New Occurrence of Rusinovite, Ca10(Si2O7)3Cl2: Composition, Structure and Raman Data of Rusinovite from Shadil-Khokh Volcano, South Ossetia and Bellerberg Volcano, Germany
Abstract
:1. Introduction
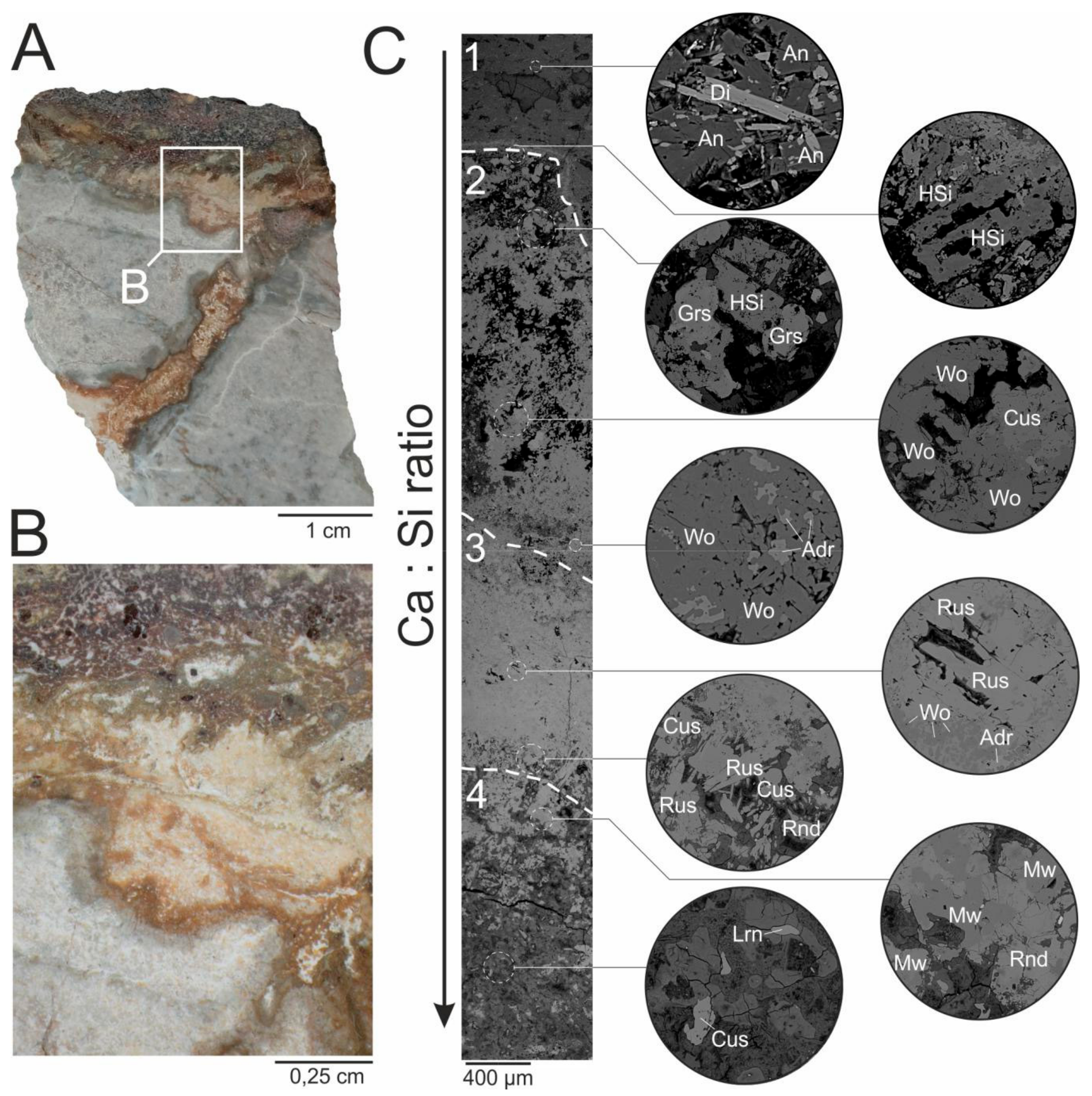
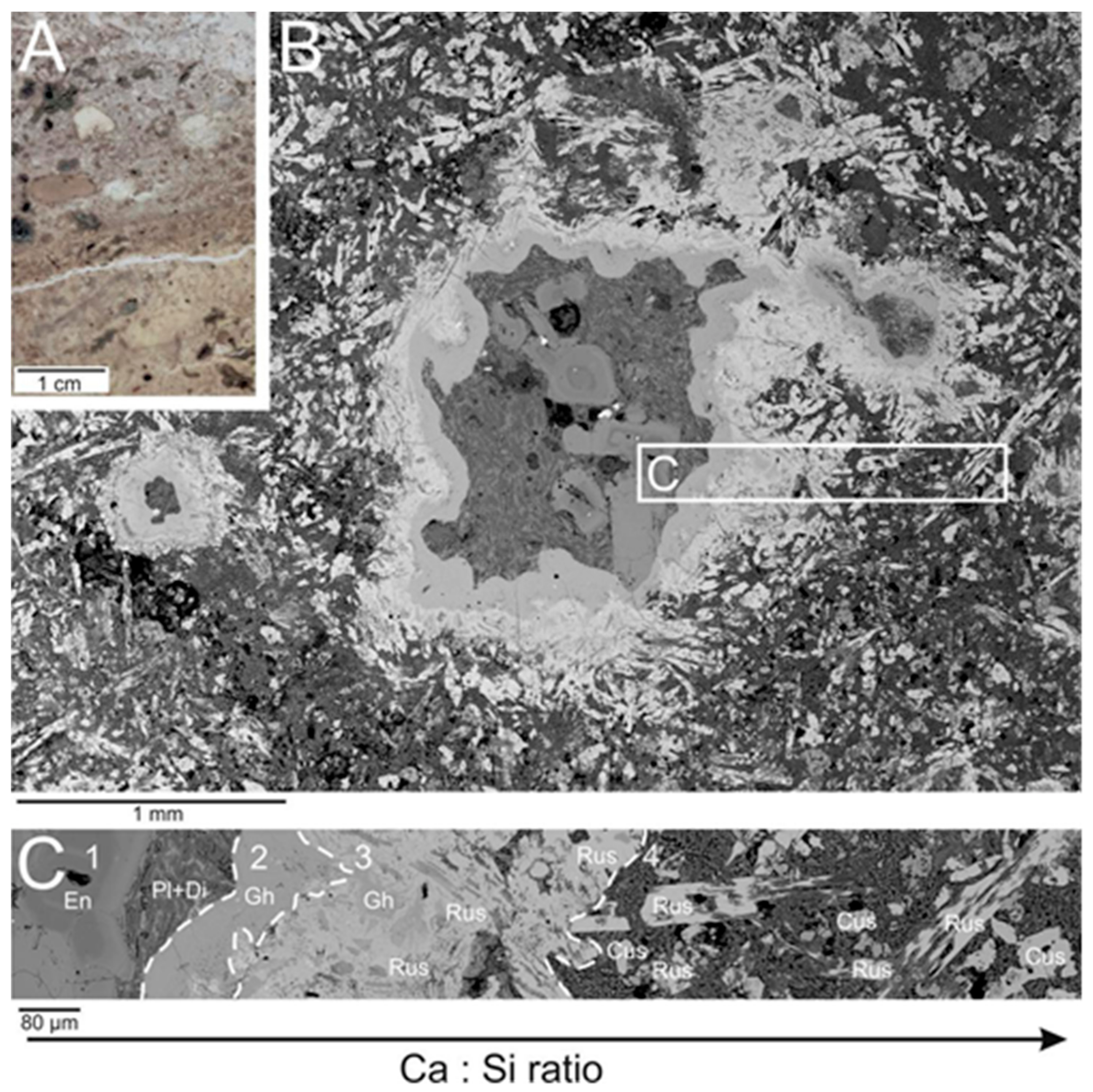
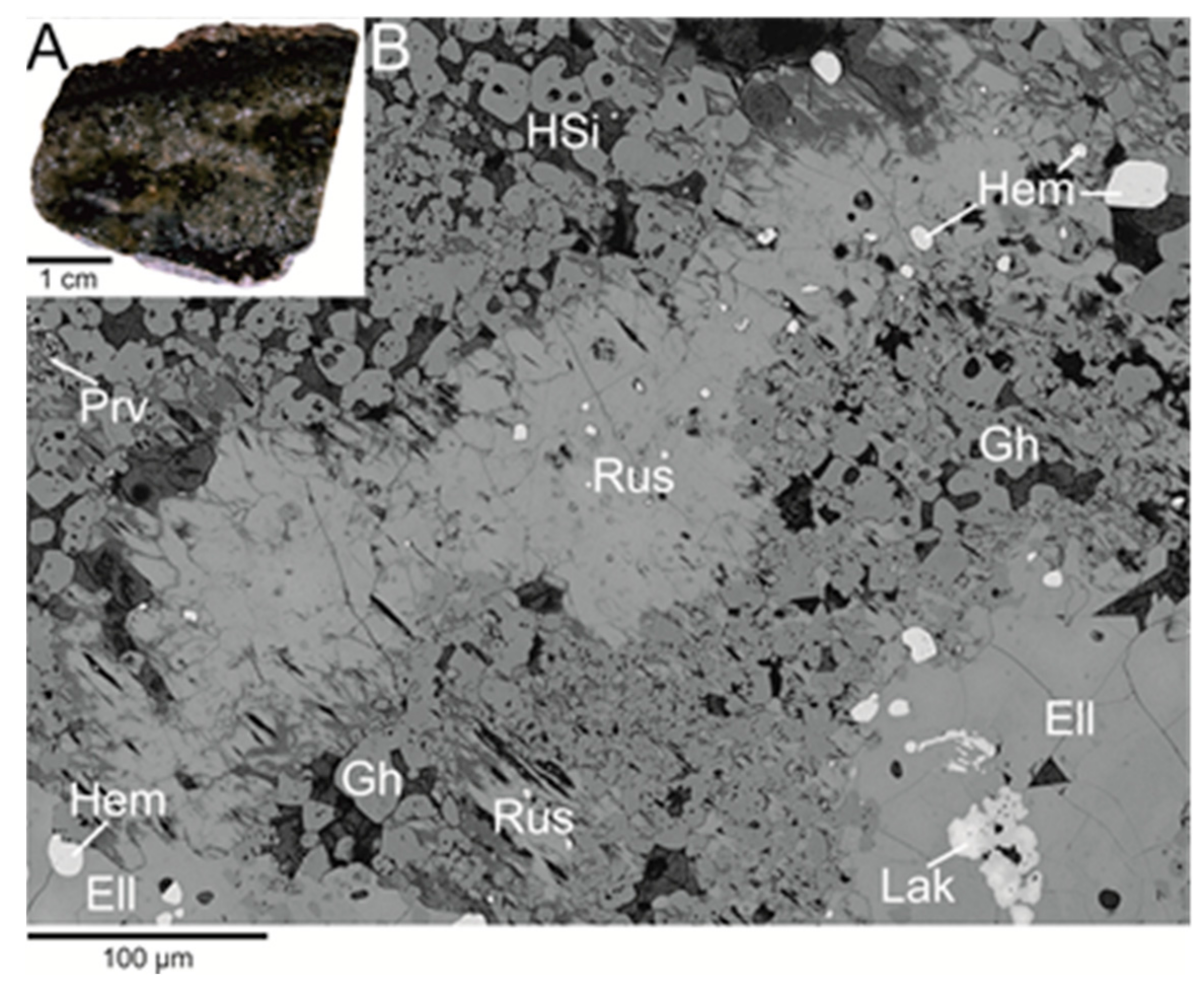
2. The Occurrence and Paragenesis of Rusinovite
3. Materials and Methods
4. Results
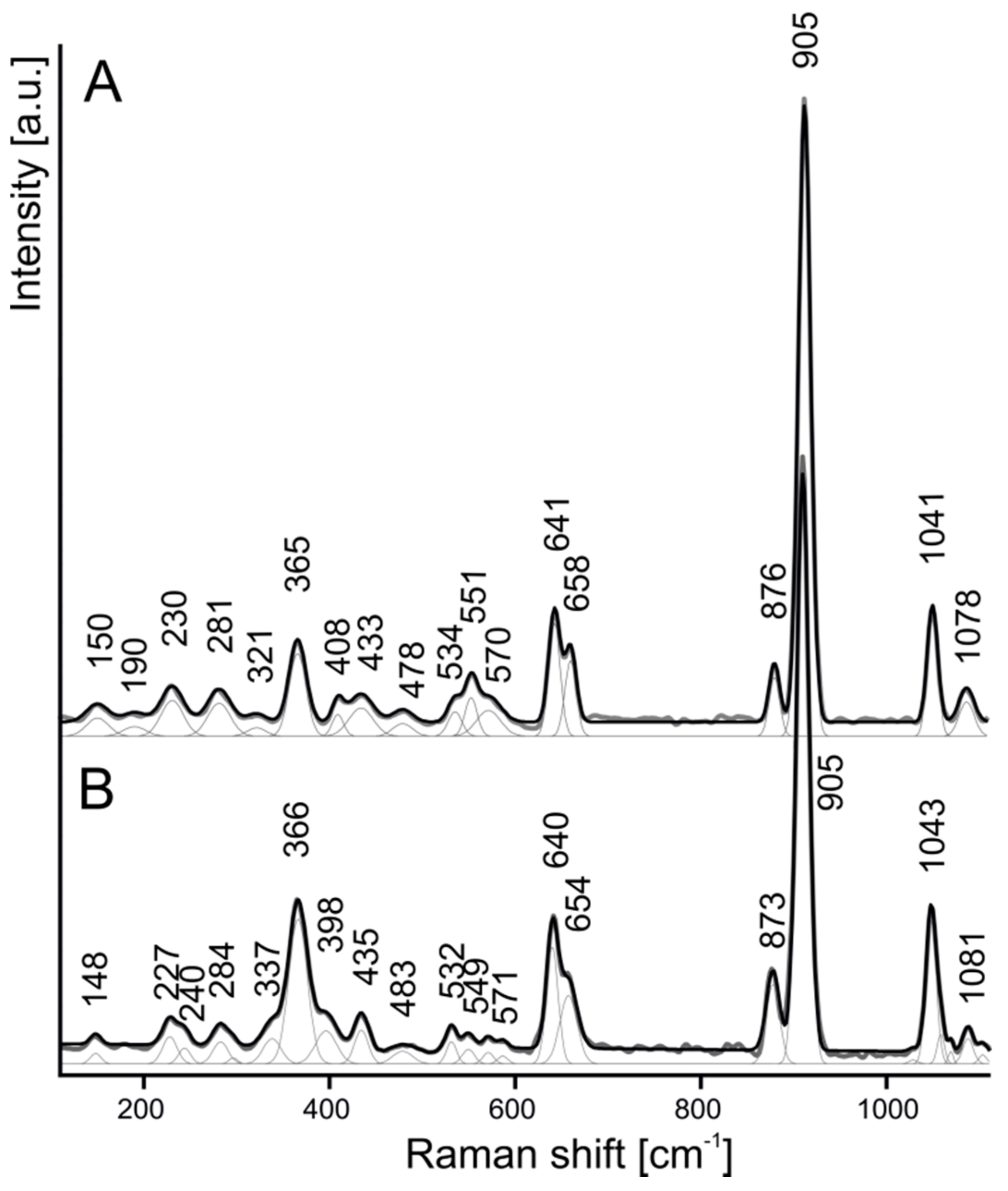
4.1. Chemical Composition and Raman Spectroscopy of Rusinovite
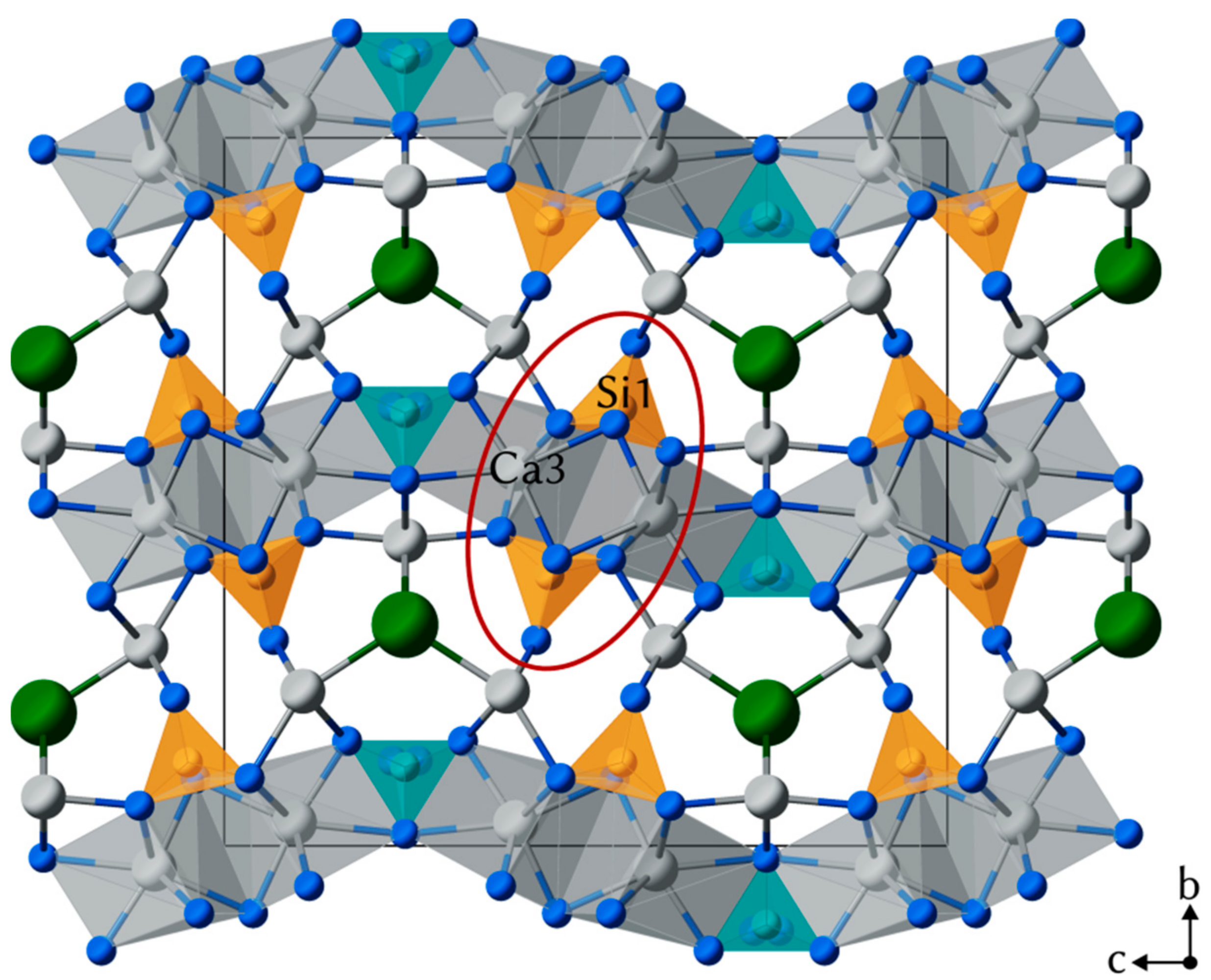
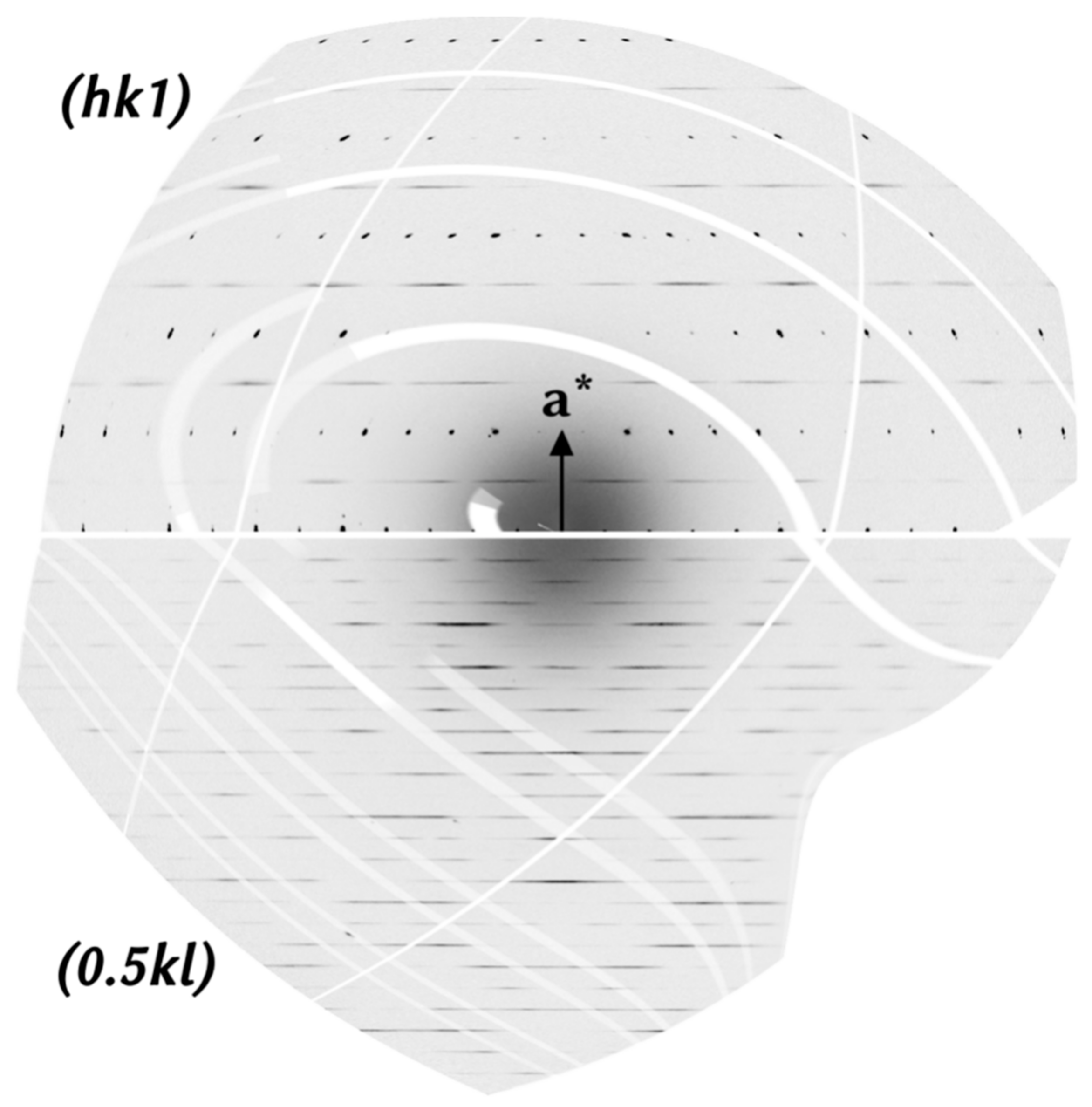
4.2. Crystal Structure of Rusinovite
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galuskin, E.V.; Galuskina, I.O.; Lazic, B.; Armbruster, T.; Zadov, A.E.; Krzykawski, T.; Banasik, K.; Gazeev, V.M.; Pertsev, N.N. Rusinovite, Ca10(Si2O7)3Cl2: A new skarn mineral from the Upper Chegem caldera, Kabardino-Balkaria, Northern Caucasus, Russia. Eur. J. Mineral. 2011, 23, 837–844. