Dynamic Disorder of Fe3+ Ions in the Crystal Structure of Natural Barioferrite
Abstract
:1. Introduction
2. Methods and Description
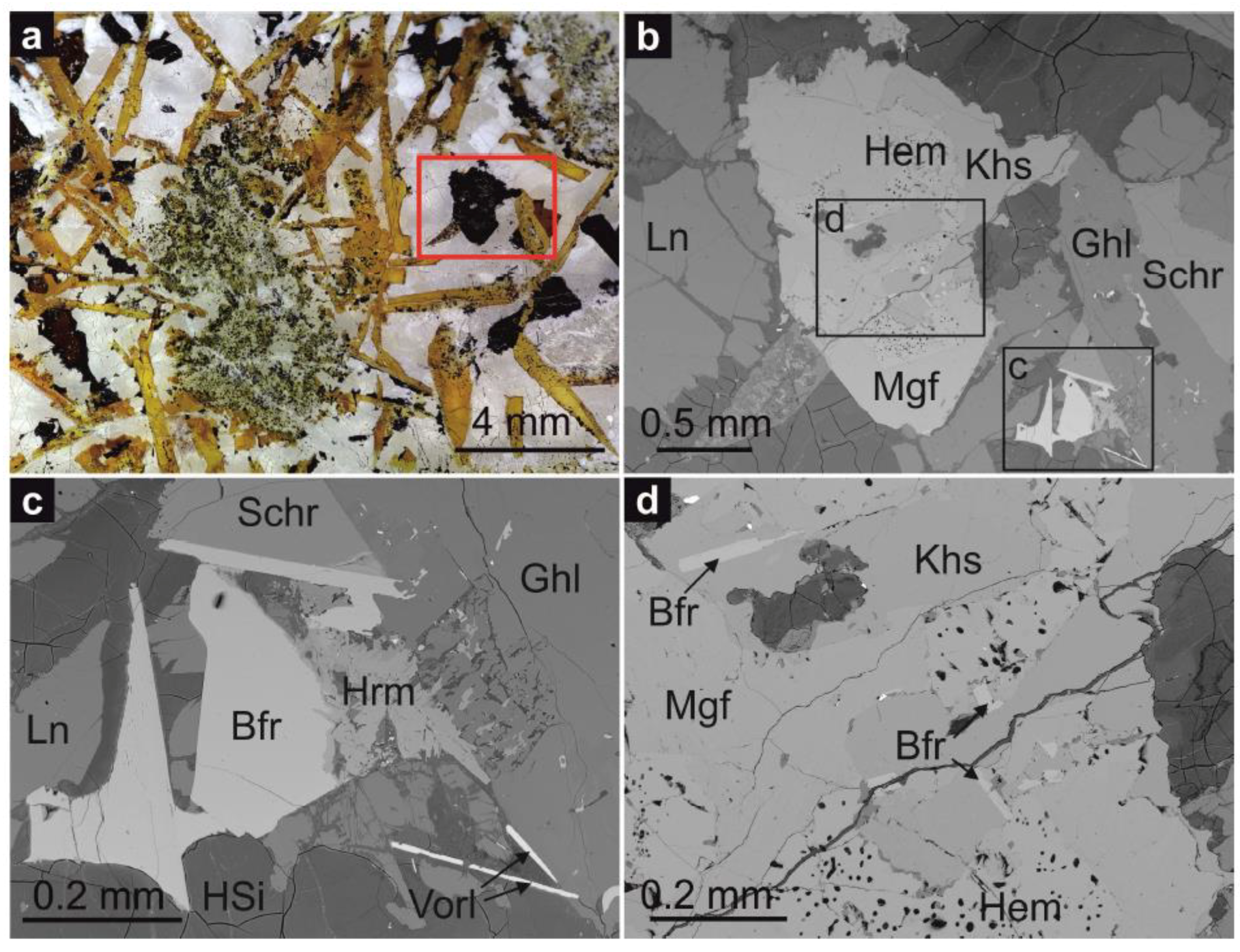
2.1. Geological Setting and Mineral Description
