Nitrogen Starvation Acclimation in Synechococcus elongatus: Redox-Control and the Role of Nitrate Reduction as an Electron Sink
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Growth Conditions
2.2. Determination of Luciferase Activity
2.3. Measurement of Glutamine Synthetase (GS) Activity
2.4. Photosynthetic Oxygen Evolution Measurement
2.5. PAM Fluorometry
2.6. Determination of Colony Forming Units (CFU)
2.7. Determination of the Glycogen Content in a Culture
3. Results
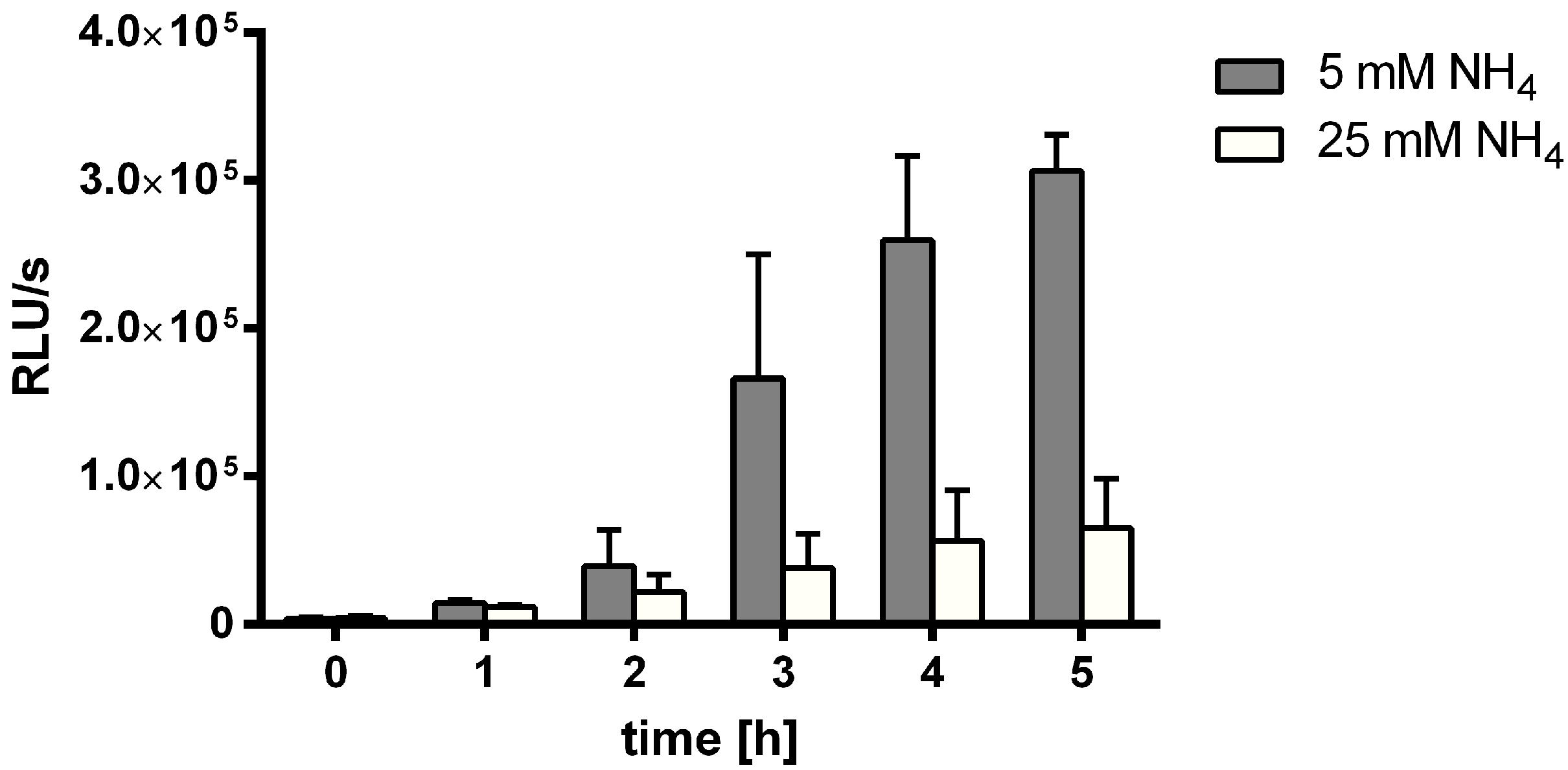
3.1. MSX-Effects on Viability, nblA and glnB Expression in Ammonium Supplemented Cells
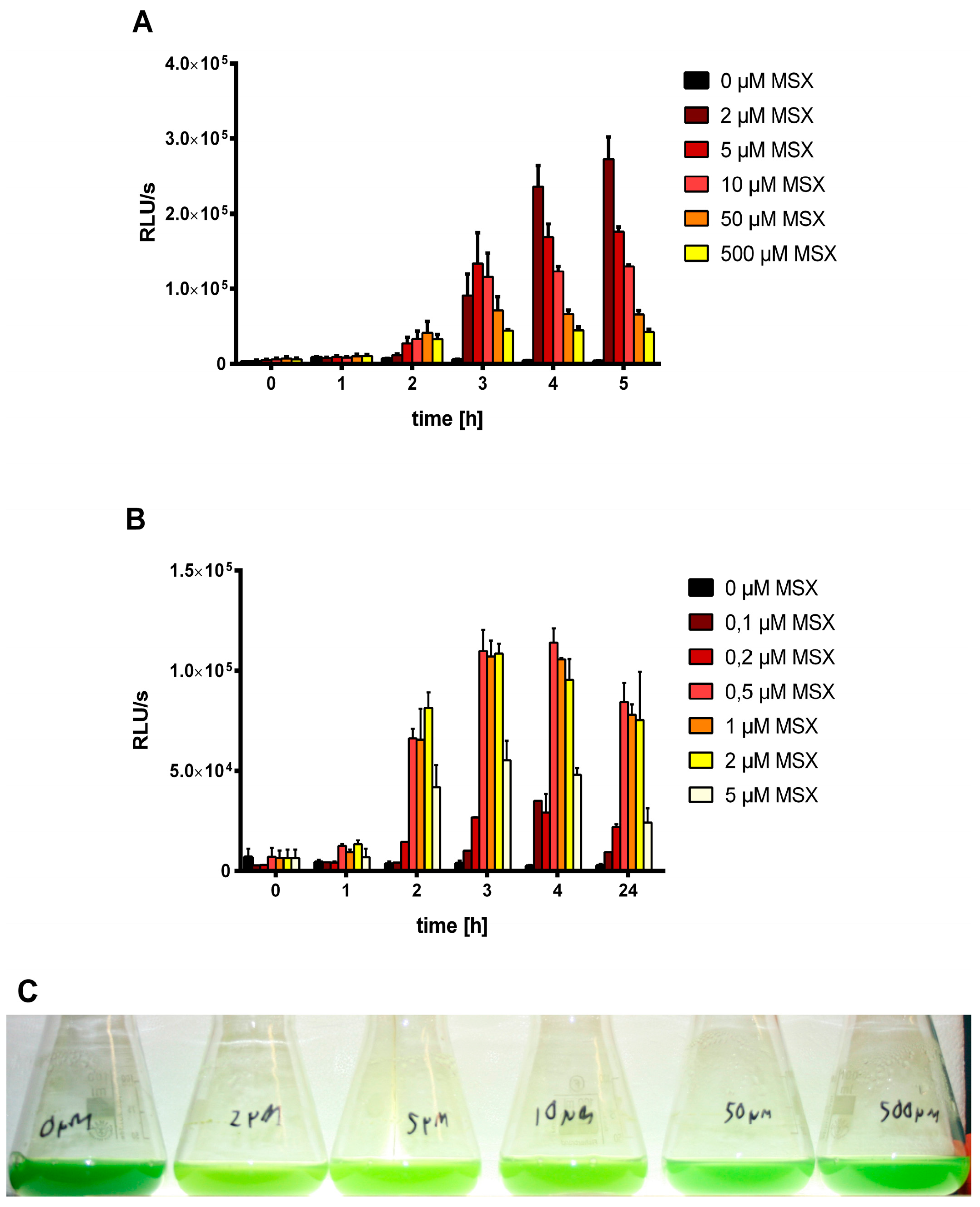
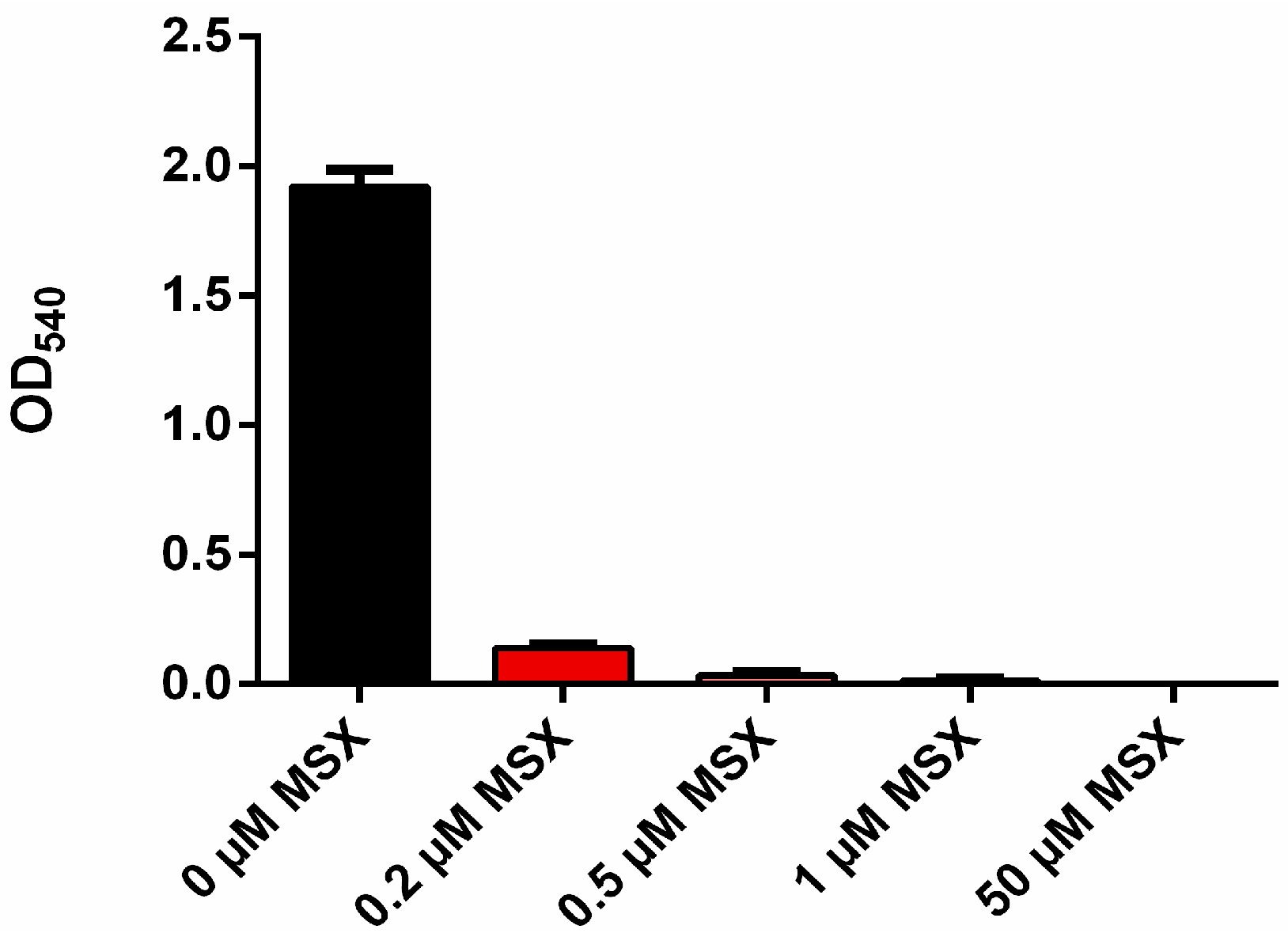
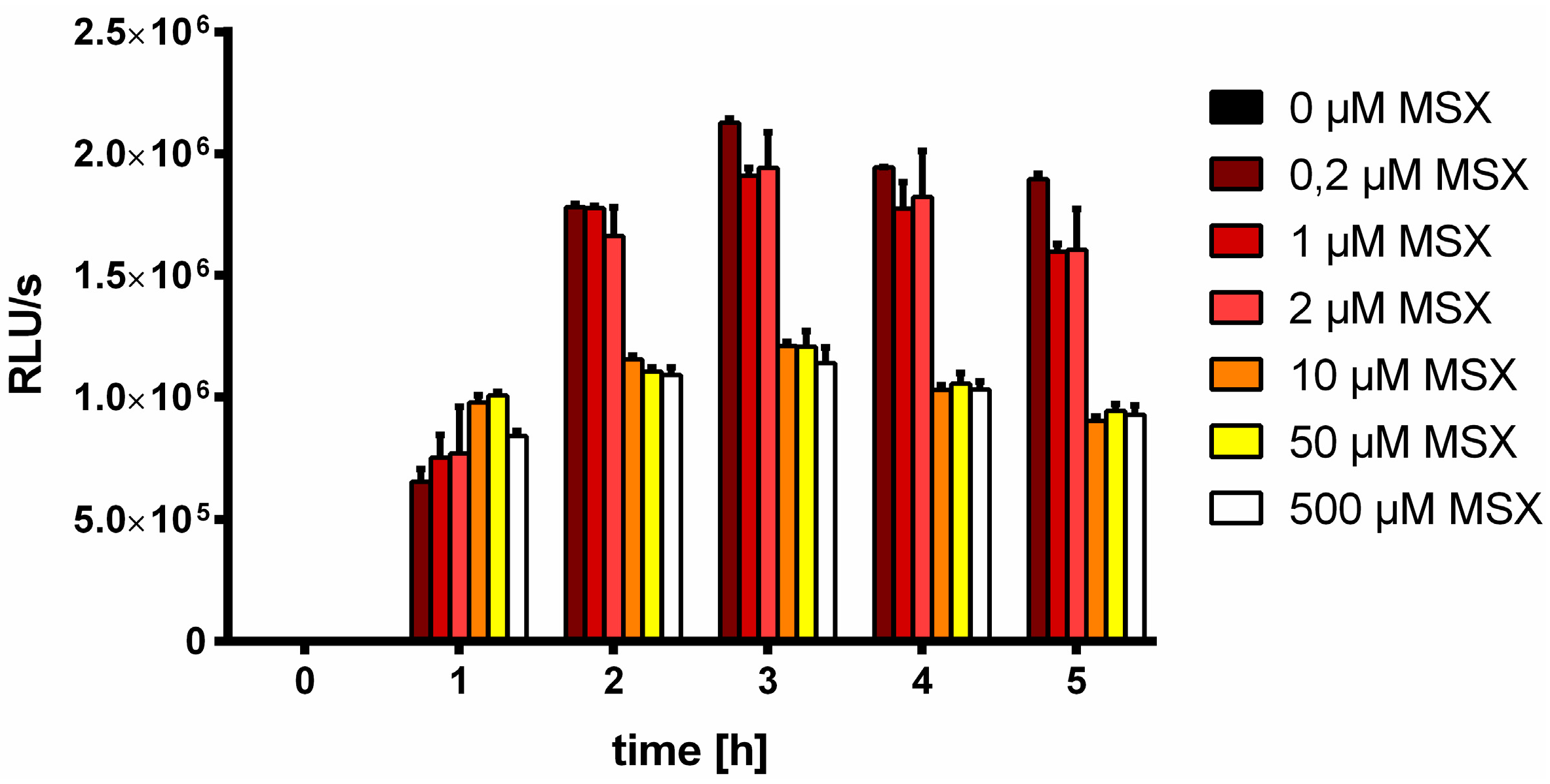
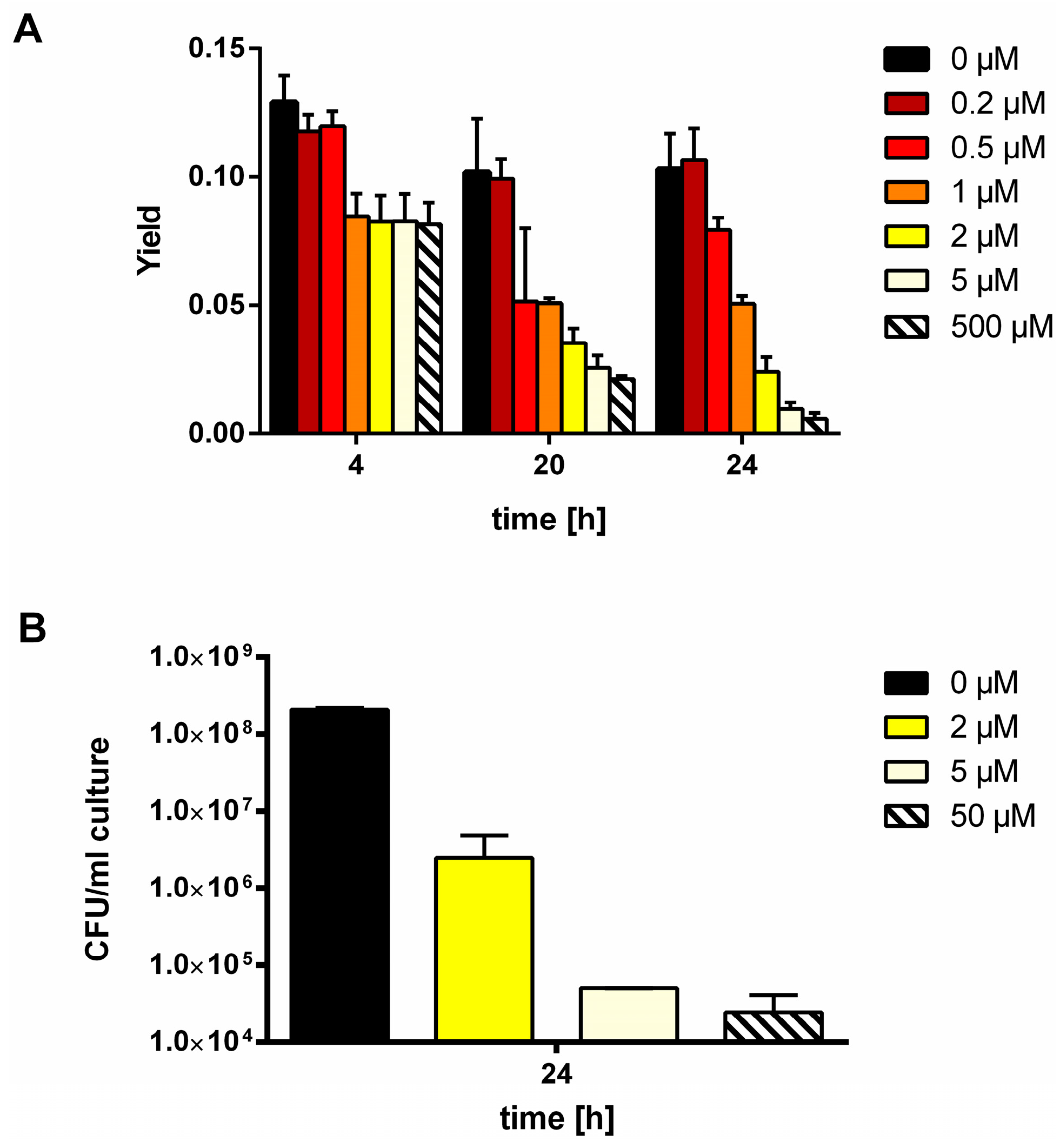
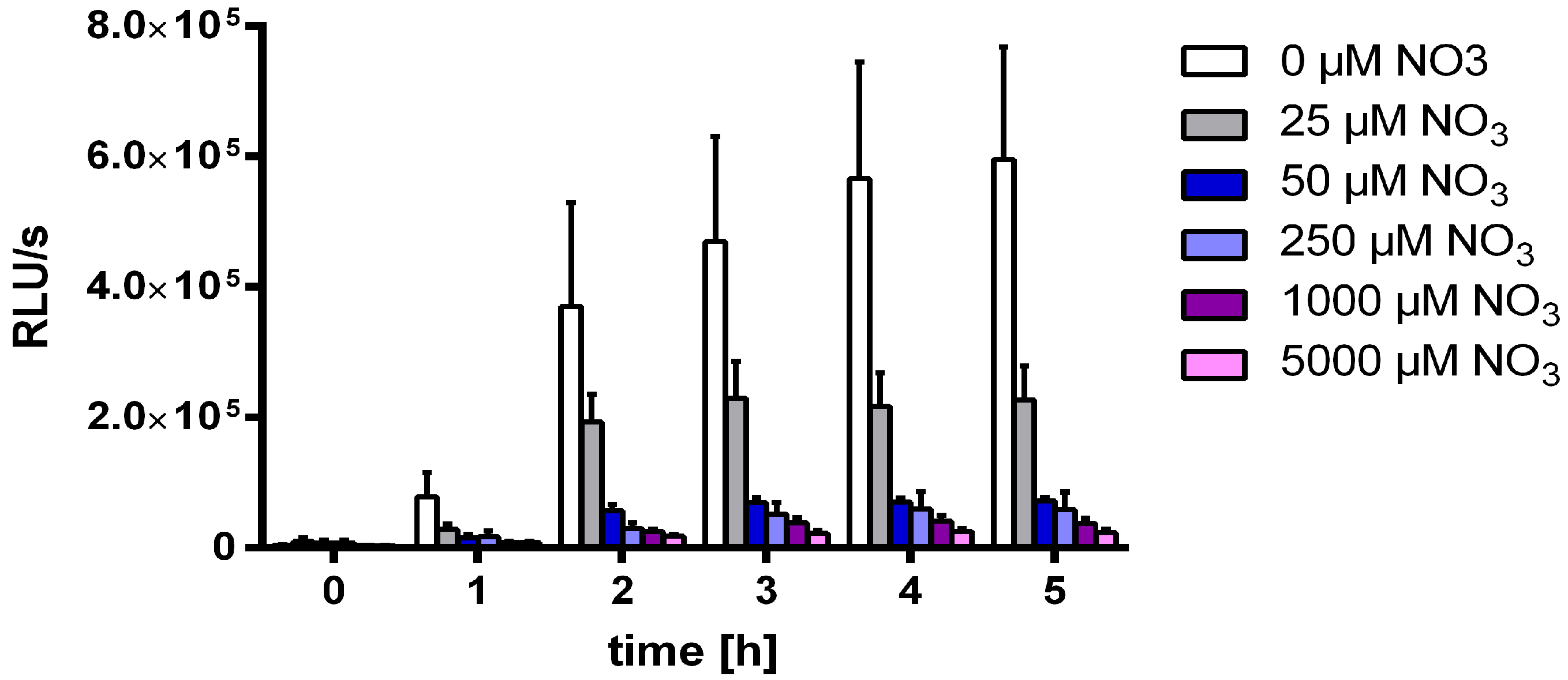
3.2. Nitrate Prevents MSX-Induced nblA-Expression

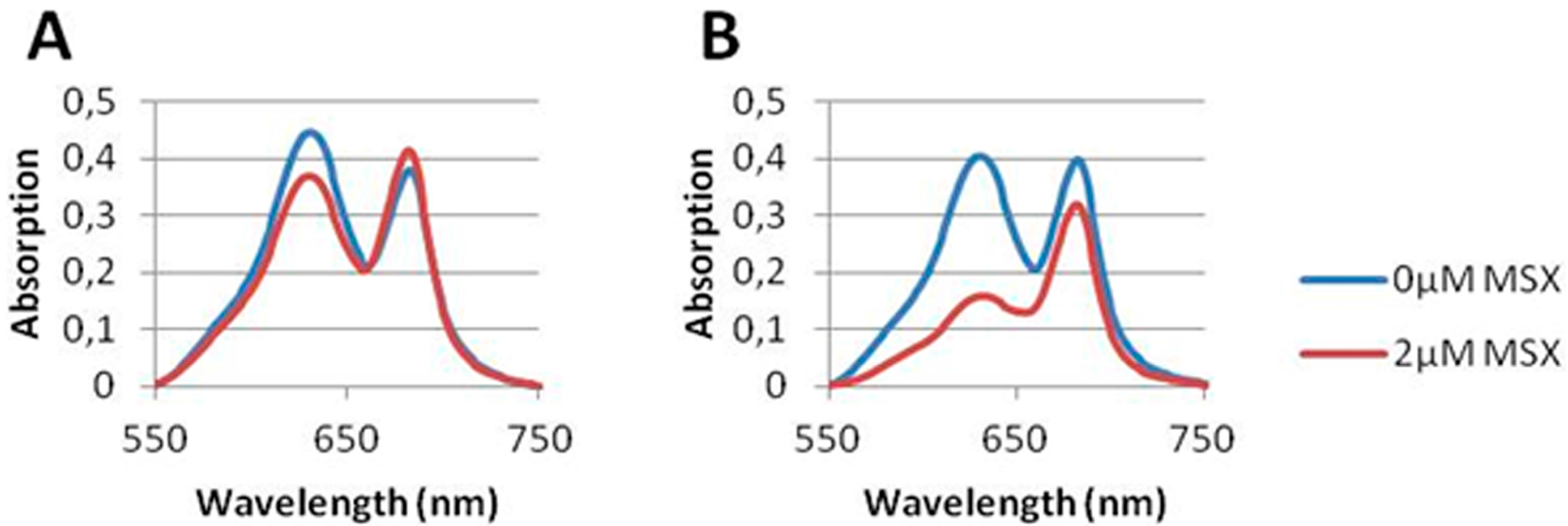
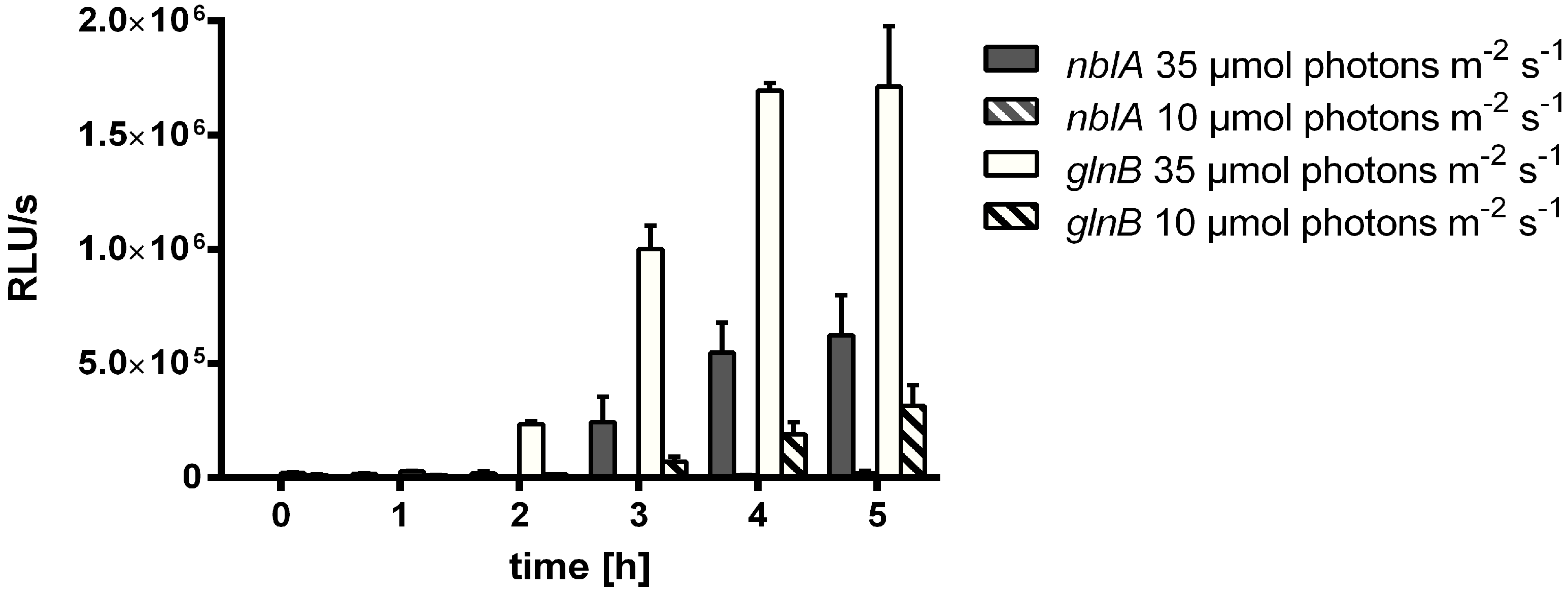
3.3. Efficient Photosynthetic Electron Transport is Necessary for MSX-Induced nblA Expression
| Conditions | Formed oxygen (nm/mL) |
|---|---|
| Darkness (0 µmol photons m−2 s−1) | 0 |
| Light (35 µmol photons m−2 s−1) | 12.3 |
| Light (35 µmol photons m−2 s−1) + 25 mM NH4 | 1.2 |

4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References
- Vitousek, P.M.; Howarth, R.W. Nitrogen limitation on land and in the sea: How can it occur? Biogeochemistry 1991, 13, 87–115. [Google Scholar]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar]
- Allen, M.M.; Smith, A.J. Nitrogen chlorosis in blue-green algae. Archiv fur Mikrobiologie 1969, 69, 114–120. [Google Scholar]
- Collier, J.L.; Grossman, A.R. Chlorosis induced by nutrient deprivation in Synechococcus sp. strain PCC 7942: Not all bleaching is the same. J. Bacteriol. 1992, 174, 4718–4726. [Google Scholar]
