Activation Mechanism of Lead Ions in Perovskite Flotation with Octyl Hydroxamic Acid Collector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Microflotation Experiments
2.3. Zeta-Potential Measurements
2.4. FT-IR Spectroscopy Analysis
2.5. Adsorption Tests
2.6. XPS Analysis
3. Results
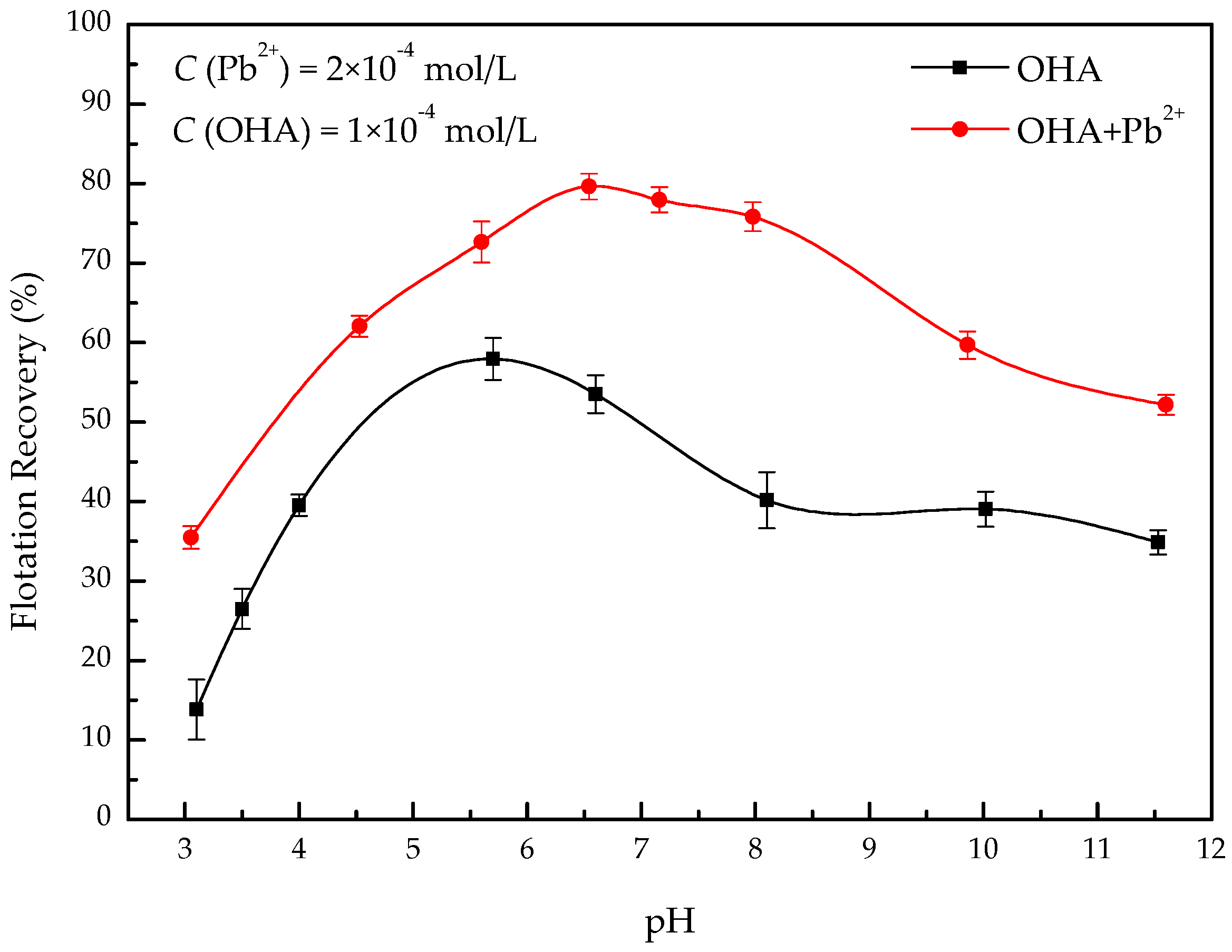
3.1. Microflotation Experiments
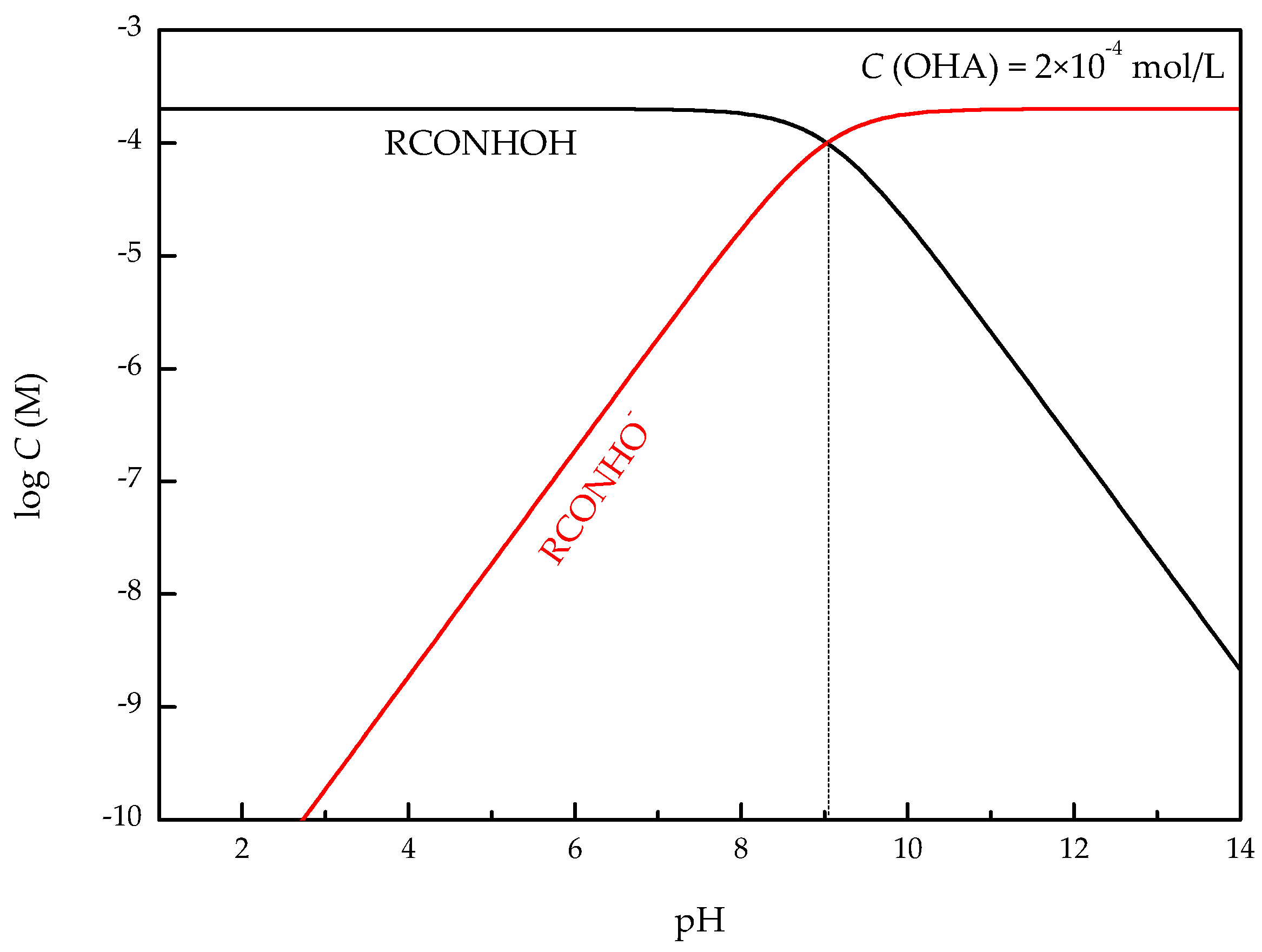
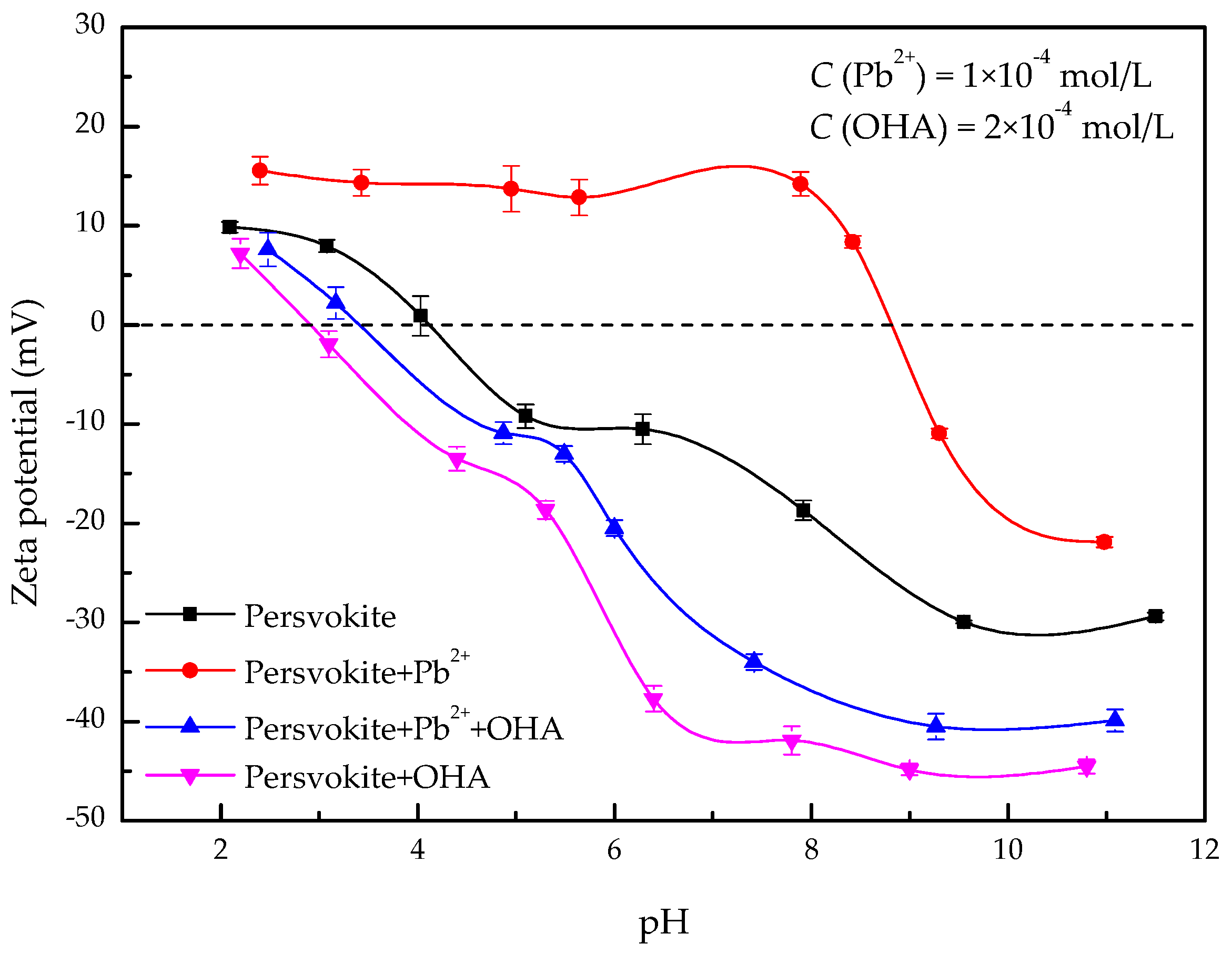
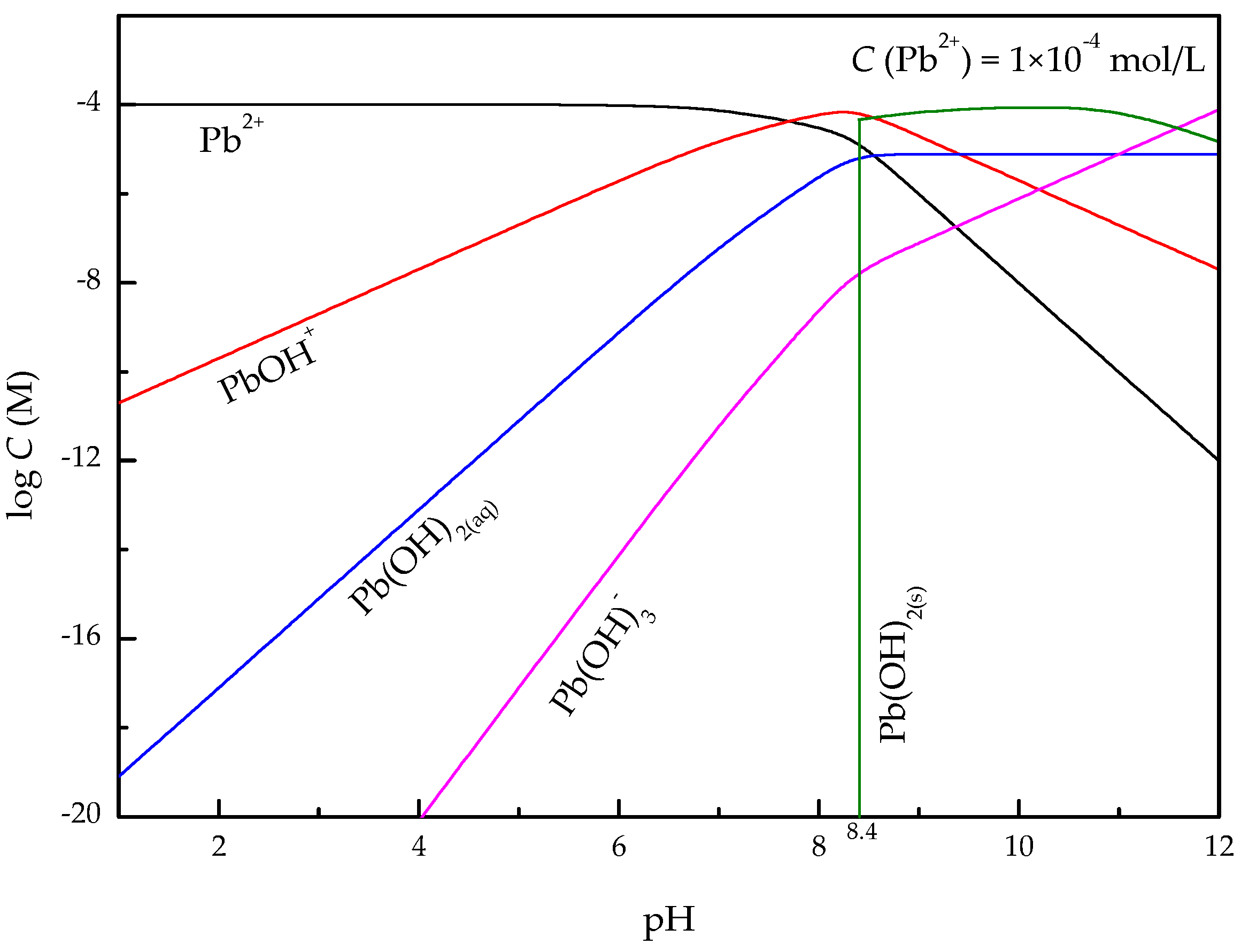
3.2. Zeta-Potential Measurements
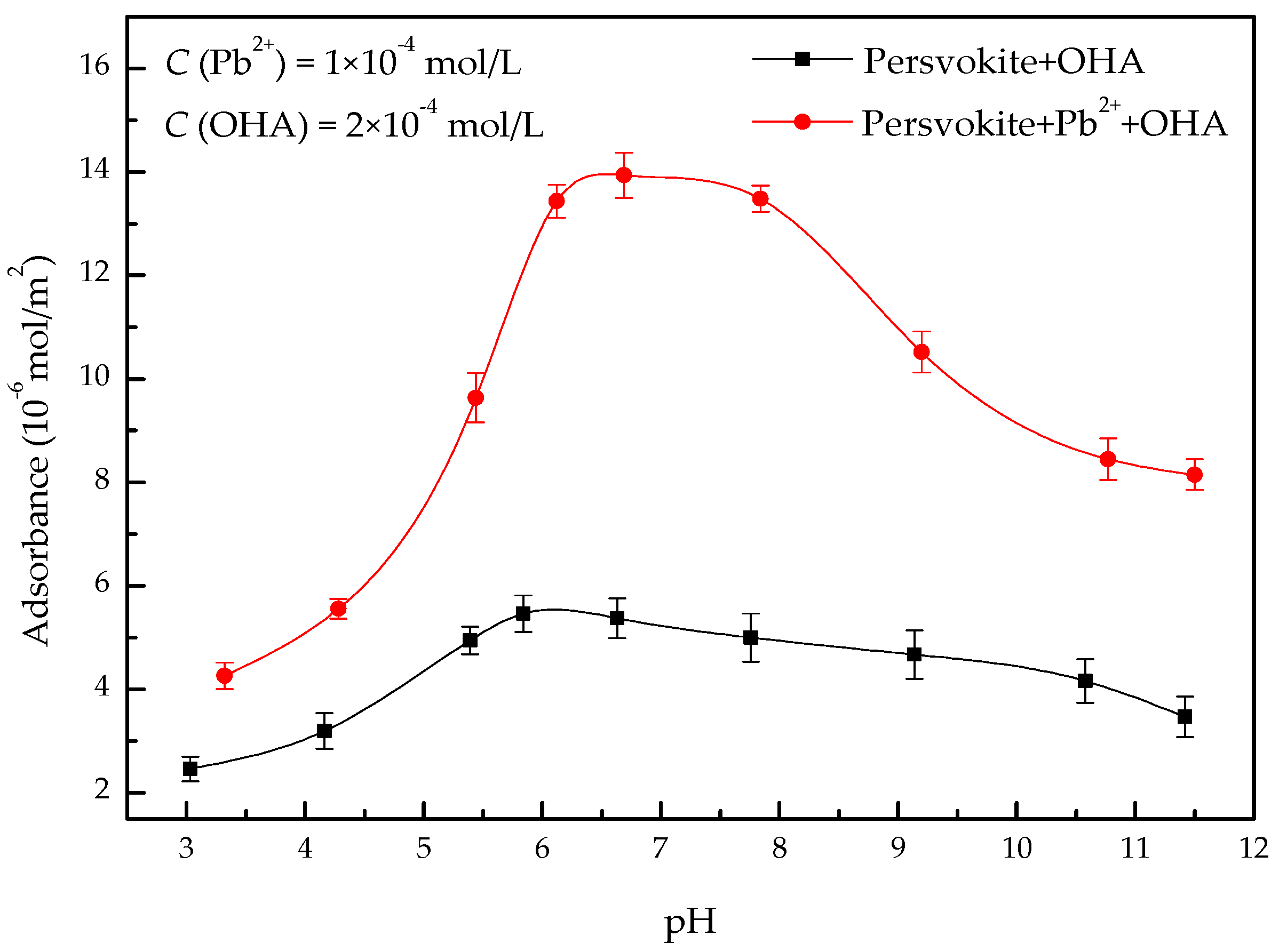
3.3. Adsorption Tests
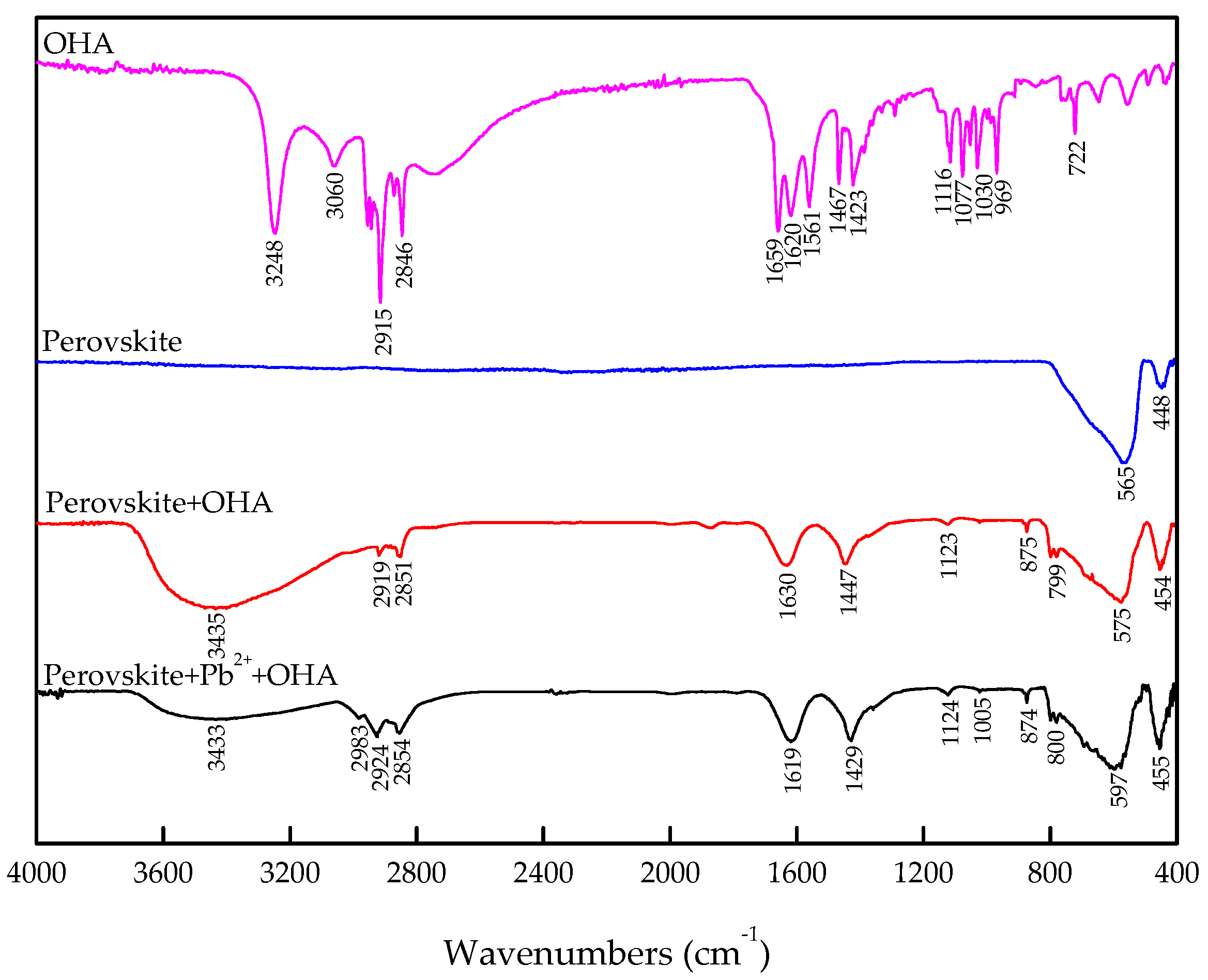
3.4. FT-IR Analysis
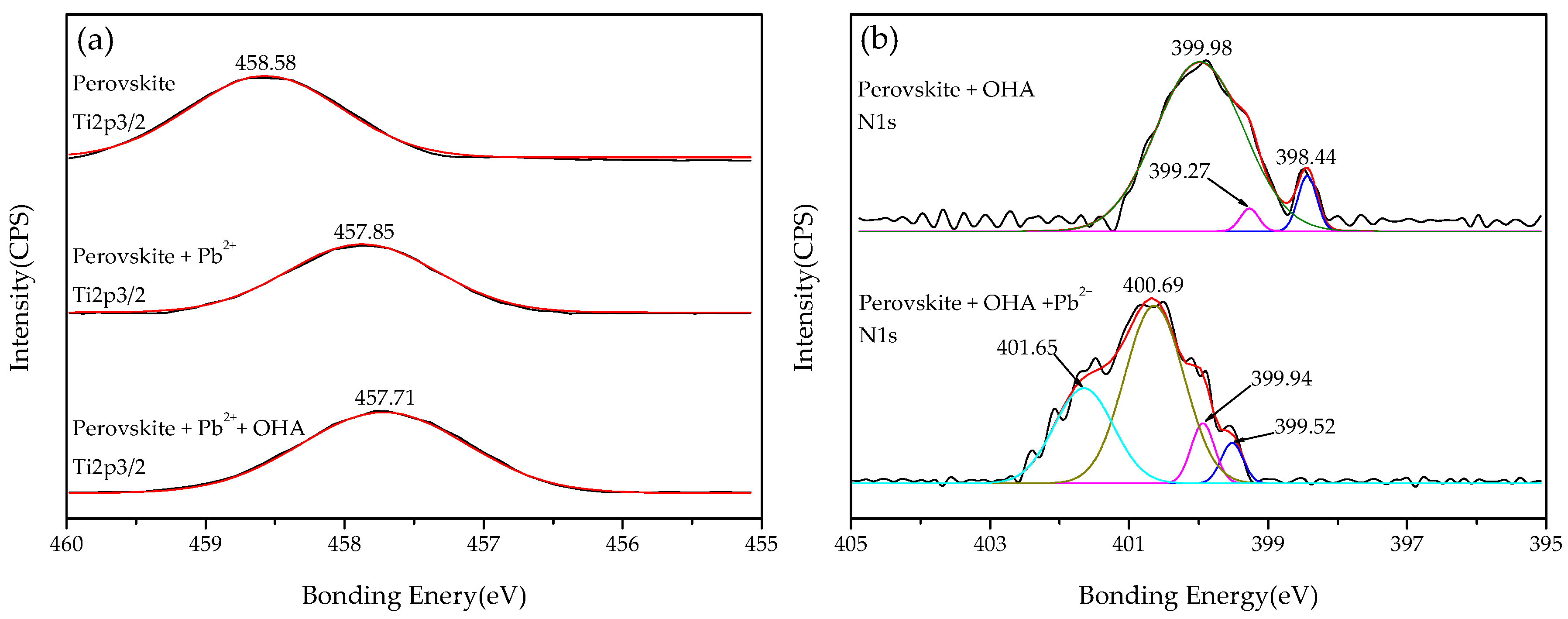
3.5. XPS Analysis
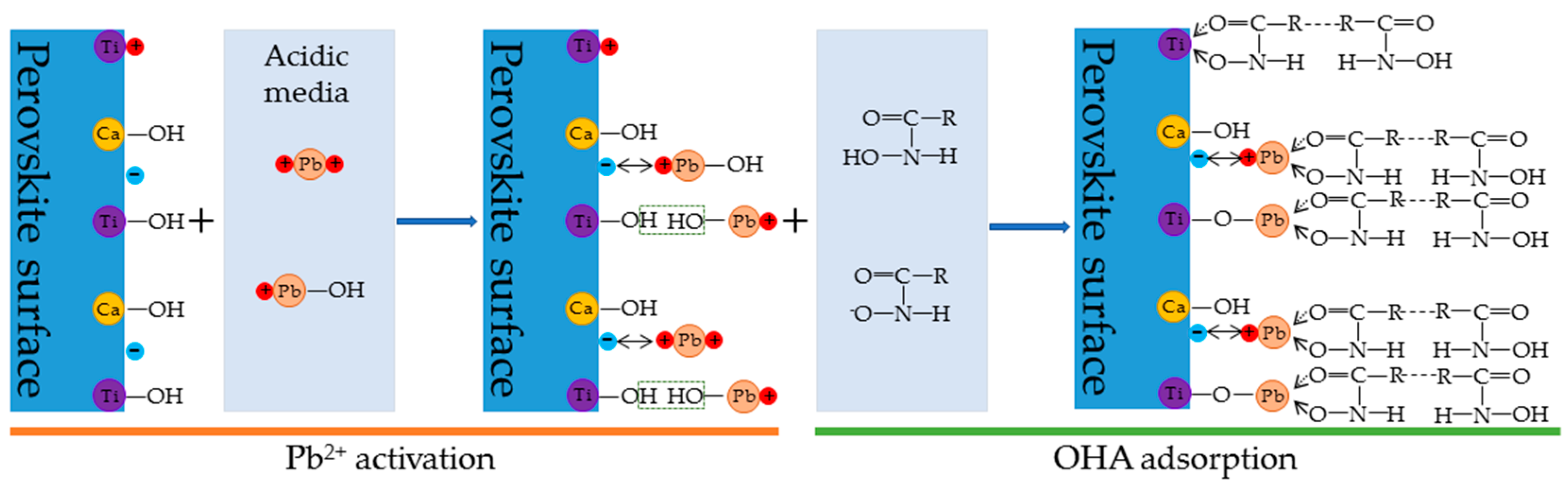
4. Discussion
5. Conclusions
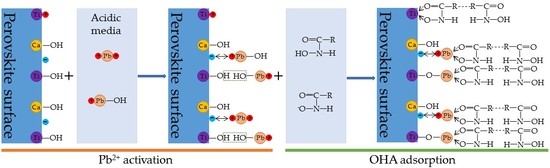
- In the present study, the effects of lead ions on the perovskite flotability and the activation mechanism of lead ions in perovskite flotation with an octyl hydroxamic acid collector were investigated using microflotation experiments, zeta-potential measurements, adsorption tests, FT-IR, and XPS analyses. The results of microflotation and adsorption tests indicate that the presence of Pb2+ can promote the adsorption of OHA on the perovskite surface and enhance the flotability of perovskite in a wide pH range. In addition, maximum recovery of 79.62% could be obtained at pH 6.5 in the presence of Pb2+.
