Consequences of Decreased Light Harvesting Capability on Photosystem II Function in Synechocystis sp. PCC 6803
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains and Growth Conditions
2.2. Low-Temperature Fluorescence Spectroscopy
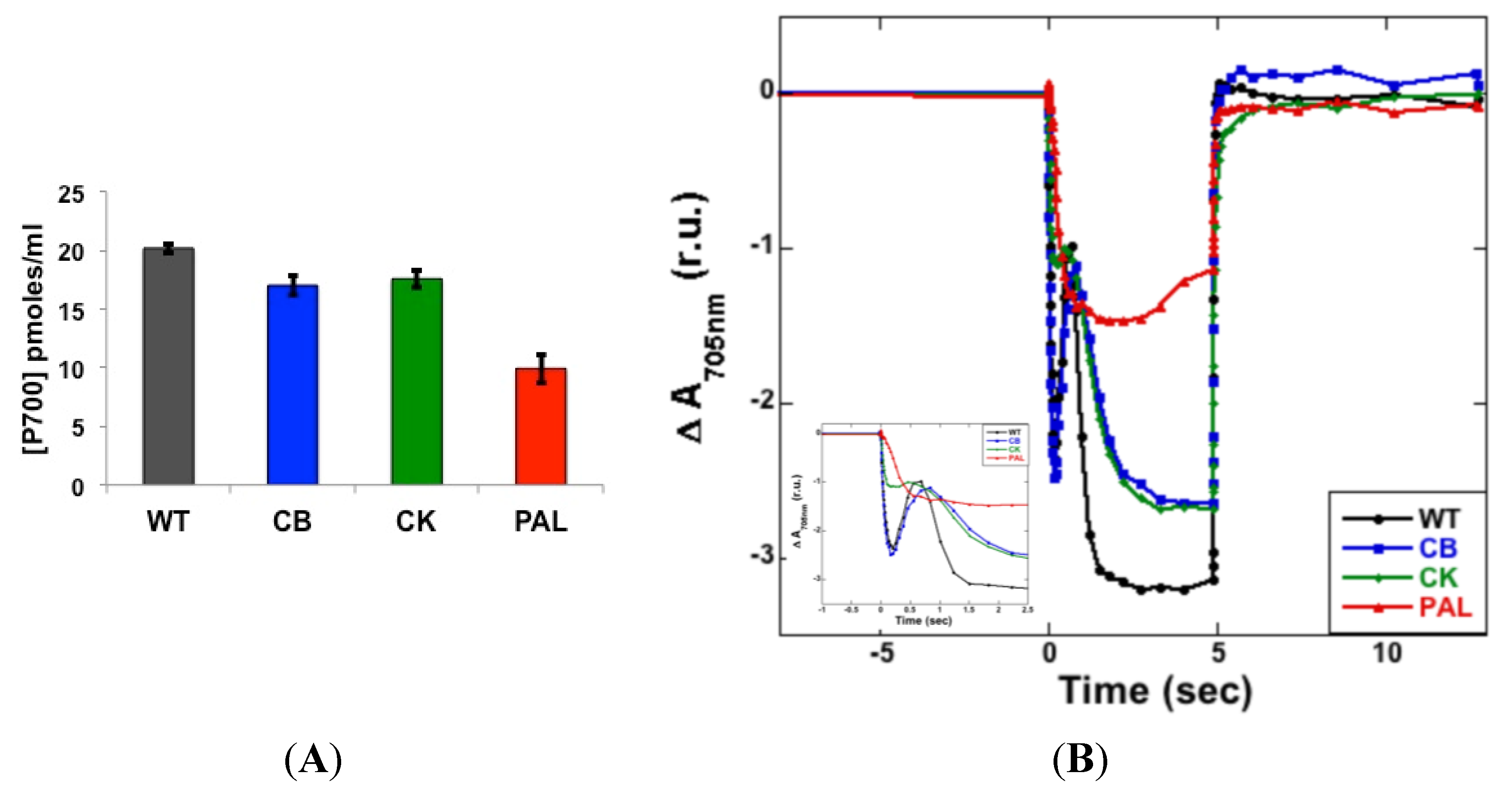
2.3. PSI Measurements
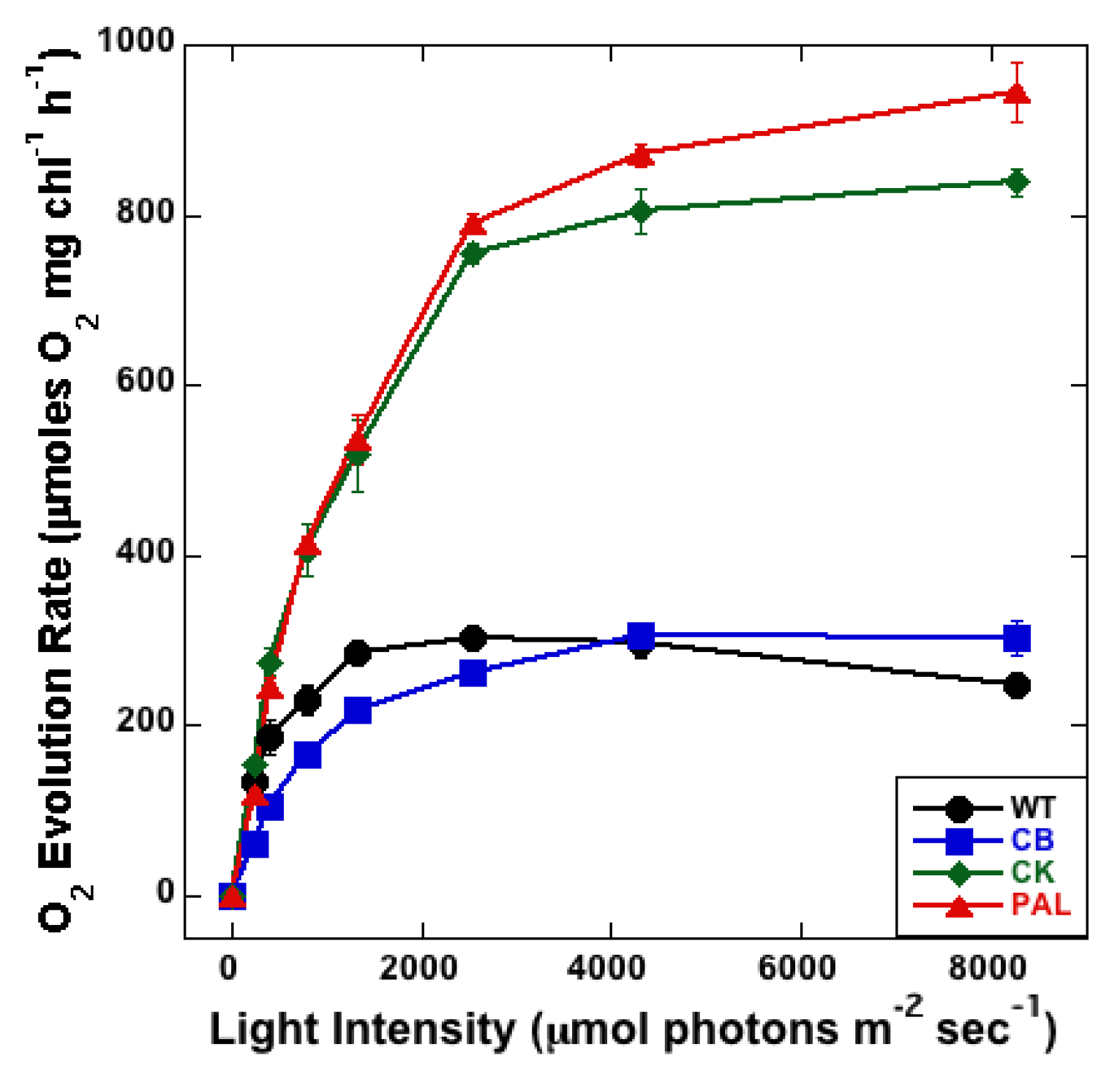
2.4. Steady State Oxygen Evolution Measurements
2.5. Western Blots
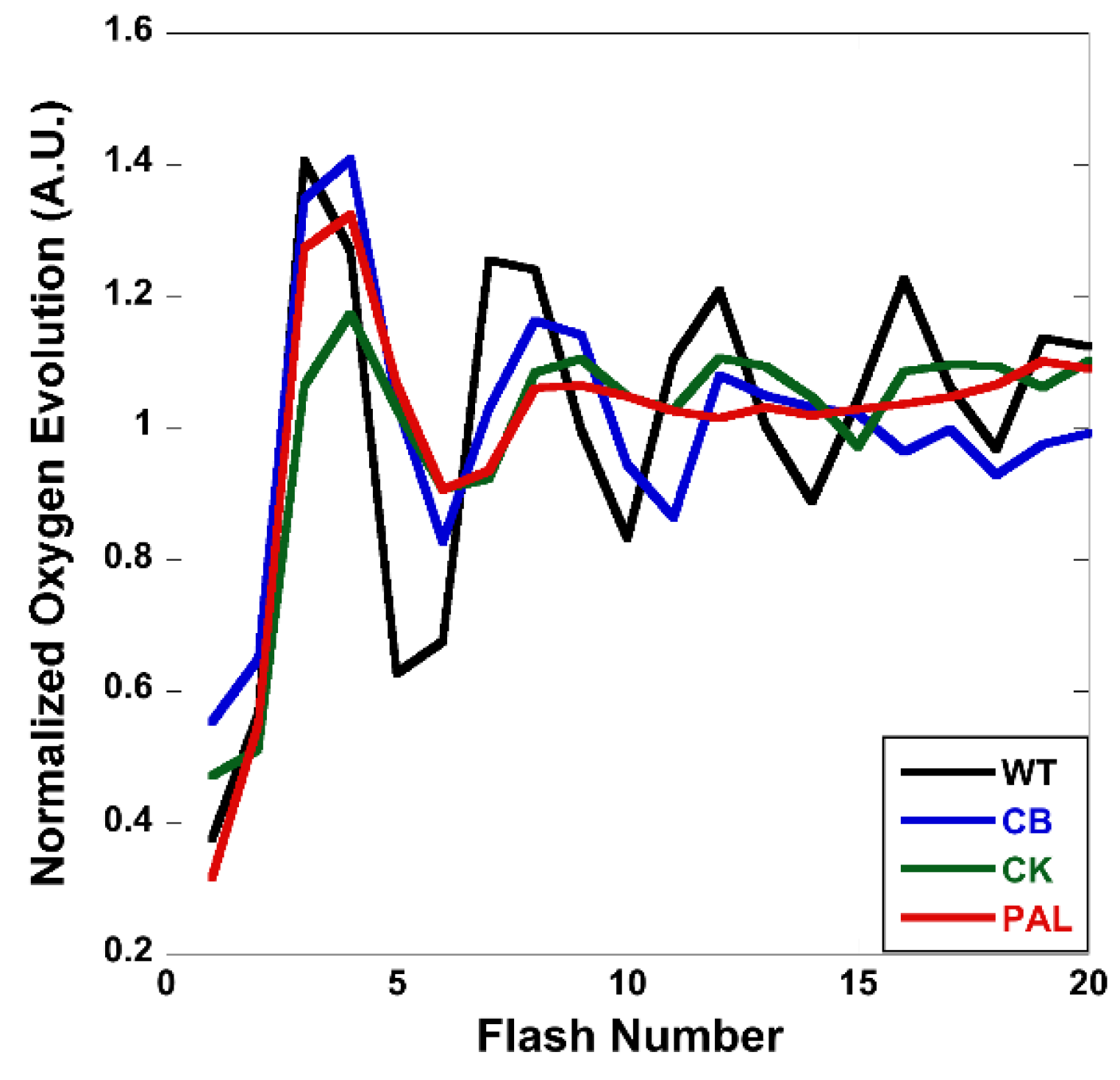
2.6. Flash-Induced Oxygen Evolution
3. Results
3.1. Low-Temperature Fluorescence
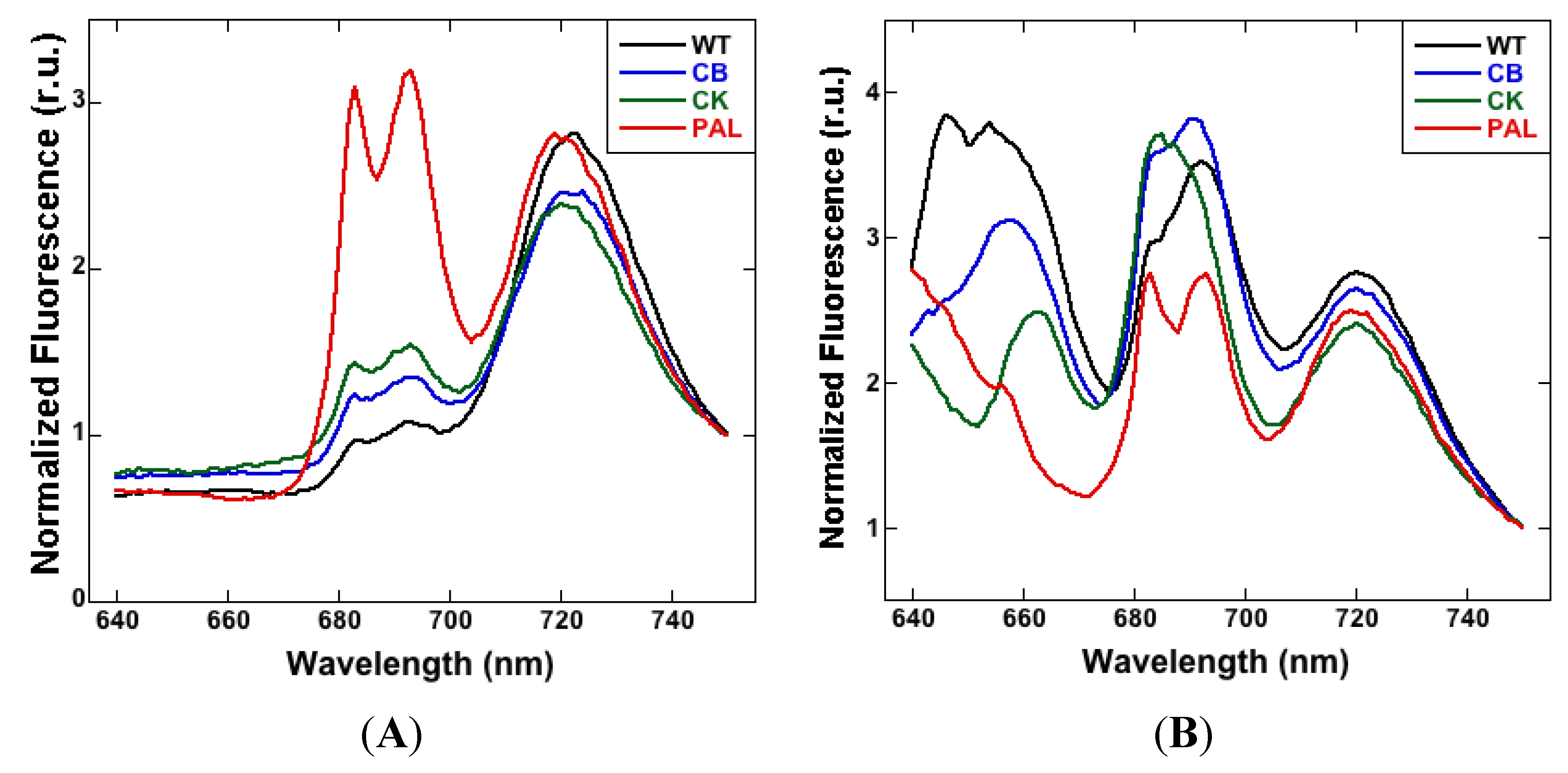
3.2. Photosystem Stoichiometry and PSI Quantitation

3.3. Measurement of PSII Activity
3.4. Flash-Induced Oxygen Evolution
| WT | CB | CK | PAL | |
|---|---|---|---|---|
| Km | 302.3 | 1345.2 | 1426.2 | 2864.5 |
| Vmax | 312.5 | 416.7 | 1111.1 | 1666.7 |
| S0 | 34.9 | 33.7 | 36.3 | 31.7 |
| S1 | 42.5 | 39.1 | 40.5 | 49.3 |
| S2 | 12.8 | 14.6 | 10.7 | 11.6 |
| S3 | 9.8 | 12.6 | 12.4 | 7.4 |
| Misses | 10.6 | 14.3 | 24.4 | 24.9 |
| Single Hits | 86.7 | 82.3 | 70.0 | 68.7 |
| Double Hits | 4.9 | −0.9 | 3.0 | 2.6 |
| Deactivation | −1.5 | 3.8 | 3.2 | 3.7 |
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Glazer, A.N. Light guides—Directional energy-transfer in a photosynthetic antenna. J. Biol. Chem. 1989, 264, 1–4. [Google Scholar]