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Kusz, J.; Gfeller, F.; Armbruster, T.; Bailau, R.; Dulski, M.; Gazeev, V.M.; Pertsev, N.N.; Zadov, A.E.; et al. Mayenite supergroup, part II: Chlorkyuygenite from Upper Chegem, Northern Caucasus, Kabardino-Balkaria, Russia, a new microporous mineral with “zeolitic” H2O. Eur. J. Mineral. 2015, 27, 113–122. [Google Scholar] [CrossRef]
- Gfeller, F.; Armbruster, T.; Galuskin, E.V.; Galuskina, I.O.; Lazic, B.; Savelyeva, V.B.; Zadov, A.E.; Dzierżanowski, P.; Gazeev, V.M. Crystal chemistry and hydrogen bonding of rustumite Ca10(Si2O7)2(SiO4)(OH)2Cl2 with variable OH, Cl, F. Am. Mineral. 2013, 98, 493–500. [Google Scholar] [CrossRef]
- Stemmermann, P.; Pöllmann, H. The system CaO-SiO2-CaCl2-phase equilibria and polymorphs below 1000 °C. An interpretation on garbage combustion ashes. Neues Jahrb. Mineral. Monatsh. 1992, 9, 409–431. [Google Scholar]
- Chesnokov, B.V.; Vilisov, V.; Bushmakin, A.; Kotlyarov, V.; Belogub, E.V. New minerals from a fired dump of the Chelyabinsk coal basin. Ural Mineral. Zbor. 1994, 3, 3–34. [Google Scholar]
- Hermoneit, B.; Ziemer, B.; Malewski, G. Single crystal growth and some properties of the new compound Ca3Si2O7·13CaCl2. J. Cryst. Growth 1981, 52, 660–664. [Google Scholar] [CrossRef]
