Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances
Abstract
:1. Introduction
2. The Nine Ferredoxins of Synechocystis Are Highly Conserved in Cyanobacteria
| Occurrence of Ferredoxin-Encoding Genes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [2Fe-2S] | [3Fe-4S] and [4Fe-4S] | |||||||||
| Cyanobacterial Species | Plant-like | Bacterial-type | ||||||||
| ∑ | fed1 | fed2 | fed3 | fed4 | fed5 | fed6 | fed7 | fed8 | fed9 | |
| Gloeobacter kilaueensis JS1 | 3 | + | + | − | − | − | − | + g | + | + |
| Gloeobacter violaceus PCC7421 | 2 | + | + | − | − | − | − | + g | + | + |
| Anabaena cylindrica PCC7122 | 5 | + a | + c | + d | − | − | + | + g | + h | + j* |
| Anabaena sp. 90 | 4 | + | + | + d | − | − | − | + g | + h' | + j* |
| Anabaena variabilis ATCC29413 | 6 | + a | + c | + d | − | − | − | + g | + h | + j |
| Cylindrospermum stagnale PCC7417 | 6 | + a | + c | + d | − | − | + f' | + g | + h | + j |
| Nostoc punctiforme PCC73102 | 6 | 2 a | + | + d | − | − | 2/1 f' | + g | + h | + |
| Nostoc sp. PCC7107 | 4 | + a | + | + d | − | − | − | + g | + h' | + j |
| Nostoc sp. PCC7120 | 4 | + a | + c | + d | − | − | + | + g | + h | + j |
| Nostoc sp. PCC7524 | 4 | + a | + c | + d’ | − | − | + f’ | + g | + h | + |
| Nostoc azollae 0708 | 5 | + a | + c | + d | − | − | + | + g | + h | + j* |
| Calothrix sp. PCC6303 | 4 | + a’ | + | + | − | − | + | + g | + h'' | + |
| Calothrix sp. PCC7507 | 6 | 2 a | + | + d | − | − | + | + g | + | + j |
| Rivularia sp. PCC7116 | 6 | 2 a' | + c | + | − | − | + f | + g | + | + j'' |
| Acaryochloris marina MBIC11017 | 8 | 3 | + c | + | − | 5/3 e* | − | + g | + i | + |
| Chamaesiphon minutus PCC6605 | 5 | + a' | + | + | − | + | − | + g | + | + |
| Cyanobacterium aponinum PC 10605 | 4 | + a' | + | + | − | + e* | + f | + g | + i | + |
| Cyanobacterium stanieri PCC7202 | 4 | + | + | + | − | − e* | − | + g | + i | + j'' |
| Cyanobium gracile PCC6307 | 4 | + | + | + | − | − | − | + g | + h | + j'' |
| Cyanothece sp. ATCC51142 | 7 | 2 a' | + | + | + e | + e | − | + g | + i | + j'' |
| Cyanothece sp. PCC7424 | 5 | + a' | + | + | + e | + e | − | + g | + i | + j'' |