- Kaňa, R.; Kotabová, E.; Komárek, O.; Šedivá, B.; Papageorgiou, G.C.; Prášil, O. The slow S to M fluorescence rise in cyanobacteria is due to a State 2 to State 1 transition. Biochim. Biophys. Acta 2012, 1817, 1237–1247. [Google Scholar]
- Liu, H.; Zhang, H.; Niedzwiedzki, D.M.; Prado, M.; He, G.; Gross, M.L.; Blankenship, R.E. Phycobilisomes supply excitations to both photosystems in a megacomplex in cyanobacteria. Science 2013, 342, 1104–1107. [Google Scholar]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar]
- Ort, D.R.; Melis, A. Optimizing antenna size to maximize photosynthetic efficiency. Plant Physiol. 2011, 155, 79–85. [Google Scholar]
- Elmorjani, K.; Thomas, J.C.; Sebban, P. Phycobilisomes of wild-type and pigment mutants of the cyanobacterium Synechocystis PCC 6803. Arch. Microbiol. 1986, 146, 186–191. [Google Scholar]
- MacColl, R. Cyanobacterial phycobilisomes. J. Struct. Biol. 1998, 124, 311–334. [Google Scholar]
- Ajlani, G.; Vernotte, C. Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 1998, 37, 577–580. [Google Scholar]
- Ughy, B.; Ajlani, G. Phycobilisome rod mutants in Synechocystis sp. strain PCC 6803. Microbiology 2004, 150, 4147–4156. [Google Scholar]