- Ding, W.; Wang, J.; Zhang, M.; Zhang, Q.; Su, Q. Luminescence properties of new Ca10(Si2O7)3Cl2:Eu2+ phosphor. Chem. Phys. Lett. 2007, 435, 301–305. [Google Scholar] [CrossRef]
- Gazeev, V.M.; Gurbanova, O.; Zadov, A.E.; Gurbanov, A.G.; Leksin, A. Mineralogy of skarned carbonate xenoliths from Shadil-Khokh volcano (Kelski Vulcan area of Great Caucasian Range). Vestn. Vladikavkazskogo Nauchnogo Cent. 2012, 2, 18–27. [Google Scholar]
- Gfeller, F.; Środek, D.; Kusz, J.; Dulski, M.; Gazeev, V.; Galuskina, I.; Galuskin, E.; Armbruster, T. Mayenite supergroup, part IV: Crystal structure and Raman investigation of Al-free eltyubyuite from the Shadil-Khokh volcano, Kel’ Plateau, Southern Ossetia, Russia. Eur. J. Mineral. 2015, 27, 137–143. [Google Scholar] [CrossRef]
- Mihajlovic, T.; Lengauer, C.L.; Ntaflos, T.; Kolitsch, U.; Tillmanns, E. Two new minerals, rondorfite, Ca8Mg[SiO4]4Cl2, and almarudite, K(□,Na)2(Mn,Fe,Mg)2(Be,Al)3[Si12O30], and a study of iron-rich wadalite, Ca12[(Al8Si4Fe2)O32]Cl6, from the Bellerberg (Bellberg) volcano, Eifel, Germany. Neues Jahrb. Mineral. Abh. 2004, 179, 265–294. [Google Scholar]
