Survival Strategies in the Aquatic and Terrestrial World: The Impact of Second Messengers on Cyanobacterial Processes
Abstract
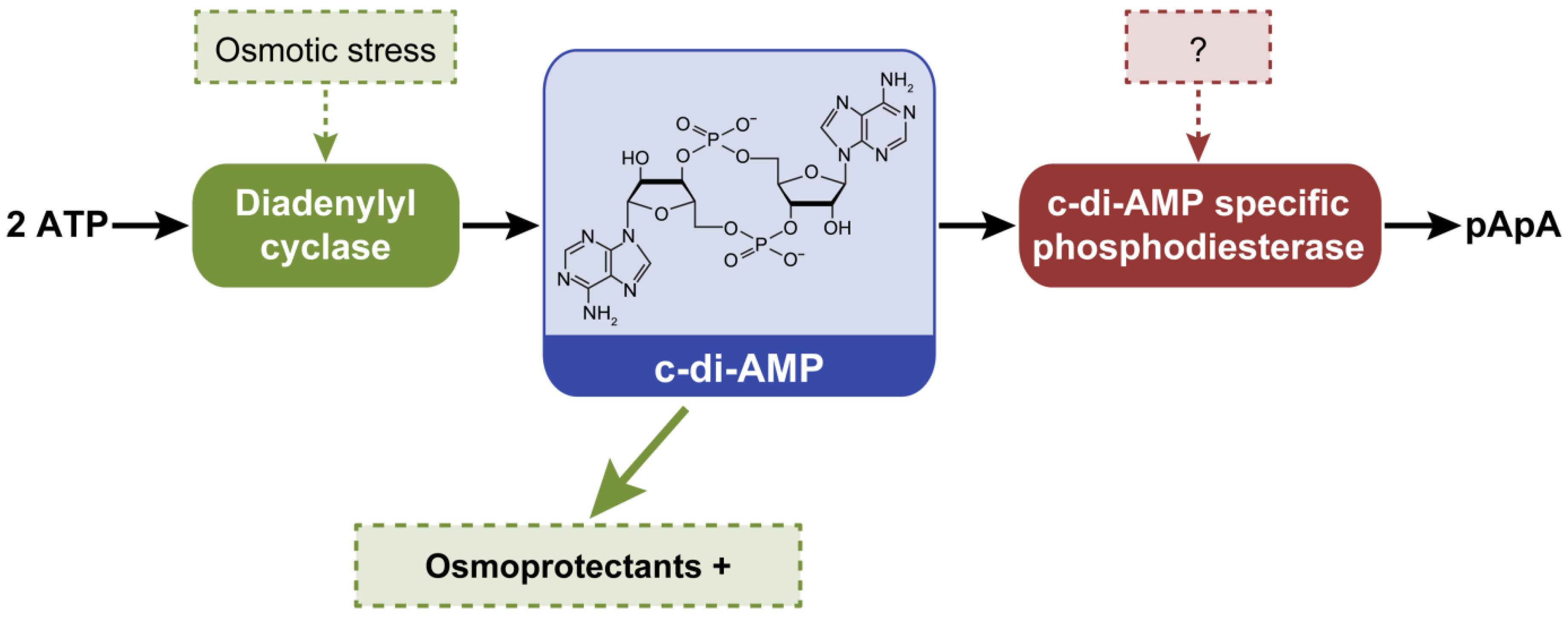
:1. Introduction
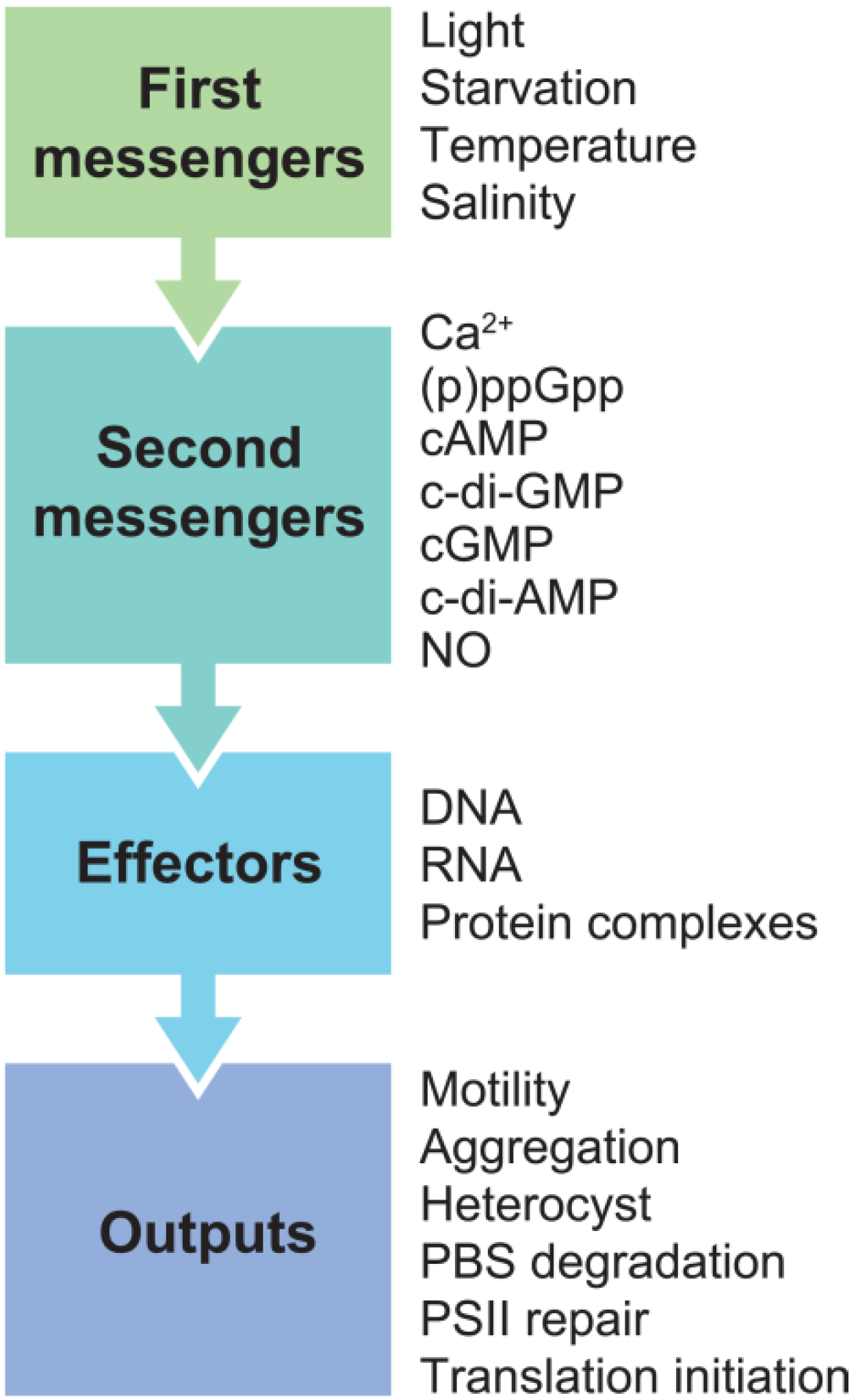
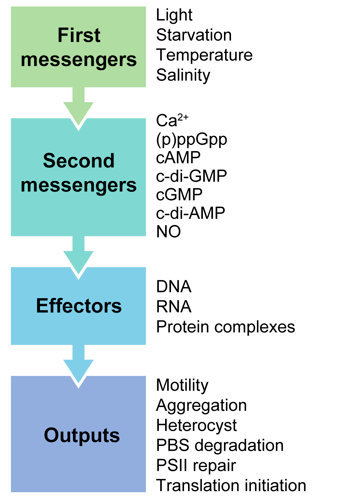
2. Second Messengers in Cyanobacteria
2.1. Calcium, Ca2+
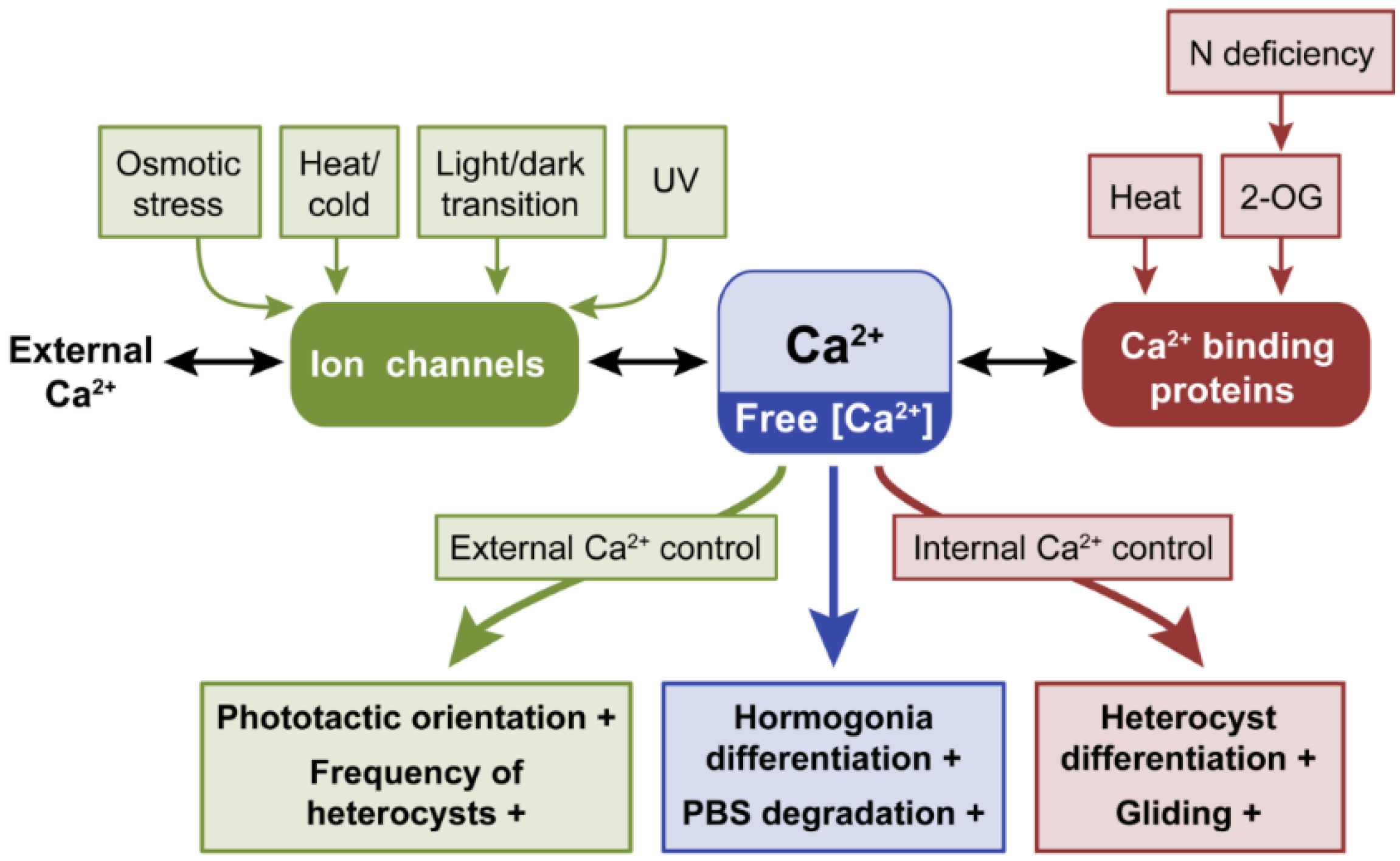
2.1.1. Calcium Controls Motility
2.1.2. The Role of Ca2+ in Heterocyst Differentiation
2.1.3. Responses to Temperature Stress and Other Stresses Are Mediated by Ca2+
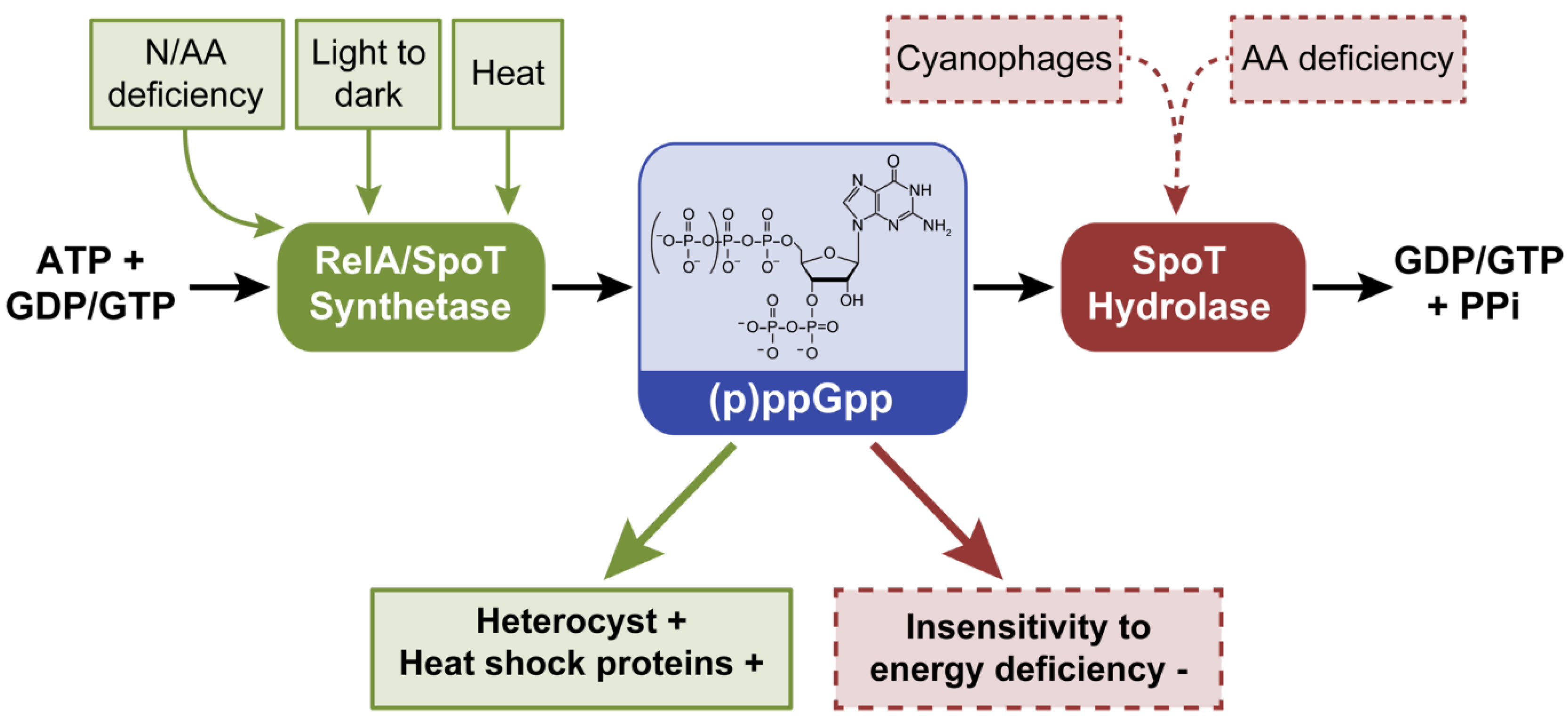
2.2. Guanosine-3’, 5’-(bis) Pyrophosphate, (p)ppGpp
| Second messenger | Pfam | Function | Number of genomes |
|---|---|---|---|
| (p)ppGpp | 04607 | (p)ppGpp synthesis and degradation | 83 |
| cAMP or cGMP | 00211 | Adenylate and guanylate cyclase | 65 |
| c-di-GMP | 00990 | Diguanylate cyclase | 61 |
| 00563 | Diguanylate phosphodiesterase | 60 | |
| Nitric oxide | 00394 | Nitrite reductase | 27 |
| 07731 | Nitrite reductase | 49 | |
| 07732 | Nitrite reductase | 47 | |
| 13442 | Nitrite reductase | 80 | |
| 00115 | Nitric oxide reductase | 83 | |
| c-di-AMP | 02457 | Diadenylyl cyclase | 83 |
2.2.1. The Role of (p)ppGpp in Cyanobacterial Cells
2.2.2. Cyanophage and (p)ppGpp
2.3. Cyclic Adenosine 3’,5’-Monophosphate, cAMP
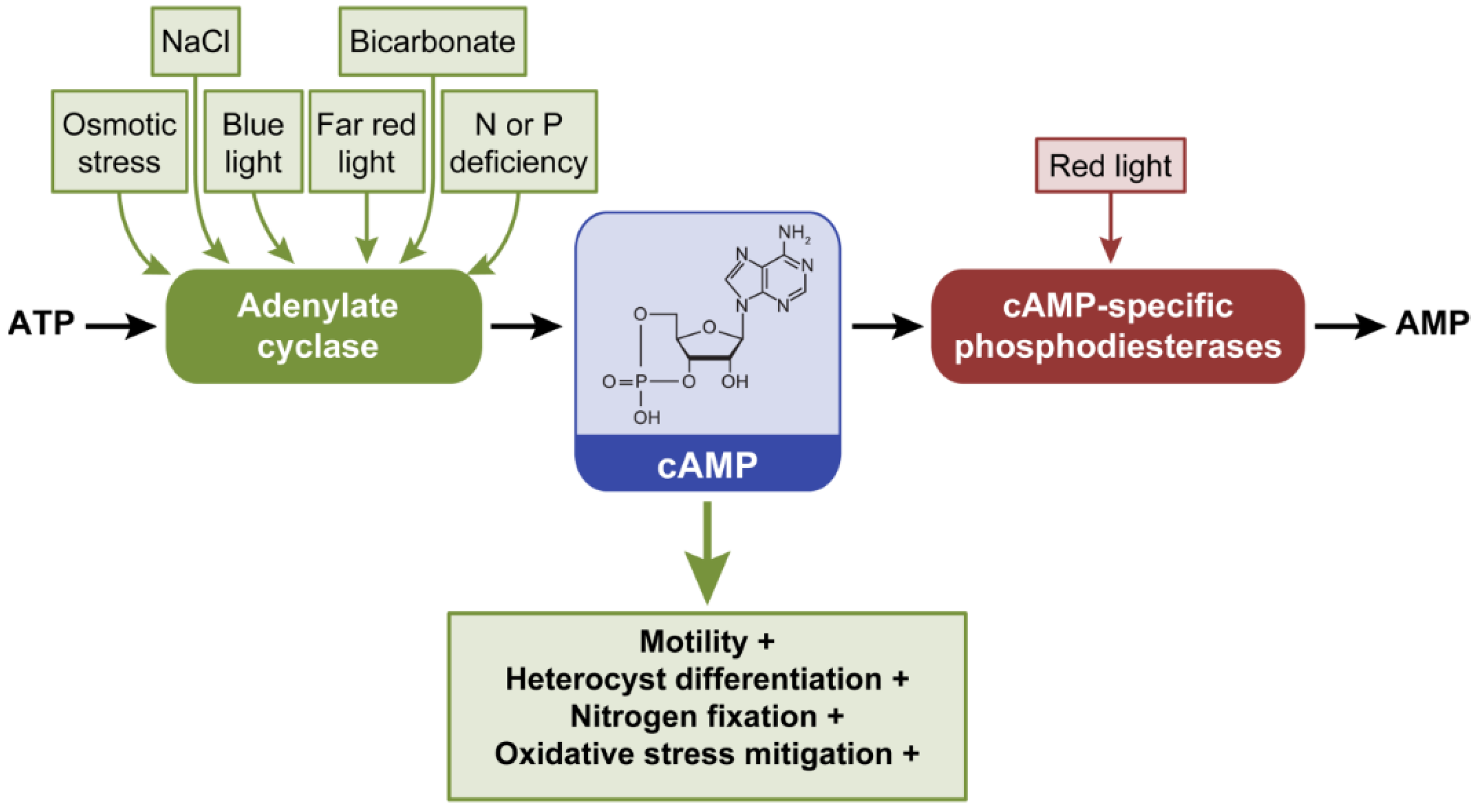
2.3.1. Cyclic AMP Regulates Motility Under Blue, Red, and Far Red Light
2.3.2. cAMP-dependent Transcriptional Regulation of Motility
2.3.3. Cyclic AMP as Nutrient Deficiency Signal
2.3.4. The Role of cAMP under Other Stresses
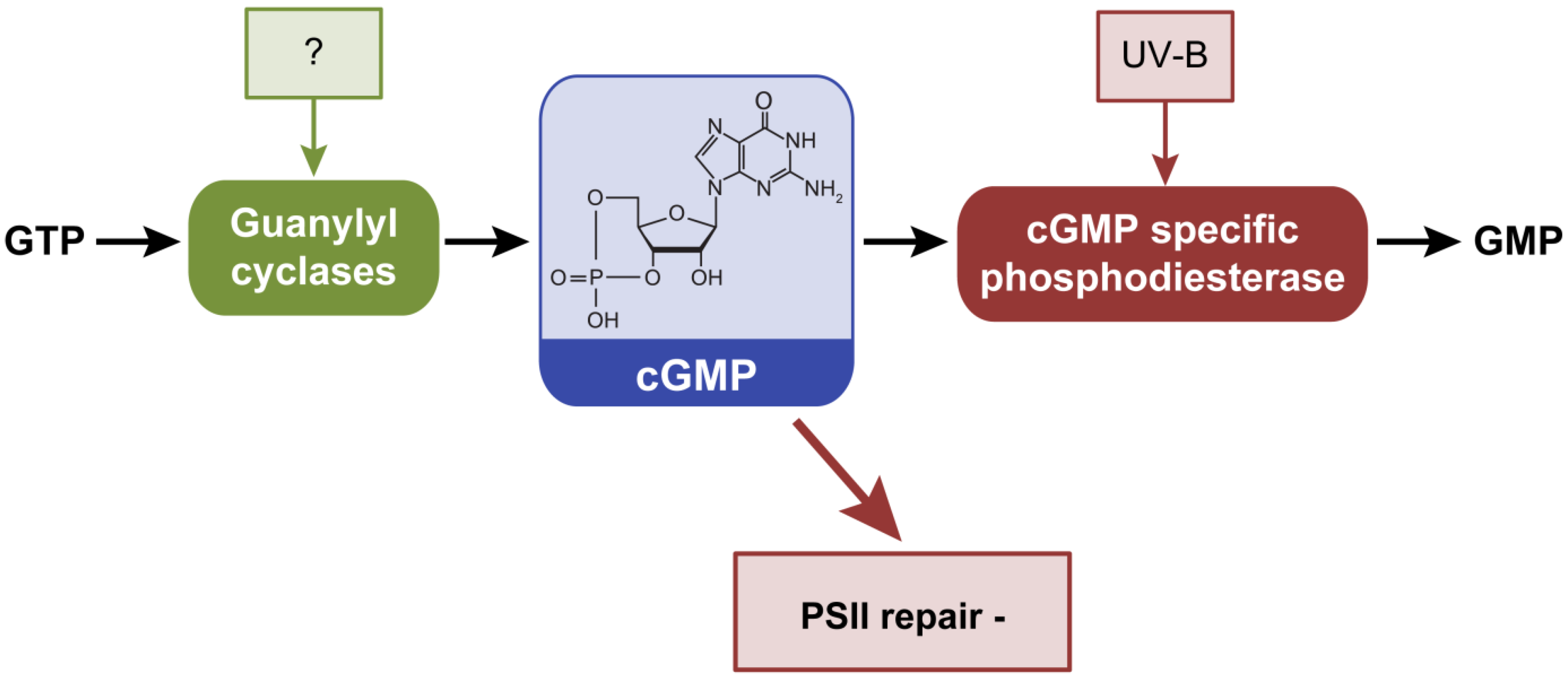
2.4. Cyclic Guanosine 3’,5’-Monophosphate, cGMP
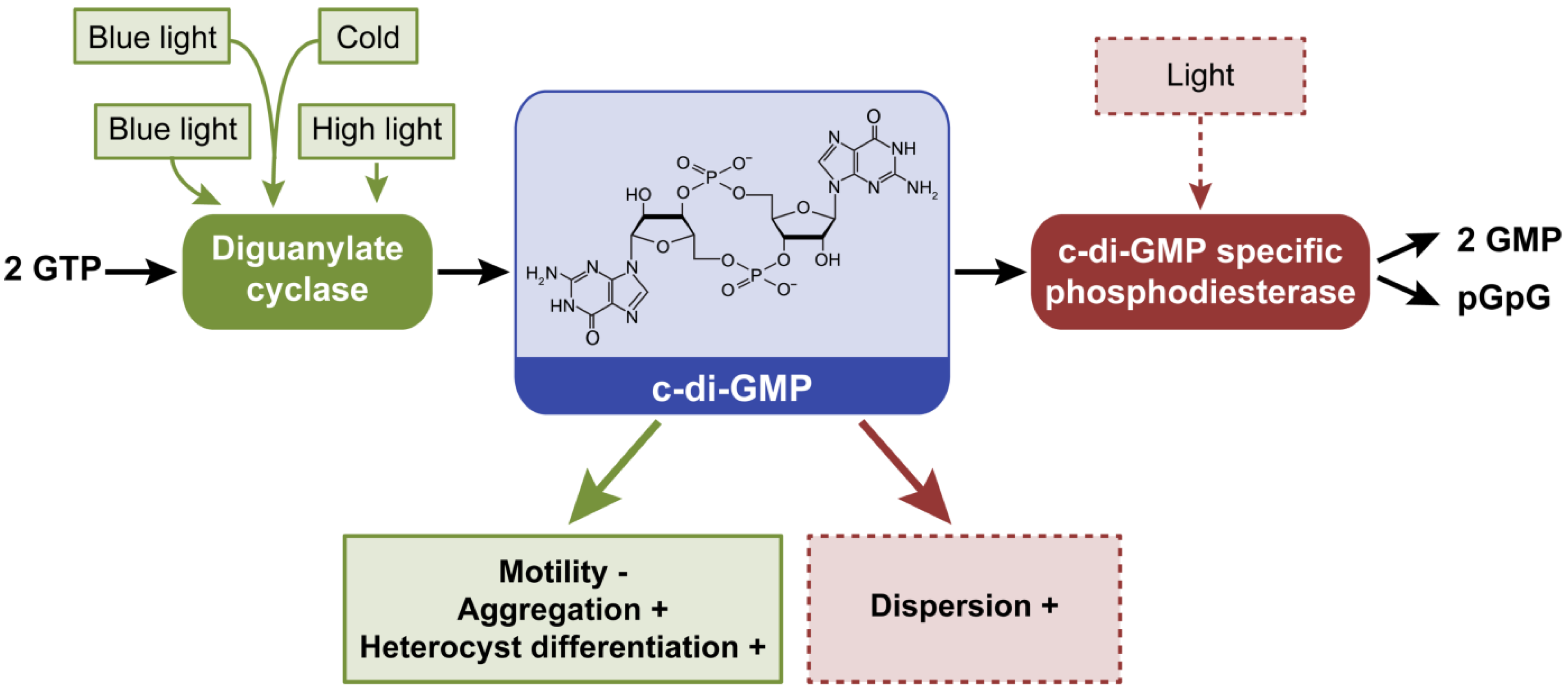
2.5. Cyclic Dimeric Guanosine 3’,5’-Monophosphate, c-di-GMP
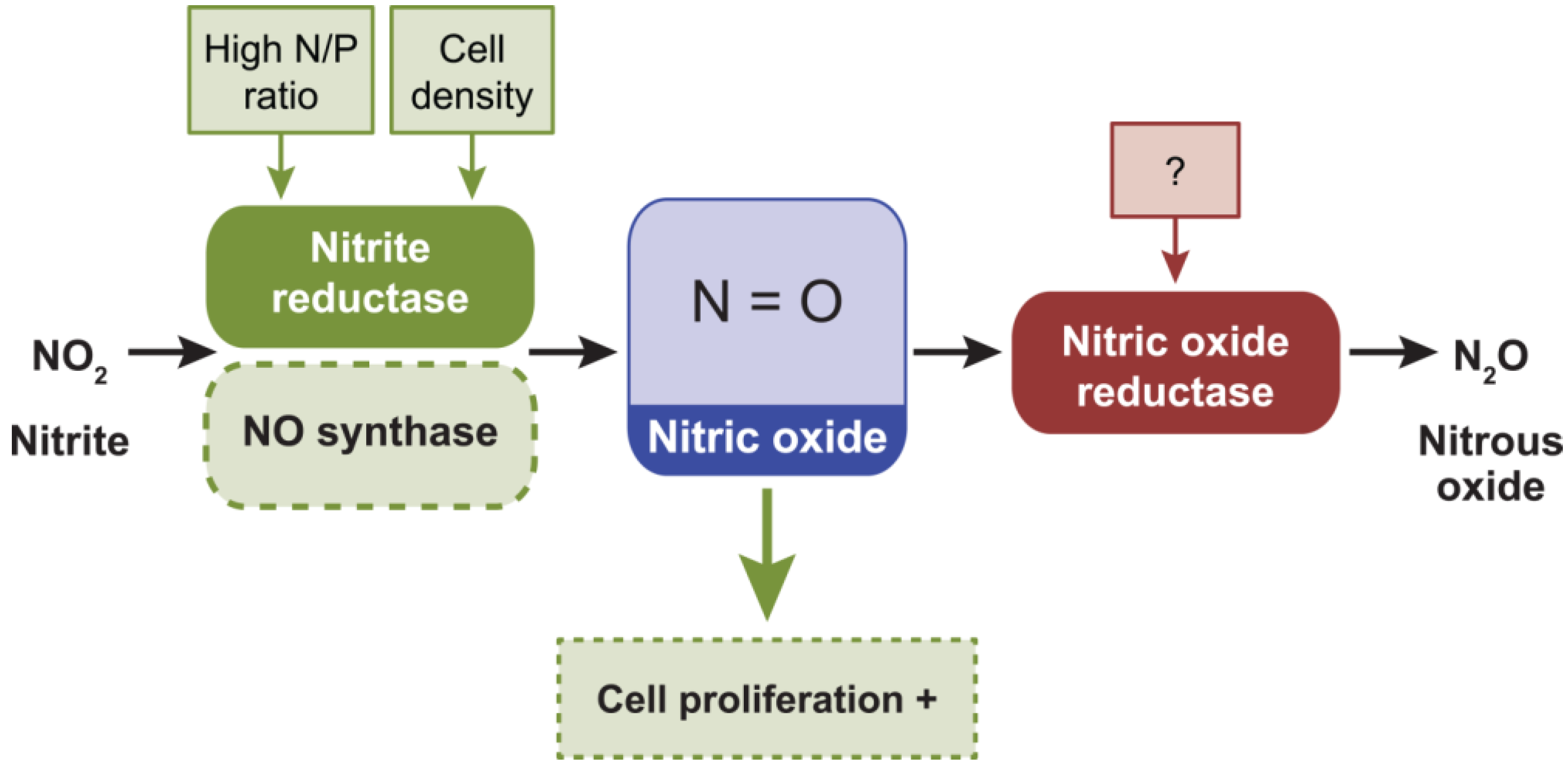
2.6. Nitric Oxide, NO
2.7. New Second Messenger-Dependent Phenotypes in Cyanobacteria
2.8. Cross Talk in Second Messenger Signaling
3. Challenges for the Future
3.1. The Complexity of Second Messenger Regulatory Networks
3.2. Second Messengers and Practical Application in Biotechnology or Therapeutics
4. Concluding Remarks
Acknowledgments
Author Contributions
Supplementary Materials
Conflicts of Interest
References and Notes
- Dominguez, D.C. Calcium signalling in bacteria. Mol. Microbiol. 2004, 54, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Kanjee, U.; Ogata, K.; Houry, W.A. Direct binding targets of the stringent response alarmone (p)ppGpp. Mol. Microbiol. 2012, 85, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Okano, H.; Hui, S.; Zhang, Z.; Kim, M.; Gunderson, C.W.; Wang, Y.P.; Lenz, P.; Yan, D.; Hwa, T. Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 2013, 500, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Soutourina, O.; Kolb, A.; Krin, E.; Laurent-Winter, C.; Rimsky, S.; Danchin, A.; Bertin, P. Multiple control of flagellum biosynthesis in Escherichia coli: Role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J. Bacteriol. 1999, 181, 7500–7508. [Google Scholar] [PubMed]