- Collins, A.M.; Liberton, M.; Jones, H.D.; Garcia, O.F.; Pakrasi, H.B.; Timlin, J.A. Photosynthetic pigment localization and thylakoid membrane morphology are altered in Synechocystis 6803 phycobilisome mutants. Plant Physiol. 2012, 158, 1600–1609. [Google Scholar]
- Liberton, M.; Page, L.E.; O’Dell, W.B.; O’Neill, H.; Mamontov, E.; Urban, V.S.; Pakrasi, H.B. Organization and flexibility of cyanobacterial thylakoid membranes examined by neutron scattering. J. Biol. Chem. 2013, 288, 3632–3640. [Google Scholar]
- Page, L.E.; Liberton, M.; Pakrasi, H.B. Reduction of photoautotrophic productivity in the cyanobacterium Synechocystis sp. strain PCC 6803 by phycobilisome antenna truncation. Appl. Environ. Microbiol 2012, 78, 6349–6351. [Google Scholar]
- Allen, M.M. Simple conditions for growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll-a and chlorophyll-b extracted with 4 different solvents—Verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochim. Biophys. Acta 1989, 975, 384–394. [Google Scholar]
- Kashino, Y.; Lauber, W.M.; Carroll, J.A.; Wang, Q.; Whitmarsh, J.; Satoh, K.; Pakrasi, H.B. Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 2002, 41, 8004–8012. [Google Scholar]
- Sonoike, K.; Katoh, S. Simple estimation of the differential absorption coefficient of P700 in detergent-treated preparations. Biochim. Biophys. Acta 1989, 976, 210–213. [Google Scholar]