| Cyanothece sp. PCC7425 | 5 | + | + c' | + | − | + e* | − | + g | + | + |
| Cyanothece sp. PCC7822 | 7 | 2 a' | + c' | + | + e | + e | − | + g | + i | + |
| Cyanothece sp. PCC8801 | 5 | + | + c'' | + | + e | + e | − | + g | + i | + j'' |
| Cyanothece sp. PCC8802 | 5 | + | + c'' | + | + e | + e | − | + g | + i | + j'' |
| Dactylococcopsis salina PCC8305 | 3 | + | + | + | − | − | − | + g | + i | + |
| Gloeocapsa sp. PCC7428 | 7 | + a' | + | + | − | + e* | − | + g | + | + j'' |
| Halothece sp. PCC7418 | 4 | + | + | + | − | − | − | + g | + i | + j'' |
| Microcystis aeruginosa NIES-843 | 4 | 2 | + c'' | + | − | − | − | + g | + | + j'' |
| Synechococcus elongatus PCC6301 | 3 | + b | + | + | − | − | − | + g | + | + j'' |
| Synechococcus elongatus PCC7942 | 3 | + b | + | + d | − | − | − | + g | + | + j'' |
| Synechococcus sp. CC9311 | 6 | 2 b | + | + d | − | − | − | + g | + | − |
| Synechococcus sp. CC9605 | 6 | + b | + | + d | − | − | − | 2 g | + | − |
| Synechococcus sp. CC9902 | 5 | + b | + | + d | − | − | − | + g | + | − |
| Synechococcus JA-2-3B' a(2-13) | 4 | + | + | + | − | 2 | − | − | + | + |
| Synechococcus JA-3-3B' Ab | 4 | + | + | + | − | 2 | − | − | + | + |
| Synechococcus sp. PCC 6312 | 4 | + | + | + d | − | + e* | − | + g | + | + |
| Synechococcus sp. PCC7002 | 4 | 2 a' | + | + | − | 2 | − | + g | + | + |
| Synechococcus sp. PCC7502 | 4 | + | + | + | − | 3 | − | + g | + | + |
| Synechococcus sp. RCC307 | 4 | + b | + c'' | + d | − | − | − | + g | + | + j'' |
| Synechococcus sp. WH7803 | 4 | + b | + c''' | + d | − | − | − | + g | + | − |
| Synechococcus sp. WH8102 | 4 | + b | +c''' | + d | − | − | − | + g | + | − |
| Synechocystis sp. PCC6803 | 4 | + | + c'' | + | + e' | + e' | + f* | + g | + i | + |
| Thermosynechococcus elongatus BP1 | 4 | + | + | + | − | + e'' | − | + g | + | + |
| Cyanobacterium UCYN-A | 4 | + | + | + | − | − | − | − | + | − |
| Arthrospira platensis NIES-39 | 3 | + | + c | + d | − | + | − | + g | + | + j'' |
| Crinalium epipsammum PCC9333 | 4 | + a'' | + c | + d | − | − | + f | + g | + | + j'' |
| Geitlerinema sp. PCC7407 | 3 | + a'' | + c | + d | − | + | + f | + g | + | + |