- Hentschel, G. Mayenit, 12CaO-7Al2O3, und Brownmillerit, 2CaO-(Al, Fe)2O3, zwei neue Minerale in den Kalksteineinschlussen der Lava des Ettringer Bellerberges. Neues Jahrb. Mineral. Mh. 1964, 22–29. (In German) [Google Scholar]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Armbruster, T.; Bailau, R.; Sharygin, V.V. Mayenite supergroup, part I: Recommended nomenclature. Eur. J. Mineral. 2015, 27, 99–111. [Google Scholar] [CrossRef]
- Schlüter, J.; Malcherek, T.; Pohl, D.; Schäfer, C. Vondechenite, a new hydrous calcium copper chloride hydroxide, from the Bellerberg, East-Eifel volcanic area, Germany. Neues Jahrb. Mineral. 2018, 195, 79–86. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. D 2010, 66, 125–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estermann, M.A.; Steurer, W. Diffuse scattering data acquisition techniques. Phase Transit. 1998, 67, 165–195. [Google Scholar] [CrossRef]
- Le Cleac’h, A.; Gillet, P. IR and Raman spectroscopic study of natual lawsonite. Eur. J. Mineral. 1990, 2, 43–53. [Google Scholar] [CrossRef]
- Dulski, M.; Bulou, A.; Marzec, K.M.; Galuskin, E.V.; Wrzalik, R. Structural characterization of rondorfite, calcium silica chlorine mineral containing magnesium in tetrahedral position [MgO4]6−, with the aid of the vibrational spectroscopies and fluorescence. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2013, 101, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Galuskin, E.V.; Krüger, B.; Krüger, H.; Blass, G.; Widmer, R.; Galuskina, I.O. Wernerkrauseite, CaFe3+2Mn4+O6: The first nonstoichiometric post-spinel mineral, from Bellerberg volcano, Eifel, Germany. Eur. J. Mineral. 2016, 28, 485–493. [Google Scholar] [CrossRef]
- Lazarev, A.N. Vibrational Spectra and Structure of Silicates; Consultants Bureau: New York, NY, USA, 1972. [Google Scholar]
- McMillan, P. Structural studies of silicate glasses and melts—applications and limitations of Raman spectroscopy. Am. Mineral. 1984, 69, 622–644. [Google Scholar]