- Collier, J.L.; Grossman, A.R. A small polypeptide triggers complete degradation of light-harvesting phycobiliproteins in nutrient-deprived cyanobacteria. EMBO J. 1994, 13, 1039–1047. [Google Scholar]
- Karradt, A.; Sobanski, J.; Mattow, J.; Lockau, W.; Baier, K. NblA, a key protein of phycobilisome degradation, interacts with ClpC, a HSP100 chaperone partner of a cyanobacterial Clp protease. J. Biol. Chem. 2008, 283, 32394–32403. [Google Scholar]
- Görl, M.; Sauer, J.; Baier, T.; Forchhammer, K. Nitrogen-starvation-induced chlorosis in Synechococcus PCC 7942: Adaptation to long-term survival. Microbiology 1998, 144, 2449–2458. [Google Scholar]
- Sauer, J.; Schreiber, U.; Schmid, R.; Volker, U.; Forchhammer, K. Nitrogen starvation-induced chlorosis in Synechococcus PCC 7942. Low-level photosynthesis as a mechanism of long-term survival. Plant Physiol. 2001, 126, 233–243. [Google Scholar] [PubMed]
- Schwarz, R.; Grossman, A.R. A response regulator of cyanobacteria integrates diverse environmental signals and is critical for survival under extreme conditions. Proc. Natl. Acad. Sci. USA 1998, 95, 11008–11013. [Google Scholar] [CrossRef] [PubMed]
- Van Waasbergen, L.G.; Dolganov, N.; Grossman, A.R. NblS, a gene involved in controlling photosynthesis-related gene expression during high light and nutrient stress in Synechococcus elongatus PCC 7942. J. Bacteriol. 2002, 184, 2481–2490. [Google Scholar]
- Lahmi, R.; Sendersky, E.; Perelman, A.; Hagemann, M.; Forchhammer, K.; Schwarz, R. Alanine dehydrogenase activity is required for adequate progression of phycobilisome degradation during nitrogen starvation in Synechococcus elongatus PCC 7942. J. Bacteriol. 2006, 188, 5258–5265. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Salinas, P.; Lopez-Redondo, M.L.; Cayuela, M.L.; Marina, A.; Contreras, A. Phosphorylation-independent activation of the atypical response regulator NblR. Microbiology 2008, 154, 3002–3015. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Chibazakura, T.; Yoshikawa, H. NblR is a novel one-component response regulator in the cyanobacterium Synechococcus elongatus PCC 7942. Biosci. Biotechnol. Biochem. 2008, 72, 1072–1079. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Kubo, T.; Hayashi, M.; Kobayashi, I.; Yagasaki, T.; Chibazakura, T.; Watanabe, S.; Yoshikawa, H. Interactions between histidine kinase NblS and the response regulators RpaB and SrrA are involved in the bleaching process of the cyanobacterium Synechococcus elongatus PCC 7942. Plant Cell Physiol. 2011, 52, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Watanabe, S.; Nimura-Matsune, K.; Chibazakura, T.; Tozawa, Y.; Yoshikawa, H. Exploration of a possible partnership among orphan two-component system proteins in cyanobacterium Synechococcus elongatus PCC 7942. Biosci. Biotechnol. Biochem. 2012, 76, 1484–1491. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.; Lopez-Redondo, M.L.; Miguel-Romero, L.; Neira, J.L.; Marina, A.; Contreras, A. Insights into the mechanism of activation of the phosphorylation-independent response regulator NblR. Role of residues Cys69 and Cys96. Biochim. Biophys. Acta 2012, 1819, 382–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashby, M.K.; Houmard, J.; Mullineaux, C.W. The ycf27 genes from cyanobacteria and eukaryotic algae: Distribution and implications for chloroplast evolution. FEMS Microbiol. Lett. 2002, 214, 25–30. [Google Scholar] [PubMed]