- Mannan, R.M.; Pakrasi, H.B. Dark heterotrophic growth-conditions result in an increase in the content of photosystem II units in the filamentous cyanobacterium Anabaena variabilis ATCC 29413. Plant Physiol. 1993, 103, 971–977. [Google Scholar]
- Norling, B.; Zak, E.; Andersson, B.; Pakrasi, H. 2D-isolation of pure plasma and thylakoid membranes from the cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 1998, 436, 189–192. [Google Scholar]
- Bricker, T.M.; Lowrance, J.; Sutton, H.; Frankel, L.K. Alterations of the oxygen-evolving apparatus in a (448)Arg > (448)S mutant in the CP47 protein of photosystem II under normal and low chloride conditions. Biochemistry 2001, 40, 11483–11489. [Google Scholar]
- Meunier, P.C. Oxygen evolution by photosystem II—The contribution of backward transitions to the anomalous behavior of double-hits revealed by a new analysis method. Photosynth. Res. 1993, 36, 111–118. [Google Scholar]
- Stadnichuk, I.; Lukashev, E.; Elanskaya, I. Fluorescence changes accompanying short-term light adaptations in photosystem I and photosystem II of the cyanobacterium Synechocystis sp. PCC 6803 and phycobiliprotein-impaired mutants: State 1/State 2 transitions and carotenoid-induced quenching of phycobilisomes. Photosynth. Res. 2009, 99, 227–241. [Google Scholar]