- The zeta-potential shows that specific adsorption of OHA and lead species on the perovskite surface can occur. FT-IR and XPS measurements indicate that the adsorption of lead ions on the perovskite surface is mainly chemical adsorption. FT-IR analysis gives further evidence that the lead species react with titanium hydroxyl compounds on the perovskite surface to form lead complexes, which are the main active sites for OHA adsorption. Meanwhile, FT-IR and XPS analyses confirm that OHA chemisorbs on the surface of Pb2+-activated perovskite and forms hydrophobic Pb-OHA complexes, and then the nonpolar part of OHA species molecules will adsorb the nonpolar part of the former OHA species through hydrogen bonds to form multi-layer adsorption. This adsorption mode improves the flotability of perovskite.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bulatovic, S.; Wyslouzil, D.M. Process development for treatment of complex perovskite, ilmenite and rutile ores. Miner. Eng. 1999, 12, 1407–1417. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhao, L.S.; Qi, T.; Hu, G.P.; Zhao, H.X.; Li, J.; Wang, L.N. Desilication from titanium–vanadium slag by alkaline leaching. Trans. Nonferr. Met. Soc. 2013, 23, 3076–3082. [Google Scholar] [CrossRef]
- Samal, S.; Mohapatra, B.K.; Mukherjee, P.S.; Chatterjee, S.K. Integrated XRD, EPMA and XRF study of ilmenite and titania slag used in pigment production. J. Alloys Compd. 2009, 474, 484–489. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, Z.; Ye, X.; Encarnacion, J.; Reichow, M.K. Noble gas isotopic systematics of Fe–Ti–V oxide ore-related mafic–ultramafic layered intrusions in the Panxi area, China: The role of recycled oceanic crust in their petrogenesis. Geochim. Cosmochim. Acta 2011, 75, 6727–6741. [Google Scholar] [CrossRef]