- Mikami, K.; Kanesaki, Y.; Suzuki, I.; Murata, N. The histidine kinase Hik33 perceives osmotic stress and cold stress in Synechocystis sp. PCC 6803. Mol. Microbiol. 2002, 46, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kanesaki, Y.; Yamamoto, H.; Paithoonrangsarid, K.; Shoumskaya, M.; Suzuki, I.; Hayashi, H.; Murata, N. Histidine kinases play important roles in the perception and signal transduction of hydrogen peroxide in the cyanobacterium Synechocystis sp. PCC 6803. Plant J. 2007, 49, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, I.; Kanesaki, Y.; Mikami, K.; Kanehisa, M.; Murata, N. Cold-regulated genes under control of the cold sensor Hik33 in Synechocystis sp. PCC 6803. Mol. Microbiol. 2001, 40, 235–244. [Google Scholar] [CrossRef] [PubMed]
- López-Redondo, M.L.; Moronta, F.; Salinas, P.; Espinosa, J.; Cantos, R.; Dixon, R.; Marina, A.; Contreras, A. Environmental control of phosphorylation pathways in a branched two-component system. Mol. Microbiol. 2010, 78, 475–489. [Google Scholar] [CrossRef] [PubMed]
- Salinas, P.; Ruiz, D.; Cantos, R.; Lopez-Redondo, M.L.; Marina, A.; Contreras, A. The regulatory factor SipA provides a link between NblS and NblR signal transduction pathways in the cyanobacterium Synechococcus sp. PCC 7942. Mol. Microbiol. 2007, 66, 1607–1619. [Google Scholar] [PubMed]
- Kappell, A.D.; Bhaya, D.; van Waasbergen, L.G. Negative control of the high light-inducible hliA gene and implications for the activities of the nbls sensor kinase in the cyanobacterium Synechococcus elongatus strain PCC 7942. Arch. Microbiol. 2006, 186, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Hanaoka, M.; Tanaka, K. Dynamics of RpaB–promoter interaction during high light stress, revealed by chromatin immunoprecipitation (ChiP) analysis in Synechococcus elongatus PCC 7942. Plant J. 2008, 56, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Hihara, Y. Acclimation to high-light conditions in cyanobacteria: From gene expression to physiological responses. J. Plant Res. 2012, 125, 11–39. [Google Scholar] [CrossRef] [PubMed]
