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Stano, P.; Luisi, P.L. Semi-synthetic minimal cells: origin and recent developments. Curr. Opin. Biotechnol. 2013, 24, 633–638. [Google Scholar] [CrossRef] [PubMed]

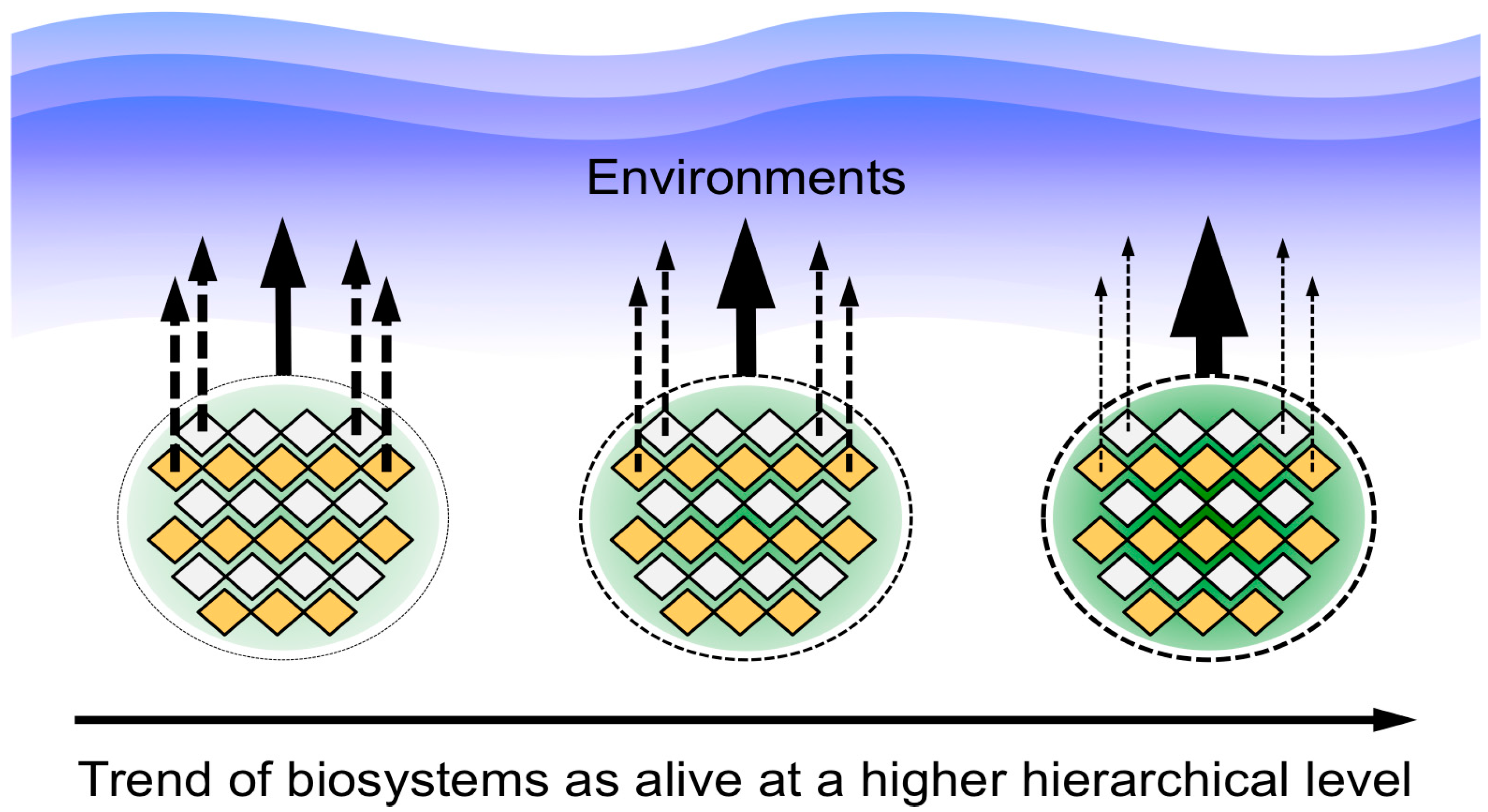
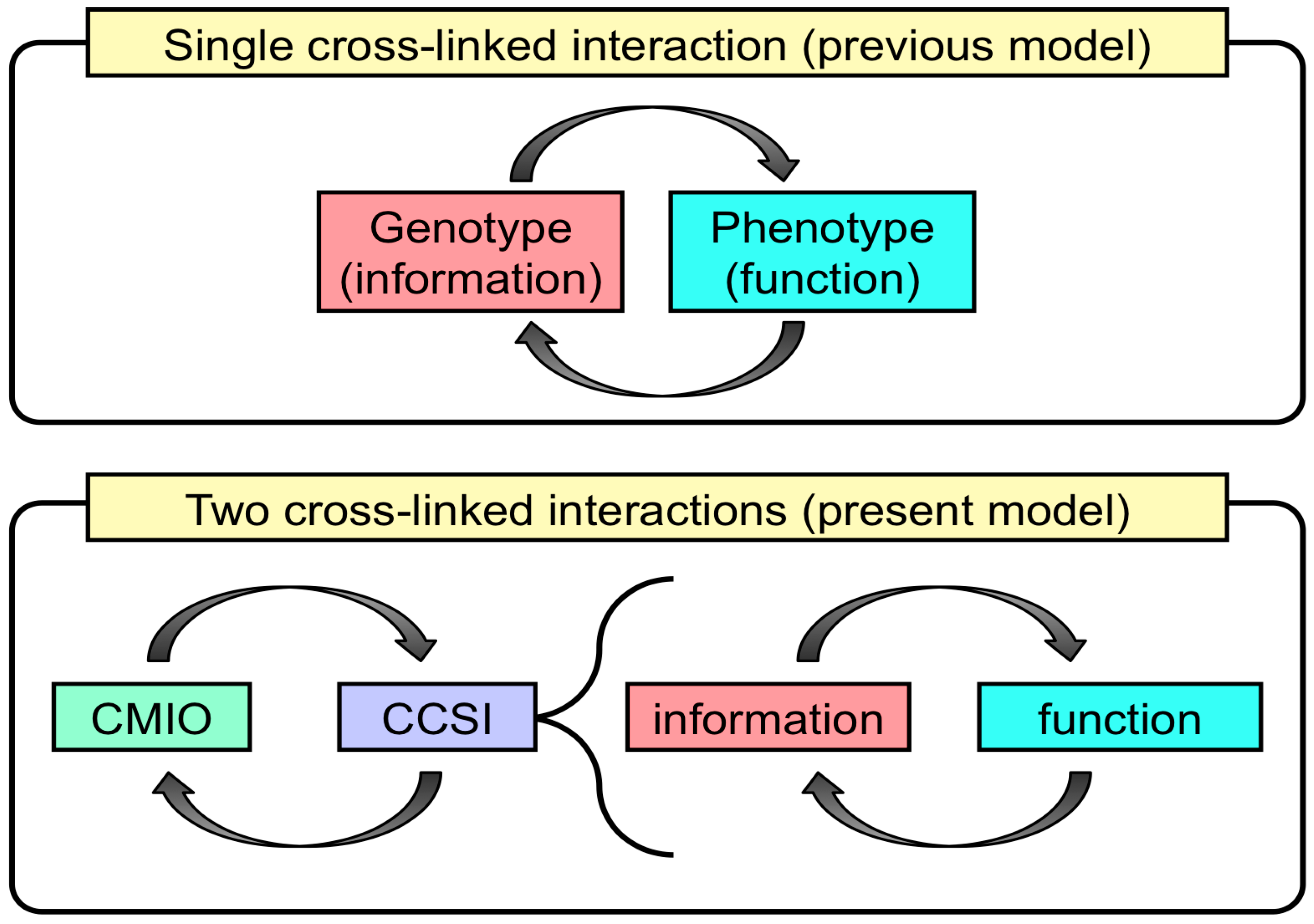

| Biosystems at Different Hierarchical Levels | Attributes |
|---|---|
| ● RNA-based life-like system (RNA world) | ● Boundary |
| ● Prokaryote | ● Metabolism |
| ● Eukaryote (single celled) | ● Information |
| ● Multicellular organism | ● Preservation of information |
| ● Social insect | ● Self-reproduction |
| ● Society of organism | ● Assignment between information and function |
| ● Ecosystem | ● Incorporation of new information and function |
| ● Civilization | ● Role of biosystem and its building blocks in relation to their environment |
| Biosystems | Inherent CCSI | Inherent CMIO |
|---|---|---|
| Prokaryote | The information flow from DNA, RNA, proteins and indirectly to other molecules, by transcription, translation and by enzymatic catalysis | Energy and material flow, including information through membrane proteins |
| Eukaryote (single celled) | Genetic information is withdrawn through nucleus and used in cytoplasm | Subcellular organelles, such as oral groove, gullet and food vacuole, are used for the incorporation of materials |
| Multicellular | Mixing of genes by means of sex for reproducing next generations | Organs, such as mouth, root and leaves, are used for gaining material and energy |