- Galuskina, I.O.; Krüger, B.; Galuskin, E.V.; Armbruster, T.; Gazeev, V.M.; Włodyka, R.; Dulski, M.; Dzierżanowski, P. Fluorchegemite Ca7(SiO4)3F2, a new mineral from the edgrewite-bearing endoskarn zone of an altered zenolith in ignimbrites from Upper Chegem caldera, Northern Caucasus, Kabardino-Balkaria, Russia: Occurrence, crystal structure and new data on the mineral assemblages. Can. Mineral. 2015, 53, 325–344. [Google Scholar] [CrossRef]


| Crystal Data | |
|---|---|
| Chemical formula | Ca10(Si2O7)3Cl2 |
| Crystal system | orthorhombic |
| Space group | Cmcm (No. 63) |
| Unit-cell dimensions | a = 3.76330(4) Å |
| b = 16.9423(3) Å | |
| c = 17.3325(2) Å | |
| Unit cell volume | 1105.10(4) Å3 |
| Formula weight | 976.2 |
| Density (calculated) | 2.934 g/cm3 |
| Z | 2 |
| Crystal size | 28 × 18 × 15 µm |
| Data collection | |
| Diffractometer | beamline X06DA, Swiss Light Source single-axis Aerotech goniometer, PILATUS 2M-F detector |
| Radiation wavelength | 0.72931 Å |
| Detector to sample distance | 80 mm |
| Oscillation range | 0.1° |
| No. of frames measured | 1800 |
| Time of exposure | 0.3 s |
| Reflection ranges | −5 ≤ h ≤ 5; −25 ≤ k ≤ 23; −25 ≤ l ≤ 23 |
| Reflection measured | 5015 |