- Moronta-Barrios, F.; Espinosa, J.; Contreras, A. In vivo features of signal transduction by the essential response regulator rpab from Synechococcus elongatus PCC 7942. Microbiology 2012, 158, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Luque, I.; Vazquez-Bermudez, M.F.; Paz-Yepes, J.; Flores, E.; Herrero, A. In vivo activity of the nitrogen control transcription factor ntca is subjected to metabolic regulation in Synechococcus sp. strain PCC 7942. FEMS Microbiol. Lett. 2004, 236, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Luque, I.; Flores, E.; Herrero, A. Molecular mechanism for the operation of nitrogen control in cyanobacteria. EMBO J. 1994, 13, 2862–2869. [Google Scholar] [PubMed]
- Espinosa, J.; Forchhammer, K.; Burillo, S.; Contreras, A. Interaction network in cyanobacterial nitrogen regulation: PipX, a protein that interacts in a 2-oxoglutarate dependent manner with PII and NtcA. Mol. Microbiol. 2006, 61, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Llacer, J.L.; Espinosa, J.; Castells, M.A.; Contreras, A.; Forchhammer, K.; Rubio, V. Structural basis for the regulation of NtcA-dependent transcription by proteins PipX and PII. Proc. Natl. Acad. Sci. USA 2010, 107, 15397–15402. [Google Scholar] [CrossRef] [PubMed]
- Forcada-Nadal, A.; Forchhammer, K.; Rubio, V. SPR analysis of promoter binding of Synechocystis PCC 6803 transcription factors NtcA and CRP suggests cross-talk and sheds light on regulation by effector molecules. FEBS Lett. 2014, 588, 2270–2276. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.; Gorl, M.; Forchhammer, K. Nitrogen starvation in Synechococcus PCC 7942: Involvement of glutamine synthetase and ntca in phycobiliprotein degradation and survival. Arch. Microbiol. 1999, 172, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gründel, M.; Scheunemann, R.; Lockau, W.; Zilliges, Y. Impaired glycogen synthesis causes metabolic overflow reactions and affects stress responses in the cyanobacterium Synechocystis sp. PCC 6803. Microbiology 2012, 158, 3032–3043. [Google Scholar] [CrossRef] [PubMed]
- Hickman, J.W.; Kotovic, K.M.; Miller, C.; Warrener, P.; Kaiser, B.; Jurista, T.; Budde, M.; Cross, F.; Roberts, J.M.; Carleton, M. Glycogen synthesis is a required component of the nitrogen stress response in Synechococcus elongatus PCC 7942. Algal Res. 2013, 2, 98–106. [Google Scholar] [CrossRef]
- Carrieri, D.; Paddock, T.; Maness, P.-C.; Seibert, M.; Yu, J. Photo-catalytic conversion of carbon dioxide to organic acids by a recombinant cyanobacterium incapable of glycogen storage. Energy Environ. Sci. 2012, 5, 9457–9461. [Google Scholar] [CrossRef]
- Espinosa, J.; Forchhammer, K.; Contreras, A. Role of the Synechococcus PCC 7942 nitrogen regulator protein pipx in ntca-controlled processes. Microbiology 2007, 153, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Aldehni, F.M.; Sauer, J.; Spielhaupter, C.; Schmid, R.; Forchhammer, K. Signal transduction protein PII is required for NtcA-regulated gene expression during nitrogen deprivation in the cyanobacterium Synechococcus elongatus strain PCC 7942. J. Bacteriol. 2003, 185, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Bender, R.A.; Janssen, K.A.; Resnick, A.D.; Blumenberg, M.; Foor, F.; Magasanik, B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J. Bacteriol. 1977, 129, 1001–1009. [Google Scholar] [PubMed]