- Wolfgang, M.C.; Lee, V.T.; Gilmore, M.E.; Lory, S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev. Cell 2003, 4, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Römling, U.; Galperin, M.Y.; Gomelsky, M. Cyclic di-GMP: The first 25 years of a universal bacterial second messenger. Microbiol. Mol. Biol. Rev. 2013, 77, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Marletta, M.A.; Spiering, M.M. Trace elements and nitric oxide function. J. Nutr. 2003, 133, 1431S–1433S. [Google Scholar] [PubMed]
- Corrigan, R.M.; Gründling, A. Cyclic di-AMP: Another second messenger enters the fray. Nat. Rev. Microbiol. 2013, 11, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Berleman, J.E.; Hasselbring, B.M.; Bauer, C.E. Hypercyst mutants in Rhodospirillum centenum identify regulatory loci involved in cyst cell differentiation. J. Bacteriol. 2004, 186, 5834–5841. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichel, F.; Belnap, J.; Neuer, S.; Schanz, F. Estimates of global cyanobacterial biomass and its distribution. Algol. Stud. 2003, 109, 213–227. [Google Scholar] [CrossRef]
- Beck, C.; Knoop, H.; Axmann, I.M.; Steuer, R. The diversity of cyanobacterial metabolism: Genome analysis of multiple phototrophic microorganisms. BMC Genomics 2012, 13. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Baracaldo, P.; Hayes, P.K.; Blank, C.E. Morphological and habitat evolution in the cyanobacteria using a compartmentalization approach. Geobiology 2005, 3, 145–165. [Google Scholar]
- Bryant, D.A.; Frigaard, N.U. Prokaryotic photosynthesis and phototrophy illuminated. Trends Microbiol. 2006, 14, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Ashby, M.K.; Houmard, J. Cyanobacterial two-component proteins: Structure, diversity, distribution, and evolution. Microbiol. Mol. Biol. Rev. 2006, 70, 472–509. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bai, J.; Bao, Q.; Zhao, F. Lineage-specific domain fusion in the evolution of purine nucleotide cyclases in cyanobacteria. J. Mol. Evol. 2008, 67, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, M.; Koestler, B.J.; Waters, C.M.; Williams, B.L.; Montgomery, B.L. Occurrence of cyclic di-GMP-modulating output domains in cyanobacteria: An illuminating perspective. mBio 2013, 4. [Google Scholar] [CrossRef]
- Xu, M.; Su, Z. Computational prediction of cAMP receptor protein (CRP) binding sites in cyanobacterial genomes. BMC Genomics 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, P.; Lindberg, P.; Oliveira, P.; Stensjö, K.; Heidorn, T. Design, engineering, and construction of photosynthetic microbial cell factories for renewable solar fuel production. Ambio 2012, 41, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Oliver, J.W.K.; Atsumi, S. Metabolic design for cyanobacterial chemical synthesis. Photosyn. Res. 2014, 120, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, I.; Leganés, F.; Bonilla, I.; Fernández-Piñas, F. Use of recombinant aequorin to study calcium homeostasis and monitor calcium transients in response to heat and cold shock in cyanobacteria. Plant Physiol. 2000, 123, 161–176. [Google Scholar] [CrossRef] [PubMed]