| Social insect | Differentiation of queen, worker, soldier, etc., for reproducing next generations | Workers gather to stock materials for the society of individuals |
| Civilization | Social systems and instruments for education and research | Social systems and instruments for production and consumption |
| CCSI supported by CMIO |
|---|
| 1. Preservation of information |
| 2. Replication (or amplification) of information |
| 3. Assignment between information and function |
| 4. Incorporation of new function linked with its information into the biosystem |
| CMIO directed by CCSI |
| 5. Central controlling machinery for inflow and outflow of energy, material and information from environments |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawamura, K. A Hypothesis: Life Initiated from Two Genes, as Deduced from the RNA World Hypothesis and the Characteristics of Life-Like Systems. Life 2016, 6, 29. https://doi.org/10.3390/life6030029
Kawamura K. A Hypothesis: Life Initiated from Two Genes, as Deduced from the RNA World Hypothesis and the Characteristics of Life-Like Systems. Life. 2016; 6(3):29. https://doi.org/10.3390/life6030029
Chicago/Turabian StyleKawamura, Kunio. 2016. "A Hypothesis: Life Initiated from Two Genes, as Deduced from the RNA World Hypothesis and the Characteristics of Life-Like Systems" Life 6, no. 3: 29. https://doi.org/10.3390/life6030029