| Rint | 0.0274 |
| Refinement of structure | full matrix least-squares on F |
| No. of unique reflections | 1087 |
| No. of observed unique refl. [I > 3s(I)] | 1032 |
| Final R values [I > 3s(I)] | R = 0.021; wR = 0.035 |
| Final R values (all data) | R = 0.022; wR = 0.036 |
| S (all data) | 2.34 |
| Refined parameters | 85 |
| Weighting scheme | w = 1/(s2(F) + 0.0001F2) |
| Δρmin [e Å−3] | −0.73 |
| Δρmax [e Å−3] | 0.32 |
| Component | Bellerberg Volcano | Shadil-Khokh Volcano | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean 18 | Mean 14 | |||||||||
| wt % | S.D. | Range | Atom | pfu | wt % | S.D. | Range | Atom | pfu | |
| SiO2 | 36.73 | 0.23 | 36.44–37.33 | Si | 6.00 | 36.89 | 0.23 | 36.12–36.93 | Si | 5.99 |
| CaO | 56.70 | 0.35 | 56.24–57.11 | Ca | 9.93 | 57.25 | 0.35 | 56.79–58.00 | Ca | 9.95 |
| FeO | 0.39 | 0.13 | 0.26–0.61 | Fe | 0.05 | 0.26 | 0.13 | 0.20–0.32 | Fe | 0.03 |
| MgO | 0.06 | 0.03 | 0.00–0.09 | Mg | 0.02 | 0.09 | 0.03 | 0.08–0.11 | Mg | 0.02 |
| Cl | 7.21 | 0.26 | 7.11–7.31 | Cl | 2.00 | 6.94 | 0.26 | 6.76–7.18 | Cl | 1.91 |
| H2O * | 0.00 | 0.08 | OH | 0.08 | ||||||