- Herbaud, M.L.; Guiseppi, A.; Denizot, F.; Haiech, J.; Kilhoffer, M.C. Calcium signalling in Bacillus subtilis. Biochim. Biophys. Acta 1998, 1448, 212–226. [Google Scholar] [CrossRef]
- Torrecilla, I.; Leganés, F.; Bonilla, I.; Fernández-Piñas, F. Calcium transients in response to salinity and osmotic stress in the nitrogen-fixing cyanobacterium Anabaena sp. PCC7120, expressing cytosolic apoaequorin. Plant Cell Environ. 2001, 24, 641–648. [Google Scholar] [CrossRef]
- Ordal, G.W. Calcium ion regulates chemotactic behaviour in bacteria. Nature 1977, 270, 66–67. [Google Scholar] [CrossRef] [PubMed]
- Tisa, L.S.; Adler, J. Calcium ions are involved in Escherichia coli chemotaxis. Proc. Natl. Acad. Sci. USA 1992, 89, 11804–11808. [Google Scholar] [CrossRef] [PubMed]
- Baryshev, V.A.; Glagolev, A.N.; Skulachev, V.P. Interrelationship between Ca2+ and a methionine-requiring step in Halobacterium Halobium taxis. FEMS Microbiol. Lett. 1982, 13, 47–50. [Google Scholar] [CrossRef]
- Murvanidze, G.V.; Glagolev, A.N. Electrical nature of the taxis signal in cyanobacteria. J. Bacteriol. 1982, 150, 239–244. [Google Scholar] [PubMed]
- Hernández-Muñiz, W.; Stevens, S.E., Jr. Characterization of the motile hormogonia of Mastigocladus laminosus. J. Bacteriol. 1987, 169, 218–223. [Google Scholar] [PubMed]
- Moon, Y.J.; Park, Y.M.; Chung, Y.H.; Choi, J.S. Calcium is involved in photomovement of cyanobacterium Synechocystis sp PCC 6803. Photochem. Photobiol. 2004, 79, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Hoiczyk, E.; Baumeister, W. Oscillin, an extracellular, Ca2+-binding glycoprotein essential for the gliding motility of cyanobacteria. Mol. Microbiol. 1997, 26, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Brahamsha, B. An abundant cell-surface polypeptide is required for swimming by the nonflagellated marine cyanobacterium Synechococcus. Proc. Natl. Acad. Sci. USA 1996, 93, 6504–6509. [Google Scholar] [CrossRef] [PubMed]
- Pitta, T.P.; Sherwood, E.E.; Kobel, A.M.; Berg, H.C. Calcium is required for swimming by the nonflagellated cyanobacterium Synechococcus strain WH8113. J. Bacteriol. 1997, 179, 2524–2528. [Google Scholar] [PubMed]
- Huang, T.-C.; Chow, T.-J. Comparative studies of some nitrogen-fixing unicellular cyanobacteria isolated from rice fields. J. Gen. Microbiol. 1988, 134, 3089–3097. [Google Scholar]
- Chen, T.-H.; Huang, T.-C.; Chow, T.-J. Calcium requirement in nitrogen fixation in the cyanobacterium Synechococcus RF-1. Planta 1988, 173, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Gallon, J.R.; Hamadi, A.F. Studies on the effects of oxygen on acetylene reduction (nitrogen fixation) in Gloeothece sp. ATCC 27152. J. Gen. Microbiol. 1984, 130, 495–503. [Google Scholar]