- Dai, G.Z.; Qiu, B.S.; Forchhammer, K. Ammonium tolerance in the cyanobacterium Synechocystis sp. strain PCC 6803 and the role of the psbA multigene family. Plant Cell Environ. 2014, 37, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Ramos, J.L.; Guerrero, M.G.; Losada, M. Optimization of conditions for photoproduction of ammonia from nitrate by anacystis nidulans. Appl. Environ. Microbiol. 1982, 44, 1013–1019. [Google Scholar] [PubMed]
- Britt, R.D.; Zimmermann, J.L.; Sauer, K.; Klein, M.P. Ammonia binds to the catalytic mn of the oxygen-evolving complex of photosystem II. Evidence by electron spin-echo envelope modulation spectroscopy. J. Am. Chem. Soc. 1989, 111, 3522–3532. [Google Scholar] [CrossRef]
- Luque, I.; Zabulon, G.; Contreras, A.; Houmard, J. Convergence of two global transcriptional regulators on nitrogen induction of the stress-acclimation gene nblA in the cyanobacterium Synechococcus sp. PCC 7942. Mol. Microbiol. 2001, 41, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Salomon, E.; Bar-Eyal, L.; Sharon, S.; Keren, N. Balancing photosynthetic electron flow is critical for cyanobacterial acclimation to nitrogen limitation. Biochim. Biophys. Acta 2013, 1827, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Krasikov, V.; Aguirre von Wobeser, E.; Dekker, H.L.; Huisman, J.; Matthijs, H.C.P. Time-series resolution of gradual nitrogen starvation and its impact on photosynthesis in the cyanobacterium Synechocystis sp. PCC 6803. Physiol. Plant. 2012, 145, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, K.; Chen, X.; Schreiber, K.; Kaspar, U.; Makam, S.; Appel, J. The bidirectional nife-hydrogenase in Synechocystis sp. PCC 6803 is reduced by flavodoxin and ferredoxin and is essential under mixotrophic, nitrate-limiting conditions. J.Biol. Chem. 2014, 289, 1930–1937. [Google Scholar] [CrossRef] [PubMed]