| Leptolyngbya sp. PCC7376 | 4 | 2 a'' | + c | + | − | + | − | − | + | + |
| Microcoleus sp. PCC7113 | 6 | 2 | + c | + | − | − | +f | + g | + | + j'' |
| Oscillatoria acuminata PCC6304 | 3 | + a’ | + c | + d | − | + | − | + g | + | + j'' |
| Oscillatoria nigroviridis PCC7112 | 4 | + a’ | + c | + | − | − | +f | + g | + | + j'' |
| Pseudanabaena sp. PCC7367 | 4 | + | + | + | − | + | − | + g | + | + |
| Trichodesmium erythraeum ISM101 | 4 | 3 a'' | − | + | − | − | − | + g | + | + j'' |
| Chroococcidiopsis thermalis PCC7203 | 5 | + | + | + d | − | + e'' | − | + g | 2 | + j'' |
| Pleurocapsa sp. PCC7327 | 5 | + | + c' | + | + e' | + e | − | + g | + i | + j'' |
| Stanieria cyanosphaera PCC7437 | 4 | + | + | + | + e' | + e' | − | + g | + i | + j'' |
| Prochlorococcus marinus AS9601 | 3 | + b | + c''' | + d | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9211 | 2 | + b | + c''' | − | − | − | − | + g' | + | − |
| Prochlorococcus marinus MIT9215 | 2 | + b | + c''' | ? | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9301 | 3 | + b | + c''' | + d | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9303 | 1 | + b | − | − | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9312 | 3 | + b | + c''' | + d | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9313 | 1 | + b | − | − | − | − | − | + g' | − | − |
| Prochlorococcus marinus MIT9515 | 2 | + b | + | − | − | − | − | + g' | − | − |
| Prochlorococcus marinus NATL1A | 3 | + b | + | − | − | − | − | + g' | − | − |
| Prochlorococcus marinus NATL2A | 3 | + b | + | − | − | − | − | + g' | − | − |
| Prochlorococcus marinus SS120 | 2 | + b | + | − | − | − | − | + g' | + | − |
| Prochlorococcus marinus MED4 | 3 | + b | + c’’ | + d | − | − | − | + g' | − | − |
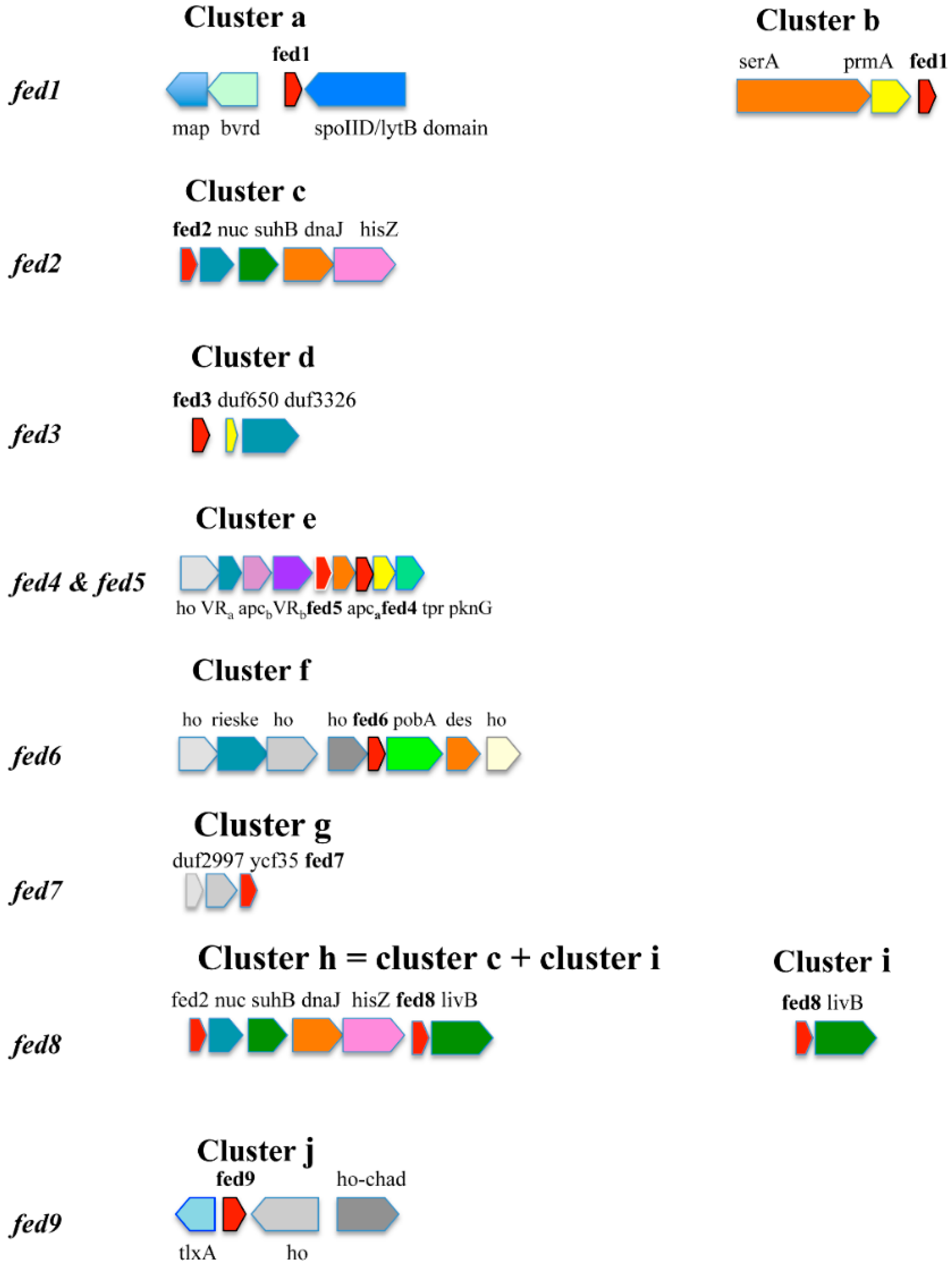
3. The Synechocystis Ferredoxins Genes Are Differently Regulated by Trophic Conditions
| Name | Conditions Triggering Upregulation of the Fed Genes | Conditions Triggering Downregulation of the Fed Genes |
|---|---|---|
| fed1 | Light [9,11]; NaHCO3 [11]; | Darkness [9]; Cd, LFe, H2O2 [11,13]; Na2SeO3, Na2SeO4 [11]; HZn [13]; DCMU, DBMIB, LiC, HT°, SS [14] |