- Smith, R.; Hobson, S.; Ellis, I. Evidence for calcium-mediated regulation of heterocyst frequency and nitrogenase activity in Nostoc 6720. New Phytol. 1987, 105, 531–541. [Google Scholar] [CrossRef]
- Knight, M.R.; Campbell, A.K.; Smith, S.M.; Trewavas, A.J. Recombinant aequorin as a probe for cytosolic free Ca2+ in Escherichia coli. FEBS Lett. 1991, 282, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, I.; Leganés, F.; Bonilla, I.; Fernández-Piñas, F. A calcium signal is involved in heterocyst differentiation in the cyanobacterium Anabaena sp. PCC7120. Microbiology 2004, 150, 3731–3739. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wei, X.; Jiang, N.; Li, H.; Dong, Y.; Hsi, K.; Zhao, J. Evidence that HetR protein is an unusual serine-type protease. Proc. Natl. Acad. Sci. USA 1998, 95, 4959–4963. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, Y.; Zhao, W.; Huang, X.; Wang, D.; Brown, N.; Brand, J.; Zhao, J. CcbP, a calcium-binding protein from Anabaena sp. PCC 7120, provides evidence that calcium ions regulate heterocyst differentiation. Proc. Natl. Acad. Sci. USA 2005, 102, 5744–5748. [Google Scholar] [CrossRef] [PubMed]
- Leganés, F.; Forchhammer, K.; Fernández-Piñas, F. Role of calcium in acclimation of the cyanobacterium Synechococcus elongatus PCC 7942 to nitrogen starvation. Microbiology 2009, 155, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Herrero, A.; Muro-Pastor, A.M.; Valladares, A.; Flores, E. Cellular differentiation and the NtcA transcription factor in filamentous cyanobacteria. FEMS Microbiol. Rev. 2004, 28, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, R.; Forchhammer, K. Acclimation of unicellular cyanobacteria to macronutrient deficiency: Emergence of a complex network of cellular responses. Microbiology 2005, 151, 2503–2514. [Google Scholar] [CrossRef] [PubMed]
- Torrecilla, I.; Leganés, F.; Bonilla, I.; Fernández-Piña, F. Light-to-dark transitions trigger a transient increase in intracellular Ca2+ modulated by the redox state of the photosynthetic electron transport chain in the cyanobacterium Anabaena sp. PCC7120. Plant Cell Environ. 2004, 27, 810–819. [Google Scholar] [CrossRef]
- Nazarenko, L.V.; Andreev, I.M.; Lyukevich, A.A.; Pisareva, T.V.; Los, D.A. Calcium release from Synechocystis cells induced by depolarization of the plasma membrane: MscL as an outward Ca2+ channel. Microbiology 2003, 149, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Richter, P.; Krywult, M.; Sinha, R.P.; Häder, D.-P. Calcium signals from heterocysts of Anabaena sp. after UV irradiation. J. Plant. Physiol. 1999, 154, 137–139. [Google Scholar] [CrossRef]