- Baebprasert, W.; Jantaro, S.; Khetkorn, W.; Lindblad, P.; Incharoensakdi, A. Increased H2 production in the cyanobacterium Synechocystis sp. strain PCC 6803 by redirecting the electron supply via genetic engineering of the nitrate assimilation pathway. Metab. Eng. 2011, 13, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Flores, E.; Herrero, A. Nitrogen assimilation and nitrogen control in cyanobacteria. Biochem. Soc. Trans. 2005, 33, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, M.; Perewoska, I.; Kirilovsky, D. Redox control of ntcA gene expression in Synechocystis sp. PCC 6803. Nitrogen availability and electron transport regulate the levels of the NtcA protein. Plant Physiol. 2001, 125, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.-M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Botany 2014. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klotz, A.; Reinhold, E.; Doello, S.; Forchhammer, K. Nitrogen Starvation Acclimation in Synechococcus elongatus: Redox-Control and the Role of Nitrate Reduction as an Electron Sink. Life 2015, 5, 888-904. https://doi.org/10.3390/life5010888
Klotz A, Reinhold E, Doello S, Forchhammer K. Nitrogen Starvation Acclimation in Synechococcus elongatus: Redox-Control and the Role of Nitrate Reduction as an Electron Sink. Life. 2015; 5(1):888-904. https://doi.org/10.3390/life5010888
Chicago/Turabian StyleKlotz, Alexander, Edgar Reinhold, Sofía Doello, and Karl Forchhammer. 2015. "Nitrogen Starvation Acclimation in Synechococcus elongatus: Redox-Control and the Role of Nitrate Reduction as an Electron Sink" Life 5, no. 1: 888-904. https://doi.org/10.3390/life5010888