- Zhang, P.; Frankel, L.K.; Bricker, T.M. Integration of apo-α-phycocyanin into phycobilisomes and its association with FNRL in the absence of the phycocyanin α-subunit lyase (CpcF) in Synechocystis sp. PCC 6803. PLoS One 2014, 9. [Google Scholar] [CrossRef]
- Joliot, P.; Barbieri, G.; Chabaud, R. Model of the system II photochemical centers. Photochem. Photobiol. 1969, 10, 309–329. [Google Scholar]
- Kok, B.; Forbush, B.; McGloin, M. Cooperation of charges in photosynthetic O2 evolution-I. A linear four step mechanism. Photochem. Photobiol. 1970, 11, 457–475. [Google Scholar]
- Liberton, M.; Collins, A.M.; Page, L.E.; O’Dell, W.B.; O’Neill, H.; Urban, V.S.; Timlin, J.A.; Pakrasi, H.B. Probing the consequences of antenna modification in cyanobacteria. Photosynth. Res. 2013, 118, 17–24. [Google Scholar]
- Lea-Smith, D.J.; Bombelli, P.; Dennis, J.S.; Scott, S.A.; Smith, A.G.; Howe, C.J. Phycobilisome-deficient strains of Synechocystis sp. PCC 6803 have reduced size and require carbon-limiting conditions to exhibit enhanced productivity. Plant Physiol. 2014, 165, 705–714. [Google Scholar]
- Kirst, H.; Formighieri, C.; Melis, A. Maximizing photosynthetic efficiency and culture productivity in cyanobacteria upon minimizing the phycobilisome light-harvesting antenna size. Biochim. Biophys. Acta 2014, 1837, 1653–1664. [Google Scholar]