| –O=Cl | 1.63 | 1.57 | ||||||||
| Total | 99.47 | 99.95 | ||||||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Środek, D.; Juroszek, R.; Krüger, H.; Krüger, B.; Galuskina, I.; Gazeev, V. New Occurrence of Rusinovite, Ca10(Si2O7)3Cl2: Composition, Structure and Raman Data of Rusinovite from Shadil-Khokh Volcano, South Ossetia and Bellerberg Volcano, Germany. Minerals 2018, 8, 399. https://doi.org/10.3390/min8090399
Środek D, Juroszek R, Krüger H, Krüger B, Galuskina I, Gazeev V. New Occurrence of Rusinovite, Ca10(Si2O7)3Cl2: Composition, Structure and Raman Data of Rusinovite from Shadil-Khokh Volcano, South Ossetia and Bellerberg Volcano, Germany. Minerals. 2018; 8(9):399. https://doi.org/10.3390/min8090399
Chicago/Turabian StyleŚrodek, Dorota, Rafał Juroszek, Hannes Krüger, Biljana Krüger, Irina Galuskina, and Viktor Gazeev. 2018. "New Occurrence of Rusinovite, Ca10(Si2O7)3Cl2: Composition, Structure and Raman Data of Rusinovite from Shadil-Khokh Volcano, South Ossetia and Bellerberg Volcano, Germany" Minerals 8, no. 9: 399. https://doi.org/10.3390/min8090399