- Kothari, N.C. Recent developments in processing ilmenite for titanium. Int. J. Miner. Process. 1974, 1, 287–305. [Google Scholar] [CrossRef]
- Noguchi, H.; Natsui, S.; Kikuchi, T.; Suzuki, R.O. Reduction of CaTiO3 by electrolysis in the molten salt CaCl2-CaO. Electrochemistry 2018, 86, 82–87. [Google Scholar] [CrossRef]
- Pan, F.; Zhu, Q.S.; Zhan, D.U.; Sun, H.Y. Oxidation kinetics, structural changes and element migration during oxidation process of vanadium-titanium magnetite ore. J. Iron Steel Res. Int. 2016, 23, 1160–1167. [Google Scholar] [CrossRef]
- Han, G.H.; Tao, J.; Zhang, Y.B.; Huang, Y.F.; Guang-Hui, L.I. High-temperature oxidation behavior of vanadium, titanium-bearing magnetite pellet. J. Iron Steel Res. Int. 2011, 18, 14–19. [Google Scholar] [CrossRef]
- He, S.; Sun, H.; Tan, D.G.; Peng, T. Recovery of titanium compounds from Ti-enriched product of alkali melting Ti-bearing blast furnace slag by dilute sulfuric acid leaching. Procedia Environ. Sci. 2016, 31, 977–984. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.N.; Wang, M.Y.; Li, G.Q.; Sui, Z.T. Recovery of titanium compounds from molten Ti-bearing blast furnace slag under the dynamic oxidation condition. Miner. Eng. 2007, 20, 684–693. [Google Scholar] [CrossRef]
- Niu, Y.; Du, X.; Yang, W.; Sui, Z. Flotation of the modified high titanium bf slag. J. Mater. Met. 2012, 11, 13–17. (In Chinese) [Google Scholar]
- Li, J.; Zhang, Z.T.; Wang, X.D. Precipitation behaviour of Ti enriched phase in Ti bearing slag. Ironmak. Steelmak. 2000, 39, 414–418. [Google Scholar] [CrossRef]