- Kwon, J.-H.; Bernát, G.; Wagner, H.; Rögner, M.; Rexroth, S. Reduced light-harvesting antenna: Consequences on cyanobacterial metabolism and photosynthetic productivity. Algal Res. 2013, 2, 188–195. [Google Scholar]
- Thomas, J.C.; Ughy, B.; Lagoutte, B.; Ajlani, G. A second isoform of the ferredoxin:NADP oxidoreductase generated by an in-frame initiation of translation. Proc. Natl. Acad. Sci. USA 2006, 103, 18368–18373. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagarajan, A.; Page, L.E.; Liberton, M.; Pakrasi, H.B. Consequences of Decreased Light Harvesting Capability on Photosystem II Function in Synechocystis sp. PCC 6803. Life 2014, 4, 903-914. https://doi.org/10.3390/life4040903
Nagarajan A, Page LE, Liberton M, Pakrasi HB. Consequences of Decreased Light Harvesting Capability on Photosystem II Function in Synechocystis sp. PCC 6803. Life. 2014; 4(4):903-914. https://doi.org/10.3390/life4040903
Chicago/Turabian StyleNagarajan, Aparna, Lawrence E. Page, Michelle Liberton, and Himadri B. Pakrasi. 2014. "Consequences of Decreased Light Harvesting Capability on Photosystem II Function in Synechocystis sp. PCC 6803" Life 4, no. 4: 903-914. https://doi.org/10.3390/life4040903