| fed2 | Cd, H2O2, HZn [13]; HL, BL, UV, SS [14] | Glc [9]; Na2SeO4 [11]; LiC [14] |
| fed3 | BL* [14] | Cd [13]; H2O2 [13,14]; LiC [14] |
| fed4 | LL [9]; H2O2 [13] | Cd, LFe, HZn [13]; HL, DCMU, LiC, SS [14] |
| fed5 | LL [9]; H2O2 [13] | Cd, LFe, HZn [13]; HL, DCMU, LiC, SS [14] |
| fed6 | BL [14] | |
| fed7 | LFe [13]; HL [14] LiC [15] | H2O2 [11,13]; Cd, HFe [13] |
| fed8 | Cd [11]; HL, LiC [14] | H2O2, LFe [14] |
| fed9 | HL, HT° [14] |
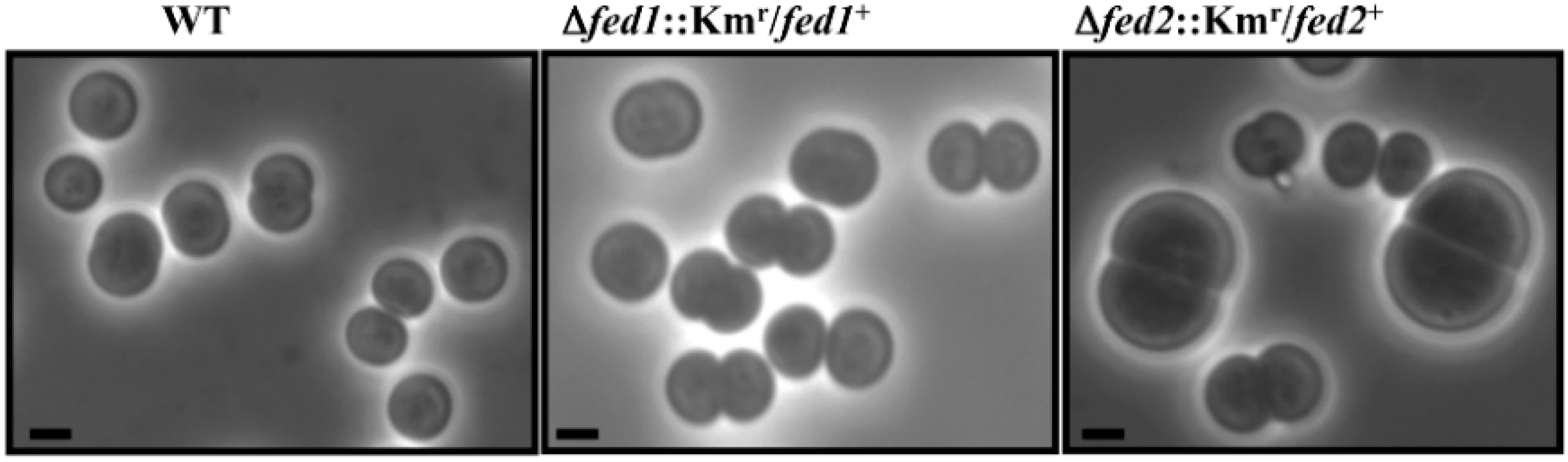
4. The Nine Synechocystis Ferredoxins Play a Crucial Role in Photoautotrophic Growth or Tolerance to Environmental Stresses
| Name | Gene ID | Type of Iron Sulfur Center | Importance for Photo-Autotrophic Growth | Reference |
|---|---|---|---|---|
| fed1 | ssl0020 | [2Fe-2S] plant-like | Essential | [4,9] |
| fed2 | sll1382 | [2Fe-2S] plant-like | Essential | This study |
| fed3 | slr1828 | [2Fe-2S] plant-like | Essential | This study |
| fed4 | slr0150 | [2Fe-2S] plant-like | Dispensable | This study, [4] |
| fed5 | slr0148 | [2Fe-2S] adrenodoxin-like | Dispensable | This study |
| fed6 | ssl2559 | [2Fe-2S] bacterial type | Essential | This study |
| fed7 | sll0662 | [4Fe-4S] | Dispensable | [15,18] |
| fed8 | ssr3184 | [3Fe-4S] [4Fe-4S] | Essential | This study |
| fed9 | slr2059 | [4Fe-4S] [4Fe-4S] | Dispensable | This study |
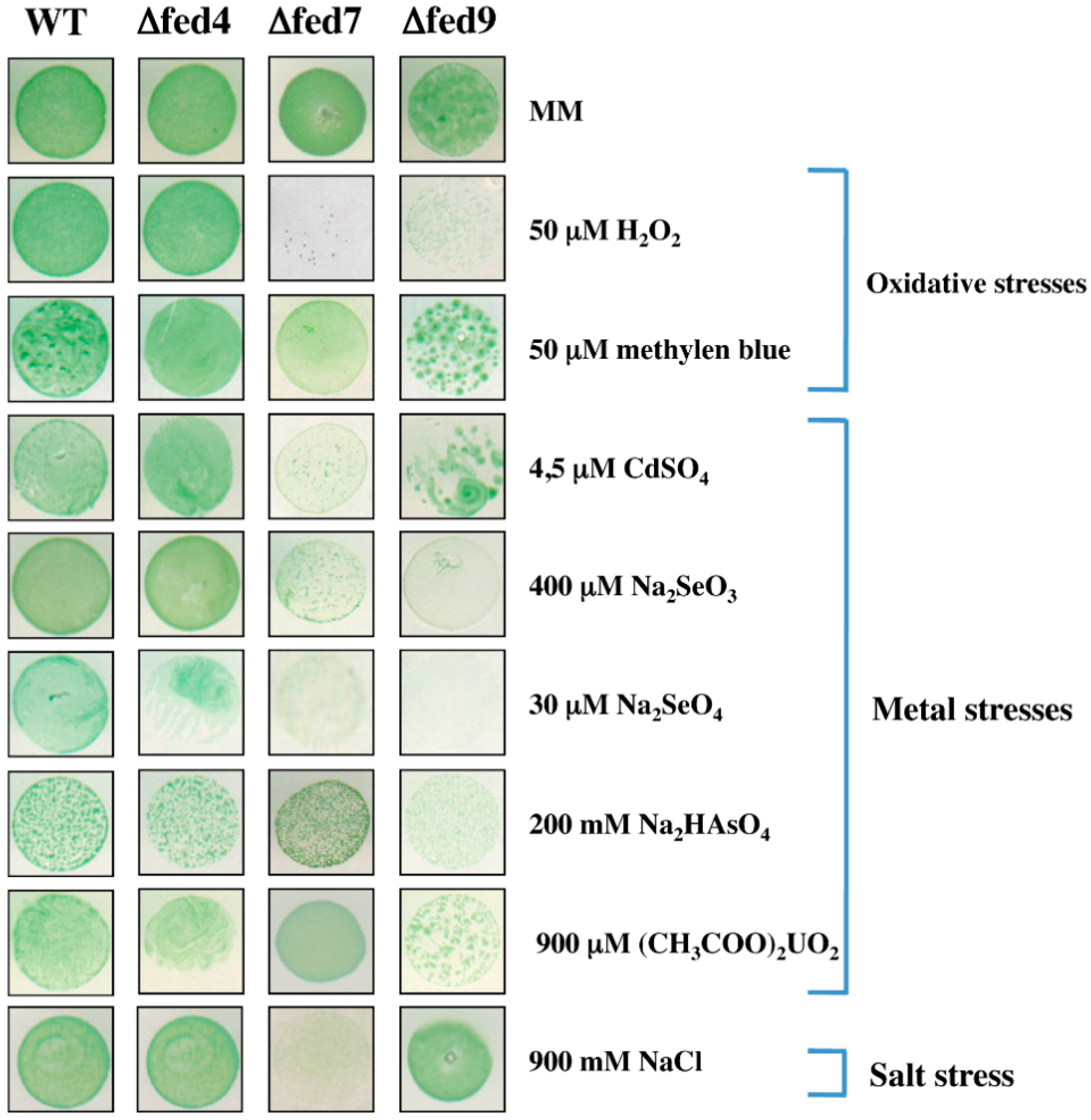
Fed7 and Fed9 Ferredoxins Plays a Prominent Role in the Tolerance to Oxidative and Metal Stresses, and the [2Fe-2S] Center of Fed7 Is Required for the Tolerance to Iron Starvation

5. Analysis of the Selectivity/Redundancy of Ferredoxins: Identification of Fed-Interacting Proteins
| Gene Cloned in pUT18 | Gene Cloned in pKT25 | β-GAL Activity (nmol·min−1·mg−1) | Reference |
|---|---|---|---|
| Controls: | |||
| zip domain | zip domain | 4213 ± 385 | This study |
| none | none | 75 ± 4 | This study |
| fed7 | none | 74 ± 8 | This study |
| none | ftrC | 82 ± 4 | [18] |
| fed9 | none | 76 ± 7 | This study |
| none | fed9 | 69 ± 6 | This study |
| Tests: | |||
| fed7 | fed9 | 92 ± 8 | This study |
| fed7 | dnaJ | 1081 ± 88 | This study |
| fed7C53S | dnaJ | 346 ± 20 | This study |