- Jiang, K.; Hu, X.; Ma, M.; Wang, D.; Qiu, G.; Jin, X.; Chen, G.Z. “Perovskitization”-assisted electrochemical reduction of solid TiO2 in molten CaCl2. Angew. Chem. Int. Ed. 2006, 45, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Lou, T.; Li, Y.H.; Li, L.S.; Sui, Z. Study of precipitation of perovskite phase from the oxide slag. Acta Metall. Sin. 2000, 36, 141–144. (In Chinese) [Google Scholar]
- Li, L.; Sui, Z. Physical chemistry behavior of enrichment selectivity of TiO2 in perovskite. Acta Phys.-Chim. Sin. 2001, 17, 845–849. (In Chinese) [Google Scholar]
- Wang, W.; Zhu, Y.; Zhang, S.; Deng, J.; Huang, Y.; Yan, W. Flotation behaviors of perovskite, titanaugite, and magnesium aluminate spinel using octyl hydroxamic acid as the collector. Minerals 2017, 7, 134. [Google Scholar] [CrossRef]
- Zhang, S. Study on High-Temperature Enrichment and Flotation Separation of Titanium Components from Ti-Bearing Blast Furnace Slag. Master’s Thesis, Southwest University of Science and Technology, Mianyang, Sichuan, China, 2017; pp. 43–47. (In Chinese). [Google Scholar]
- Meng, Q.; Feng, Q.; Shi, Q.; Ou, L. Studies on interaction mechanism of fine wolframite with octyl hydroxamic acid. Miner. Eng. 2015, 79, 133–138. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Liu, B.; Wang, X.; Du, H.; Liu, J.; Zheng, S.; Zhang, Y.; Miller, J.D. The surface features of lead activation in amyl xanthate flotation of quartz. Int. J. Miner. Process. 2016, 151, 33–39. [Google Scholar] [CrossRef]
- Crumbliss, A.L. Iron bioavailability and the coordination chemistry of hydroxamic acids. Coord. Chem. Rev. 1990, 105, 155–179. [Google Scholar] [CrossRef]