- Srivatsan, A.; Wang, J.D. Control of bacterial transcription, translation and replication by (p)ppGpp. Curr. Opin. Microbiol. 2008, 11, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Potrykus, K.; Cashel, M. (p)ppGpp: Still magical? Annu. Rev. Microbiol. 2008, 62, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Toulokhonov, I.I.; Shulgina, I.; Hernandez, V.J. Binding of the transcription effector ppGpp to Escherichia coli RNA polymerase is allosteric, modular, and occurs near the N terminus of the β’-subunit. J. Biol. Chem. 2001, 276, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Takahashi, K.; Ochiai, Y.; Hosaka, T.; Ochi, K.; Nabeta, K. Bacterial alarmone, guanosine 5’-diphosphate 3’-diphosphate (ppGpp), predominantly binds the β’ subunit of plastid-encoded plastid RNA polymerase in chloroplasts. Chembiochem 2009, 10, 1227–1233. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, D.; Archibald, J.M.; Weber, A.P.; Reyes-Prieto, A. How do endosymbionts become organelles? Understanding early events in plastid evolution. Bioessays 2007, 29, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, V.M.; Chen, I.M.; Palaniappan, K.; Chu, K.; Szeto, E.; Grechkin, Y.; Ratner, A.; Jacob, B.; Huang, J.; Williams, P.; et al. IMG: The Integrated Microbial Genomes database and comparative analysis system. Nucleic Acids Res. 2012, 40, D115–D122. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Qian, Y.; Miao, X.; Wen, C. Role of the all1549 (ana-rsh) gene, a relA/spoT homolog, of the cyanobacterium Anabaena sp. PCC 7120. Curr. Microbiol. 2011, 62, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Akinyanju, J.; Smith, R.J. Accumulation of ppGpp and pppGpp during nitrogen deprivation of the cyanophyte Anabaena cylindrica. FEBS Lett. 1979, 107, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Friga, G.M.; Borbély, G.; Farkas, G.L. Accumulation of guanosine tetraphosphate (ppGpp) under nitrogen starvation in Anacystis nidulans, a cyanobacterium. Arch. Microbiol. 1981, 129, 341–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-R.; Lin, G.-M.; Chen, W.-L.; Wang, L.; Zhang, C.-C. ppGpp metabolism is involved in heterocyst development in the cyanobacterium Anabaena sp. strain PCC 7120. J. Bacteriol. 2013, 195, 4536–4544. [Google Scholar] [CrossRef] [PubMed]
- Mann, N.; Carr, N.G.; Midgley, J.E. RNA synthesis and the accumulation of guanine nucleotides during growth shift down in the blue-green alga Anacystis nidulans. Biochim. Biophys. Acta 1975, 402, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.J.; Carr, N.G. The regulation of stable RNA synthesis in the blue-green alga Anacystis nidulans: effect of leucine deprivation and 5-methyltryptophan inhibition. J. Gen. Microbiol. 1977, 103, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Surányi, G.; Korcz, A.; Pálfi, Z.; Borbély, G. Effects of light deprivation on RNA synthesis, accumulation of guanosine 3’(2’)-diphosphate 5’-diphosphate, and protein synthesis in heat-shocked Synechococcus sp. strain PCC 6301, a cyanobacterium. J. Bacteriol. 1987, 169, 632–639. [Google Scholar] [PubMed]
- Borbély, G.; Kaki, C.; Gulyás, A.; Farkas, G.L. Bacteriophage infection interferes with guanosine 3’-diphosphate-5’-diphosphate accumulation induced by energy and nitrogen starvation in the cyanobacterium Anacystis nidulans. J. Bacteriol. 1980, 144, 859–864. [Google Scholar] [PubMed]