| fed7C53S C56S C59S | dnaJ | 413 ± 69 | This study |
| fed7C100S | dnaJ | 781 ± 26 | This study |
| fed9 | dnaJ | 78 ± 6 | This study |
| fed7 | ftrC | 1766 ± 164 | [18] |
| fed7C53S | ftrC | 567 ± 87 | This study |
| fed7C53S C56S C59S | ftrC | 587 ± 104 | This study |
| fed7C96S | ftrC | 652 ± 47 | This study |
| fed7C100S | ftrC | 237 ± 32 | This study |
| fed7 | ftrCC31S | 1428 ± 16 | This study |
| fed7 | ftrCC56S | 1460 ± 52 | This study |
| fed7 | ftrCC58S | 228 ± 32 | [18] |
| fed7 | ftrCC75S | 1597 ± 116 | This study |
| fed7 | ftrCC77S | 1223 ± 17 | This study |
| fed7 | ftrCC86S | 1475 ± 24 | This study |
| fed7 | ftrCC88S | 1613 ± 231 | [18] |
| fed9 | ftrC | 2728 ± 184 | This study |
| fed9C84S C87S C90S C125S | ftrC | 2649 ± 42 | This study |
| fed9C94S C115S C118S C121S | ftrC | 2058 ± 12 | This study |
| fed9D80A | ftrC | 73 ± 3 | This study |
| fed9 | ftrCC31S | 2531 ± 128 | This study |
| fed9 | ftrCC56S | 2312 ± 99 | This study |
| fed9 | ftrCC58S | 3181 ± 113 | This study |
| fed9 | ftrCC75S | 2159 ± 53 | This study |
| fed9 | ftrCC77S | 2350 ± 52 | This study |
| fed9 | ftrCC86S | 2241 ± 120 | This study |
| fed9 | ftrCC88S | 121 ± 2 | This study |
| fed7 | flv3 | 89 ± 9 | This study |
| fed9 | flv3 | 2797 ± 175 | This study |
| fed9C84S C87S C90S C125S | flv3 | 2008 ± 123 | This study |
| fed9C94S C115S C118S C121S | flv3 | 2311 ± 28 | This study |
| fed9D80A | flv3 | 2253 ± 84 | This study |
| fed9 | fed9 | 2472 ± 190 | This study |
| fed9 | fed9D80A | 3057 ± 250 | This study |
| fed9 | sll0330 | 1934 ± 42 | This study |
5.1. Fed1, Fed7 and Fed9 Belong to a Ferredoxin-Glutaredoxin-Thioredoxin Crosstalk Pathway Operating in Stress Resistance
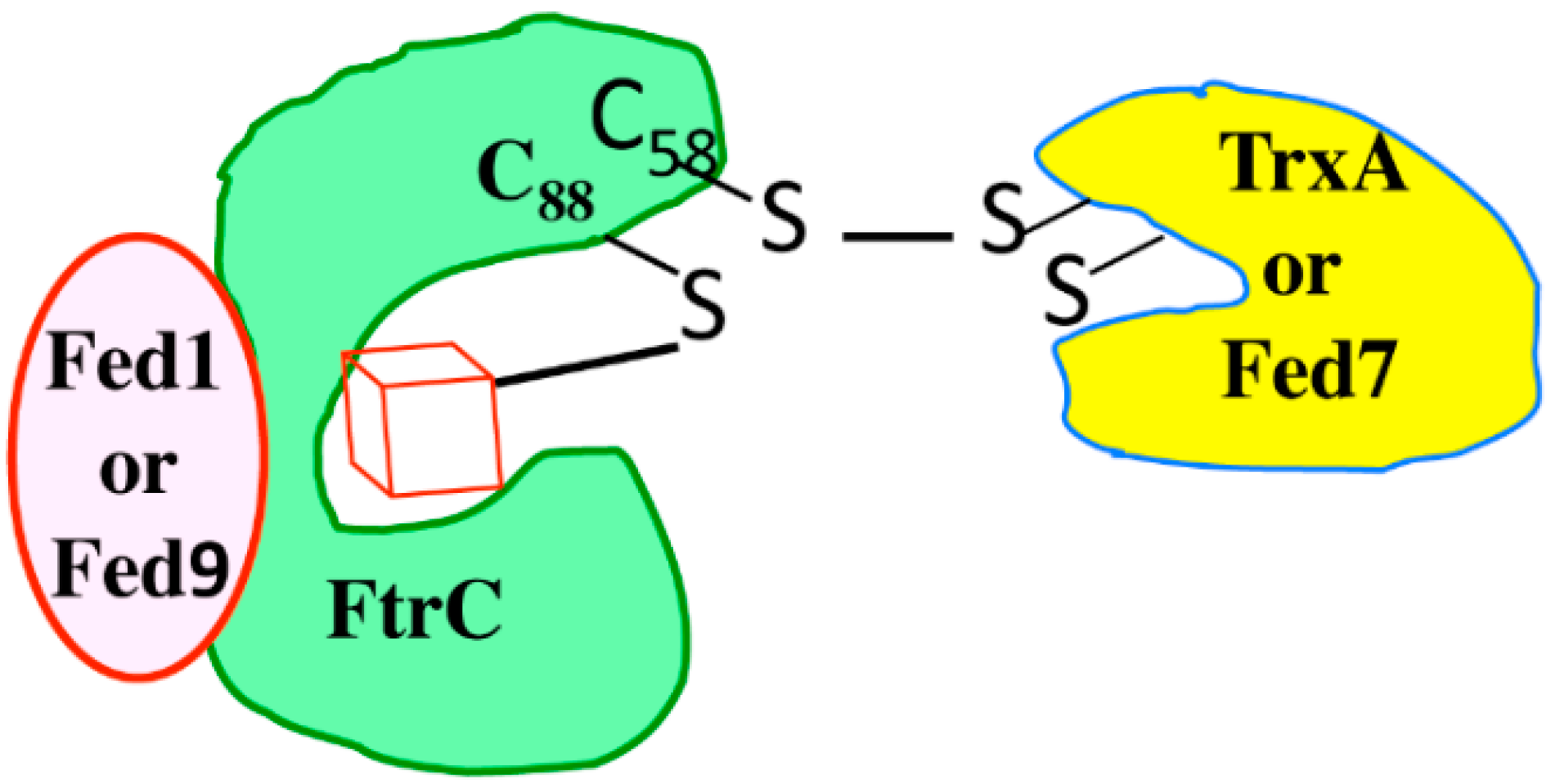
5.2. Identification of Proteins Selectively Interacting with Either Fed7 or Fed9, but Not Both
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sticht, H.; Rosch, P. The structure of iron-sulfur proteins. Prog. Biophys. Mol. Biol. 1998, 70, 95–136. [Google Scholar]
- Hanke, G.; Mulo, P. Plant type ferredoxins and ferredoxin-dependent metabolism. Plant Cell Environ. 2013, 36, 1071–1084. [Google Scholar] [CrossRef] [PubMed]