- Chen, G.L.; Tao, D.; Ren, H.; Ji, F.F.; Qiao, J.K. An investigation of niobite flotation with octyl diphosphonic acid as collector. Int. J. Miner. Process. 2005, 76, 111–122. [Google Scholar] [CrossRef]
- Agrawal, Y.K. Hydroxamic acids and their metal complexes. Russ. Chem. Rev. 1979, 48, 948–963. [Google Scholar] [CrossRef]
- Ni, X.; Liu, Q. The adsorption and configuration of octyl hydroxamic acid on pyrochlore and calcite. Colloids Surf. A 2012, 411, 80–86. [Google Scholar] [CrossRef]
- Abaka-Wood, G.B.; Addai-Mensah, J.; Skinner, W. A study of selective flotation recovery of rare earth oxides from hematite and quartz using hydroxamic acid as a collector. Adv. Powder Technol. 2018, 29, 1886–1899. [Google Scholar] [CrossRef]
- Pavez, O.; Brandao, P.R.G.; Peres, A.E.C. Adsorption of oleate and octyl-hydroxamate on to rare-earths minerals. Miner. Eng. 1996, 9, 357–366. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q.; Groppo, J.G. Flotation of monazite in the presence of calcite part I: Calcium ion effects on the adsorption of hydroxamic acid. Miner. Eng. 2017, 100, 40–48. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R.Q. Flotation of monazite in the presence of calcite part II: Enhanced separation performance using sodium silicate and EDTA. Miner. Eng. 2018. [Google Scholar] [CrossRef]
- Xu, L.; Tian, J.; Wu, H.; Lu, Z.; Yang, Y.; Sun, W.; Hu, Y. Effect of Pb2+ ions on ilmenite flotation and adsorption of benzohydroxamic acid as a collector. Appl. Surf. Sci. 2017, 425, 796–802. [Google Scholar] [CrossRef]
- Fan, X.; Rowson, N.A. The effect of Pb(NO3)2 on ilmenite flotation. Miner. Eng. 2000, 13, 205–215. [Google Scholar] [CrossRef]