- Bryan, M.J.; Burroughs, N.J.; Spence, E.M.; Clokie, M.R.; Mann, N.H.; Bryan, S.J. Evidence for the intense exchange of MazG in marine cyanophages by horizontal gene transfer. PLoS One 2008, 3, e2048. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Marianovsky, I.; Glaser, G. MazG–a regulator of programmed cell death in Escherichia coli. Mol. Microbiol. 2006, 59, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Clokie, M.R.; Mann, N.H. Marine cyanophages and light. Environ. Microbiol. 2006, 8, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Hirose, M.; Ohmori, M. Function of cAMP as a mat-forming factor in the cyanobacterium Spirulina platensis. Plant Cell Physiol. 1992, 33, 21–25. [Google Scholar]
- Terauchi, K.; Ohmori, M. An adenylate cyclase, Cya1, regulates cell motility in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1999, 40, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Terauchi, K.; Ohmori, M. Blue light stimulates cyanobacterial motility via a cAMP signal transduction system. Mol. Microbiol. 2004, 52, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Raffelberg, S.; Wang, L.; Gao, S.; Losi, A.; Gärtner, W.; Nagel, G. A LOV-domain-mediated blue-light-activated adenylate (adenylyl) cyclase from the cyanobacterium Microcoleus chthonoplastes PCC 7420. Biochem. J. 2013, 455, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, M.; Terauchi, K.; Okamoto, S.; Watanabe, M. Regulation of cAMP-mediated photosignaling by a phytochrome in the cyanobacterium Anabaena cylindrica. Photochem. Photobiol. 2002, 75, 675–679. [Google Scholar] [PubMed]
- Okamoto, S.; Kasahara, M.; Kamiya, A.; Nakahira, Y.; Ohmori, M. A phytochrome-like protein AphC triggers the cAMP signaling induced by far-red light in the cyanobacterium Anabaena sp. strain PCC7120. Photochem. Photobiol. 2004, 80, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Yoshihara, S.; Okamoto, S.; Ikeuchi, M.; Ohmori, M. A cAMP receptor protein, SYCRP1, is responsible for the cell motility of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Yoshihara, S.; Okamoto, S.; Ikeuchi, M.; Ohmori, M. A cAMP receptor protein, SYCRP1, is responsible for the cell motility of Synechocystis sp. PCC 6803. Plant Cell Physiol. 2002, 43, 460–463. [Google Scholar] [PubMed]
- Omagari, K.; Yoshimura, H.; Takano, M.; Hao, D.; Ohmori, M.; Sarai, A.; Suyama, A. Systematic single base-pair substitution analysis of DNA binding by the cAMP receptor protein in cyanobacterium Synechocystis sp. PCC 6803. FEBS Lett. 2004, 563, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Dienst, D.; Dühring, U.; Mollenkopf, H.J.; Vogel, J.; Golecki, J.; Hess, W.R.; Wilde, A. The cyanobacterial homologue of the RNA chaperone Hfq is essential for motility of Synechocystis sp PCC 6803. Microbiology 2008, 154, 3134–3143. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, H.; Kaneko, Y.; Ehira, S.; Yoshihara, S.; Ikeuchi, M.; Ohmori, M. CccS and CccP are involved in construction of cell surface components in the cyanobacterium Synechocystis sp. strain PCC 6803. Plant Cell Physiol. 2010, 51, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Francko, D.A.; Wetzel, R.G. Dynamics of cellular and extracellular cAMP in Anabaena flos-aquae (Cyanophyta): Intrinsic culture variability and correlation with metabolic variables. J. Phycol. 1981, 17, 129–134. [Google Scholar] [CrossRef]
- Hood, E.E.; Armour, S.; Ownby, J.D.; Handa, A.K.; Bressan, R.A. Effect of nitrogen starvation on the level of adenosine 3’,5’-monophosphate in Anabaena variabilis. Biochim. Biophys. Acta 1979, 588, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshimura, H.; Ehira, S.; Ikeuchi, M.; Ohmori, M. AnCrpA, a cAMP receptor protein, regulates nif-related gene expression in the cyanobacterium Anabaena sp. strain PCC 7120 grown with nitrate. FEBS Lett. 2007, 581, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshimura, H.; Hisabori, T.; Ohmori, M. Two cAMP receptor proteins with different biochemical properties in the filamentous cyanobacterium Anabaena sp. PCC 7120. FEBS Lett. 2004, 571, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Imashimizu, M.; Yoshimura, H.; Katoh, H.; Ehira, S.; Ohmori, M. NaCl enhances cellular cAMP and upregulates genes related to heterocyst development in the cyanobacterium, Anabaena sp. strain PCC 7120. FEMS Microbiol. Lett. 2005, 252, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Higo, A.; Ikeuchi, M.; Ohmori, M. cAMP regulates respiration and oxidative stress during rehydration in Anabaena sp. PCC 7120. FEBS Lett. 2008, 582, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar] [CrossRef] [PubMed]
- Gomelsky, M. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol. Microbiol. 2011, 79, 562–565. [Google Scholar] [CrossRef] [PubMed]
- Kasahara, M.; Unno, T.; Yashiro, K.; Ohmori, M. CyaG, a novel cyanobacterial adenylyl cyclase and a possible ancestor of mammalian guanylyl cyclases. J. Biol.Chem. 2001, 276, 10564–10569. [Google Scholar] [CrossRef] [PubMed]
- Herdman, M.; Elmorjani, K. Cyclic nucleotides. Methods Enzymol. 1988, 167, 584–591. [Google Scholar]
- Ochoa de Alda, J.A.; Ajlani, G.; Houmard, J. Synechocystis strain PCC 6803 cya2, a prokaryotic gene that encodes a guanylyl cyclase. J. Bacteriol. 2000, 182, 3839–3842. [Google Scholar]
- Rauch, A.; Leipelt, M.; Russwurm, M.; Steegborn, C. Crystal structure of the guanylyl cyclase Cya2. Proc. Natl. Acad. Sci. USA 2008, 105, 15720–15725. [Google Scholar] [CrossRef]
- Cadoret, J.C.; Rousseau, B.; Perewoska, I.; Sicora, C.; Cheregi, O.; Vass, I.; Houmard, J. Cyclic nucleotides, the photosynthetic apparatus and response to a UV-B stress in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2005, 280, 33935–33944. [Google Scholar] [CrossRef] [PubMed]
- Cyanobase. Available online: http://genome.kazusa.or.jp/cyanobase (accessed on 5 November 2014).