- Grinter, R.; Josts, I.; Zeth, K.; Roszak, A.W.; McCaughey, L.C.; Cogdell, R.J.; Milner, J.J.; Kelly, S.M.; Byron, O.; Walker, D. Structure of the atypical bacteriocin pectocin M2 implies a novel mechanism of protein uptake. Mol. Microbiol. 2014, 93, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Gutekunst, K.; Chen, X.; Schreiber, K.; Kaspar, U.; Makam, S.; Appel, J. The bidirectional NiFe-hydrogenase in Synechocystis sp. PCC 6803 is reduced by flavodoxin and ferredoxin and is essential under mixotrophic, nitrate-limiting conditions. J. Biol. Chem. 2014, 289, 1930–1937. [Google Scholar]
- Nakamura, Y.; Kaneko, T.; Hirosawa, M.; Miyajima, N.; Tabata, S. CyanoBase, a www database containing the complete nucleotide sequence of the genome of Synechocystis sp. strain PCC6803. Nucleic Acids Res. 1998, 26, 63–67. [Google Scholar] [CrossRef]
- Grigorieva, G.; Shestakov, S. Transformation in the cyanobacterium Synechocystis sp. 6803. FEMS Microbiol. Lett. 1982, 13, 367–370. [Google Scholar] [CrossRef]
- Marraccini, P.; Bulteau, S.; Cassierchauvat, C.; Mermetbouvier, P.; Chauvat, F. A Conjugative Plasmid Vector for Promoter Analysis in Several Cyanobacteria of the Genera Synechococcus and Synechocystis. Plant Mol. Biol. 1993, 23, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Mermet-Bouvier, P.; Chauvat, F. A Conditional Expression Vector for the Cyanobacteria Synechocystis sp. Strains PCC6803 and PCC6714 or Synechococcus sp. Strains PCC7942 and PCC6301. Curr. Microbiol. 1994, 28, 145–148. [Google Scholar] [PubMed]
- Poncelet, M.; Cassier-Chauvat, C.; Leschelle, X.; Bottin, H.; Chauvat, F. Targeted deletion and mutational analysis of the essential (2Fe-2S) plant-like ferredoxin in Synechocystis PCC6803 by plasmid shuffling. Mol. Microbiol. 1998, 28, 813–821. [Google Scholar] [CrossRef] [PubMed]
- Bottin, H.; Lagoutte, B. Ferredoxin and flavodoxin from the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 1992, 1101, 48–56. [Google Scholar] [CrossRef]