- Li, H.; Mu, S.; Weng, X.; Zhao, Y.; Song, S. Rutile flotation with Pb2+ ions as activator: Adsorption of Pb2+ at rutile/water interface. Colloids Surf. A 2016, 506, 431–437. [Google Scholar] [CrossRef]
- Han, H.; Hu, Y.; Sun, W.; Li, X.; Cao, C.; Liu, R.; Yue, T.; Meng, X.; Guo, Y.; Wang, J. Fatty acid flotation versus BHA flotation of tungsten minerals and their performance in flotation practice. Int. J. Miner. Process. 2017, 159, 22–29. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Ma, W.; Meng, Q.; Chen, Y. Effects of lead ions on the flotation of hemimorphite using sodium oleate. Miner. Eng. 2016, 89, 163–167. [Google Scholar] [CrossRef]
- Yang, S.; Qiu, X.; Peng, T.; Chang, Z.; Feng, Q.; Zhong, C. Beneficial effects and mechanism of lead ion on wolframite flotation. Physicochem. Probl. Miner. Process. 2016, 52, 855–873. [Google Scholar]
- Kay, H.F.; Bailey, P.C. Structure and properties of CaTiO3. Acta Crystallogr. 1957, 10, 219–226. [Google Scholar] [CrossRef]
- Mehdilo, A.; Irannajad, M.; Rezai, B. Effect of crystal chemistry and surface properties on ilmenite flotation behavior. Int. J. Miner. Process. 2015, 137, 71–81. [Google Scholar] [CrossRef]
- Fan, X.; Waters, K.E.; Rowson, N.A.; Parker, D.J. Modification of ilmenite surface chemistry for enhancing surfactants adsorption and bubble attachment. J. Colloid Interface Sci. 2009, 329, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Kurzak, B.; Kozłowski, H.; Farkas, E.; Kurzak, B.; Kozłowski, H.; Farkas, E. Hydroxamic and aminohydroxamic acids and their complexes with metal ions. Coord. Chem. Rev. 1992, 114, 169–200. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A Physicochem. Eng. Asp. 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Marabini, A.M.; Ciriachi, M.; Plescia, P.; Barbaro, M. Chelating reagents for flotation. Miner. Eng. 2007, 20, 1014–1025. [Google Scholar] [CrossRef]
- Zhang, W.; Honaker, R. A fundamental study of octanohydroxamic acid adsorption on monazite surfaces. Int. J. Miner. Process. 2017, 164, 26–36. [Google Scholar] [CrossRef]
- Meng, Q.; Yuan, Z.; Yu, L.; Xu, Y.; Du, Y. Study on the activation mechanism of lead ions in the flotation of ilmenite using benzyl hydroxamic acid as collector. J. Ind. Eng. Chem. 2018, 62, 209–216. [Google Scholar] [CrossRef]
- Han, H.S.; Liu, W.L.; Hu, Y.H.; Sun, W.; Li, X.D. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb–BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Ouerd, A.; Alemany-Dumont, C.; Berthomé, G.; Normand, B.; Szunerits, S. Reactivity of titanium in physiological medium I. Electrochemical characterization of the metal/protein interface. J. Electrochem. Soc. 2007, 35, C593–C601. [Google Scholar] [CrossRef]
- Soccol, D.; Martens, J.; Claessens, S.; Fransaer, J. Effect of carbon modification of particles on their incorporation rate during electrodeposition. J. Electrochem. Soc. 2011, 158, D515–D523. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]


| Sample | TiO2 | CaO | Total |
|---|---|---|---|
| Perovskite | 58.77 | 41.23 | 100.00 |
| Sample | Surface Atomic Composition (%) | |||||
|---|---|---|---|---|---|---|
| C1s | Ti2p | Ca2p | O1s | Pb4f | N1s | |
| Perovskite | 43.18 | 11.02 | 7.72 | 36.69 | - | - |