- Krupke, A.; Lavik, G.; Halm, H.; Fuchs, B.M.; Amann, R.I.; Kuypers, M.M. Distribution of a consortium between unicellular algae and the N2 fixing cyanobacterium UCYN-A in the North Atlantic Ocean. Environ. Microbiol. 2014, 16, 3153–3167. [Google Scholar] [CrossRef] [PubMed]
- Savakis, P.; De Causmaecker, S.; Angerer, V.; Ruppert, U.; Anders, K.; Essen, L.O.; Wilde, A. Light-induced alteration of c-di-GMP level controls motility of Synechocystis sp. PCC 6803. Mol. Microbiol. 2012, 85, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, G.; Nomura, R.; Shimada, T.; Win, N.-N.; Narikawa, R.; Ikeuchi, M. Cyanobacteriochrome SesA is a diguanylate cyclase that induces cell aggregation in Thermosynechococcus. J. Biol. Chem. 2014, 289, 24801–24809. [Google Scholar] [CrossRef] [PubMed]
- Neunuebel, M.R.; Golden, J.W. The Anabaena sp. strain PCC 7120 gene all2874 encodes a diguanylate cyclase and is required for normal heterocyst development under high-light growth conditions. J. Bacteriol. 2008, 190, 6829–6836. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.H.; Lagarias, J.C. Defining the bilin lyase domain: lessons from the extended phytochrome superfamily. Biochemistry 2000, 39, 13487–13495. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, B.L.; Lagarias, J.C. Phytochrome ancestry: sensors of bilins and light. Trends Plant Sci. 2002, 7, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Anders, K.; von Stetten, D.; Mailliet, J.; Kiontke, S.; Sineshchekov, V.A.; Hildebrandt, P.; Hughes, J.; Essen, L.-O. Spectroscopic and photochemical characterization of the red-light sensitive photosensory module of Cph2 from Synechocystis PCC 6803. Photochem. Photobiol. 2011, 87, 160–173. [Google Scholar] [CrossRef] [PubMed]
- Feelisch, M.; Martin, J.F. The early role of nitric oxide in evolution. Trends Ecol. Evol. 1995, 10, 496–499. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, J.; Wang, W.H.; Liu, T.W.; Zhang, J.; Gao, Y.H.; Pei, Z.M.; Zheng, H.L. The changes of nitric oxide production during the growth of Microcystis aerugrinosa. Environ. Pollut. 2011, 159, 3784–3792. [Google Scholar] [CrossRef] [PubMed]
- Mallick, N.; Rai, L.C.; Mohn, F.H.; Soeder, C.J. Studies on nitric oxide (NO) formation by the green alga Scenedesmus obliquus and the diazotrophic cyanobacterium Anabaena doliolum. Chemosphere 1999, 39, 1601–1610. [Google Scholar] [CrossRef] [PubMed]
- Sureka, K.; Choi, P.H.; Precit, M.; Delince, M.; Pensinger, D.A.; Huynh, T.N.; Jurado, A.R.; Goo, Y.A.; Sadilek, M.; Iavarone, A.T.; et al. The cyclic dinucleotide c-di-AMP is an allosteric regulator of metabolic enzyme function. Cell 2014, 158, 1389–1401. [Google Scholar] [CrossRef] [PubMed]
- Nelson, J.W.; Sudarsan, N.; Furukawa, K.; Weinberg, Z.; Wang, J.X.; Breaker, R.R. Riboswitches in eubacteria sense the second messenger c-di-AMP. Nat. Chem. Biol. 2013, 9, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Davies, B.W.; Bogard, R.W.; Young, T.S.; Mekalanos, J.J. Coordinated regulation of accessory genetic elements produces cyclic di-nucleotides for V. cholerae virulence. Cell 2012, 149, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, G.M.; Pastini, A.C.; Muschietti, J.P.; Téllez-Iñón, M.T.; Martinetto, H.E.; Torres, H.N.; Flawiá, M.M. Adenylate cyclase activity in cyanobacteria: Activation by Ca2+-calmodulin and a calmodulin-like activity. Biochim. Biophys. Acta 1990, 1055, 75–81. [Google Scholar] [CrossRef]
- Weber, H.; Pesavento, C.; Possling, A.; Tischendorf, G.; Hengge, R. Cyclic-di-GMP-mediated signalling within the σS network of Escherichia coli. Mol. Microbiol. 2006, 62, 1014–1034. [Google Scholar] [CrossRef] [PubMed]
- Christen, M.; Kulasekara, H.D.; Christen, B.; Kulasekara, B.R.; Hoffman, L.R.; Miller, S.I. Asymmetrical distribution of the second messenger c-di-GMP upon bacterial cell division. Science 2010, 328, 1295–1297. [Google Scholar] [CrossRef] [PubMed]
- Massie, J.P.; Reynolds, E.L.; Koestler, B.J.; Cong, J.P.; Agostoni, M.; Waters, C.M. Quantification of high-specificity cyclic diguanylate signaling. Proc. Natl. Acad. Sci. USA 2012, 109, 12746–12751. [Google Scholar] [CrossRef] [PubMed]
- Pultz, I.S.; Christen, M.; Kulasekara, H.D.; Kennard, A.; Kulasekara, B.; Miller, S.I. The response threshold of Salmonella PilZ domain proteins is determined by their binding affinities for c-di-GMP. Mol. Microbiol. 2012, 86, 1424–1440. [Google Scholar] [CrossRef] [PubMed]
- McCormick, K.; Baillie, G.S. Compartmentalisation of second messenger signalling pathways. Curr. Opin. Genet. Dev. 2014, 27, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Odaka, H.; Arai, S.; Inoue, T.; Kitaguchi, T. Genetically-encoded yellow fluorescent cAMP indicator with an expanded dynamic range for dual-color imaging. PLoS One 2014, 9, e100252. [Google Scholar] [CrossRef] [PubMed]
- Ducat, D.C.; Way, J.C.; Silver, P.A. Engineering cyanobacteria to generate high-value products. Trends Biotechnol. 2011, 29, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Nozzi, N.E.; Oliver, J.W.; Atsumi, S. Cyanobacteria as a platform for biofuel production. Front. Bioeng. Biotechnol. 2013, 1. [Google Scholar] [CrossRef]
- De Philippis, R.; Colica, G.; Micheletti, E. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Appl. Microbiol. Biotechnol. 2011, 92, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.H.; Moskvin, O.V.; Siltberg-Liberles, J.; Gomelsky, M. Natural and engineered photoactivated nucleotidyl cyclases for optogenetic applications. J. Biol. Chem. 2010, 285, 41501–41508. [Google Scholar] [CrossRef] [PubMed]
- Gasser, C.; Taiber, S.; Yeh, C.M.; Wittig, C.H.; Hegemann, P.; Ryu, S.; Wunder, F.; Möglich, A. Engineering of a red-light-activated human cAMP/cGMP-specific phosphodiesterase. Proc. Natl. Acad. Sci. USA 2014, 111, 8803–8808. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.H.; Kang, I.H.; Nelson, M.D.; Jensen, T.M.; Lyuksyutova, A.I.; Siltberg-Liberles, J.; Raizen, D.M.; Gomelsky, M. Engineering adenylate cyclases regulated by near-infrared window light. Proc. Natl. Acad. Sci. USA 2014, 111, 10167–10172. [Google Scholar] [CrossRef] [PubMed]
- Stierl, M.; Penzkofer, A.; Kennis, J.T.; Hegemann, P.; Mathes, T. Key residues for the light regulation of the blue light-activated adenylyl cyclase from Beggiatoa sp. Biochemistry 2014, 53, 5121–5130. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Raffelberg, S.; Losi, A.; Schaap, P.; Gärtner, W. A cyanobacterial light activated adenylyl cyclase partially restores development of a Dictyostelium discoideum, adenylyl cyclase a null mutant. J. Biotechnol. 2014. [Google Scholar] [CrossRef]
- Ryu, M.-H.; Gomelsky, M. Near-infrared light responsive synthetic c-di-GMP module for optogenetic applications. ACS Synth. Biol. 2014. [Google Scholar] [CrossRef]
- Burdette, D.L.; Monroe, K.M.; Sotelo-Troha, K.; Iwig, J.S.; Eckert, B.; Hyodo, M.; Hayakawa, Y.; Vance, R.E. STING is a direct innate immune sensor of cyclic di-GMP. Nature 2011, 478, 515–518. [Google Scholar] [CrossRef]
- Rockwell, N.C.; Martin, S.S.; Feoktistova, K.; Lagarias, J.C. Diverse two-cysteine photocycles in phytochromes and cyanobacteriochromes. Proc. Natl. Acad. Sci. USA 2011, 108, 11854–11859. [Google Scholar] [CrossRef] [PubMed]
- Petrov, G.I.; Doronin, A.; Whelan, H.T.; Meglinski, I.; Yakovlev, V.V. Human tissue color as viewed in high dynamic range optical spectral transmission measurements. Biomed. Opt. Express. 2012, 3, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agostoni, M.; Montgomery, B.L. Survival Strategies in the Aquatic and Terrestrial World: The Impact of Second Messengers on Cyanobacterial Processes. Life 2014, 4, 745-769. https://doi.org/10.3390/life4040745
Agostoni M, Montgomery BL. Survival Strategies in the Aquatic and Terrestrial World: The Impact of Second Messengers on Cyanobacterial Processes. Life. 2014; 4(4):745-769. https://doi.org/10.3390/life4040745
Chicago/Turabian StyleAgostoni, Marco, and Beronda L. Montgomery. 2014. "Survival Strategies in the Aquatic and Terrestrial World: The Impact of Second Messengers on Cyanobacterial Processes" Life 4, no. 4: 745-769. https://doi.org/10.3390/life4040745