- Mazouni, K.; Domain, F.; Chauvat, F.; Cassier-Chauvat, C. Expression and regulation of the crucial plant-like ferredoxin of cyanobacteria. Mol. Microbiol. 2003, 49, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Figge, R.M.; Cassier-Chauvat, C.; Chauvat, F.; Cerff, R. Characterization and analysis of an NAD(P)H dehydrogenase transcriptional regulator critical for the survival of cyanobacteria facing inorganic carbon starvation and osmotic stress. Mol. Microbiol. 2001, 39, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Houot, L.; Floutier, M.; Marteyn, B.; Michaut, M.; Picciocchi, A.; Legrain, P.; Aude, J.C.; Cassier-Chauvat, C.; Chauvat, F. Cadmium triggers an integrated reprogramming of the metabolism of Synechocystis PCC6803, under the control of the Slr1738 regulator. BMC Genomics 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Elvitigala, T.; Cameron, J.C.; Ghosh, B.K.; Bhattacharyya-Pakrasi, M.; Pakrasi, H.B. Integrative analysis of large scale expression profiles reveals core transcriptional response and coordination between multiple cellular processes in a cyanobacterium. BMC Syst. Biol. 2010, 4. [Google Scholar] [CrossRef]
- Mustila, H.; Allahverdiyeva, Y.; Isojarvi, J.; Aro, E.M.; Eisenhut, M. The bacterial-type [4Fe-4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta 2014, 1837, 1293–1304. [Google Scholar] [CrossRef]
- Labarre, J.; Chauvat, F.; Thuriaux, P. Insertional Mutagenesis by Random Cloning of Antibiotic-Resistance Genes into the Genome of the Cyanobacterium Synechocystis Strain Pcc-6803. J. Bacteriol. 1989, 171, 3449–3457. [Google Scholar]
- Van der Plas, J.; de Groot, R.; Woortman, M.; Cremers, F.; Borrias, M.; van Arkel, G.; Weisbeek, P. Genes encoding ferredoxins from Anabaena sp. PCC 7937 and Synechococcus sp. PCC 7942: Structure and regulation. Photosynth. Res. 1988, 18, 179–204. [Google Scholar]
- Marteyn, B.; Domain, F.; Legrain, P.; Chauvat, F.; Cassier-Chauvat, C. The thioredoxin reductase-glutaredoxins-ferredoxin crossroad pathway for selenate tolerance in Synechocystis PCC6803. Mol. Microbiol. 2009, 71, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Karimova, G.; Pidoux, J.; Ullmann, A.; Ladant, D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 1998, 95, 5752–5756. [Google Scholar] [CrossRef] [PubMed]