| Perovskite (pH = 6.5) | 33.37 | 13.48 | 8.49 | 43.10 | - | - |
| Perovskite + Pb2+ (pH = 6.5) | 37.64 | 12.58 | 8.35 | 39.86 | 0.44 | |
| Perovskite + OHA (pH = 6.5) | 49.37 | 10.05 | 6.43 | 32.02 | 0.55 | |
| Perovskite + Pb2+ + OHA (pH = 6.5) | 58.44 | 8.08 | 5.33 | 26.24 | 0.52 | 0.86 |
| Sample | Binding Energy (eV) | |||||
|---|---|---|---|---|---|---|
| C1s | Ti2p | C1s | O1s | C1s | N1s | |
| Perovskite | 284.78 | 458.58 | 346.48 | 529.58 | - | - |
| Perovskite + Pb2+ | 284.77 | 457.85 | 346.55 | 529.93 | 138.50 | - |
| Perovskite + OHA | 284.73 | 458.07 | 346.57 | 529.83 | - | 399.98 |
| Perovskite + Pb2+ + OHA | 284.75 | 457.71 | 346.54 | 529.85 | 138.16 | 400.69 |
| Sample | Chemical Shift (eV) | |||||
|---|---|---|---|---|---|---|
| C1s | Ti2p | Ca2p | O1s | Pb4f | N1s | |
| Perovskite + Pb2+ | −0.01 | −0.73 | +0.07 | +0.35 | - | - |
| Perovskite + OHA | −0.05 | −0.51 | +0.09 | +0.25 | - | - |
| Perovskite + Pb2+ + OHA | −0.03 | −0.87 | +0.06 | +0.27 | −0.34 | +0.71 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Cui, Y.; Wang, W. Activation Mechanism of Lead Ions in Perovskite Flotation with Octyl Hydroxamic Acid Collector. Minerals 2018, 8, 341. https://doi.org/10.3390/min8080341
Zheng Y, Cui Y, Wang W. Activation Mechanism of Lead Ions in Perovskite Flotation with Octyl Hydroxamic Acid Collector. Minerals. 2018; 8(8):341. https://doi.org/10.3390/min8080341
Chicago/Turabian StyleZheng, Yu, Yating Cui, and Weiqing Wang. 2018. "Activation Mechanism of Lead Ions in Perovskite Flotation with Octyl Hydroxamic Acid Collector" Minerals 8, no. 8: 341. https://doi.org/10.3390/min8080341