- Marbouty, M.; Saguez, C.; Cassier-Chauvat, C.; Chauvat, F. ZipN, an FtsA-like orchestrator of divisome assembly in the model cyanobacterium Synechocystis PCC6803. Mol. Microbiol. 2009, 74, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Marteyn, B.; Sakr, S.; Farci, S.; Bedhomme, M.; Chardonnet, S.; Decottignies, P.; Lemaire, S.D.; Cassier-Chauvat, C.; Chauvat, F. The Synechocystis PCC6803 MerA-Like Enzyme Operates in the Reduction of Both Mercury and Uranium under the Control of the Glutaredoxin 1 Enzyme. J. Bacteriol. 2013, 195, 4138–4145. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Schwendtmayer, C.; Schurmann, P.; Ramaswamy, S.; Eklund, H. Redox signaling in chloroplasts: Cleavage of disulfides by an iron-sulfur cluster. Science 2000, 287, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Duppre, E.; Rupprecht, E.; Schneider, D. Specific and promiscuous functions of multiple DnaJ proteins in Synechocystis sp. PCC 6803. Microbiology 2011, 157, 1269–1278. [Google Scholar]
- Petitjean, C.; Moreira, D.; Lopez-Garcia, P.; Brochier-Armanet, C. Horizontal gene transfer of a chloroplast DnaJ-Fer protein to Thaumarchaeota and the evolutionary history of the DnaK chaperone system in Archaea. BMC Evolut. Biol. 2012, 12. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Ermakova, M.; Eisenhut, M.; Zhang, P.; Richaud, P.; Hagemann, M.; Cournac, L.; Aro, E.M. Interplay between flavodiiron proteins and photorespiration in Synechocystis sp. PCC 6803. J. Biol. Chem. 2011, 286, 24007–24014. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassier-Chauvat, C.; Chauvat, F. Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances. Life 2014, 4, 666-680. https://doi.org/10.3390/life4040666
Cassier-Chauvat C, Chauvat F. Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances. Life. 2014; 4(4):666-680. https://doi.org/10.3390/life4040666
Chicago/Turabian StyleCassier-Chauvat, Corinne, and Franck Chauvat. 2014. "Function and Regulation of Ferredoxins in the Cyanobacterium, Synechocystis PCC6803: Recent Advances" Life 4, no. 4: 666-680. https://doi.org/10.3390/life4040666