2.2. Methods of Investigation
2.3. Chemical Composition and Raman Spectroscopy
3. X-ray Diffraction Studies
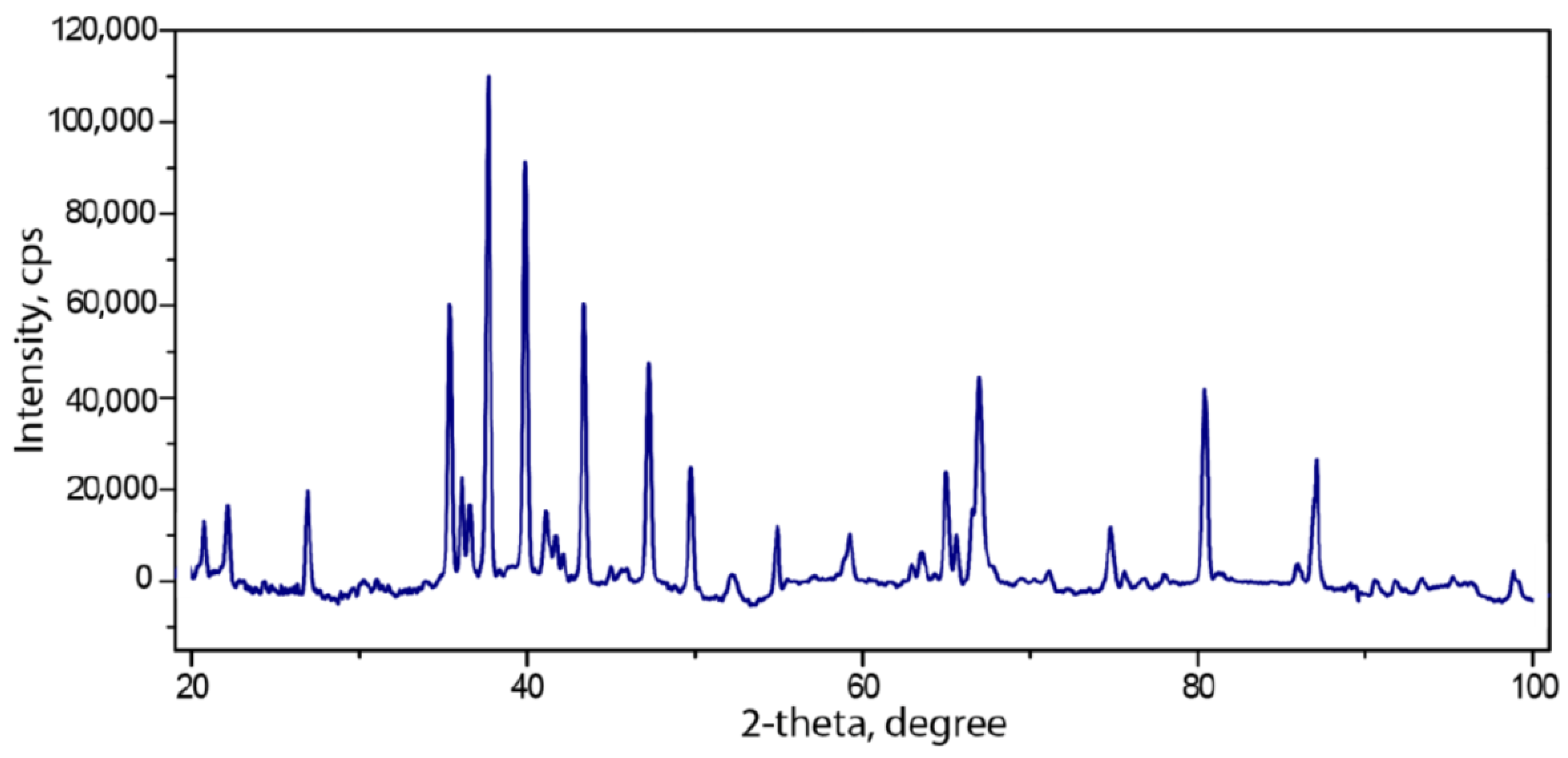
3.1. Powder Diffraction Data
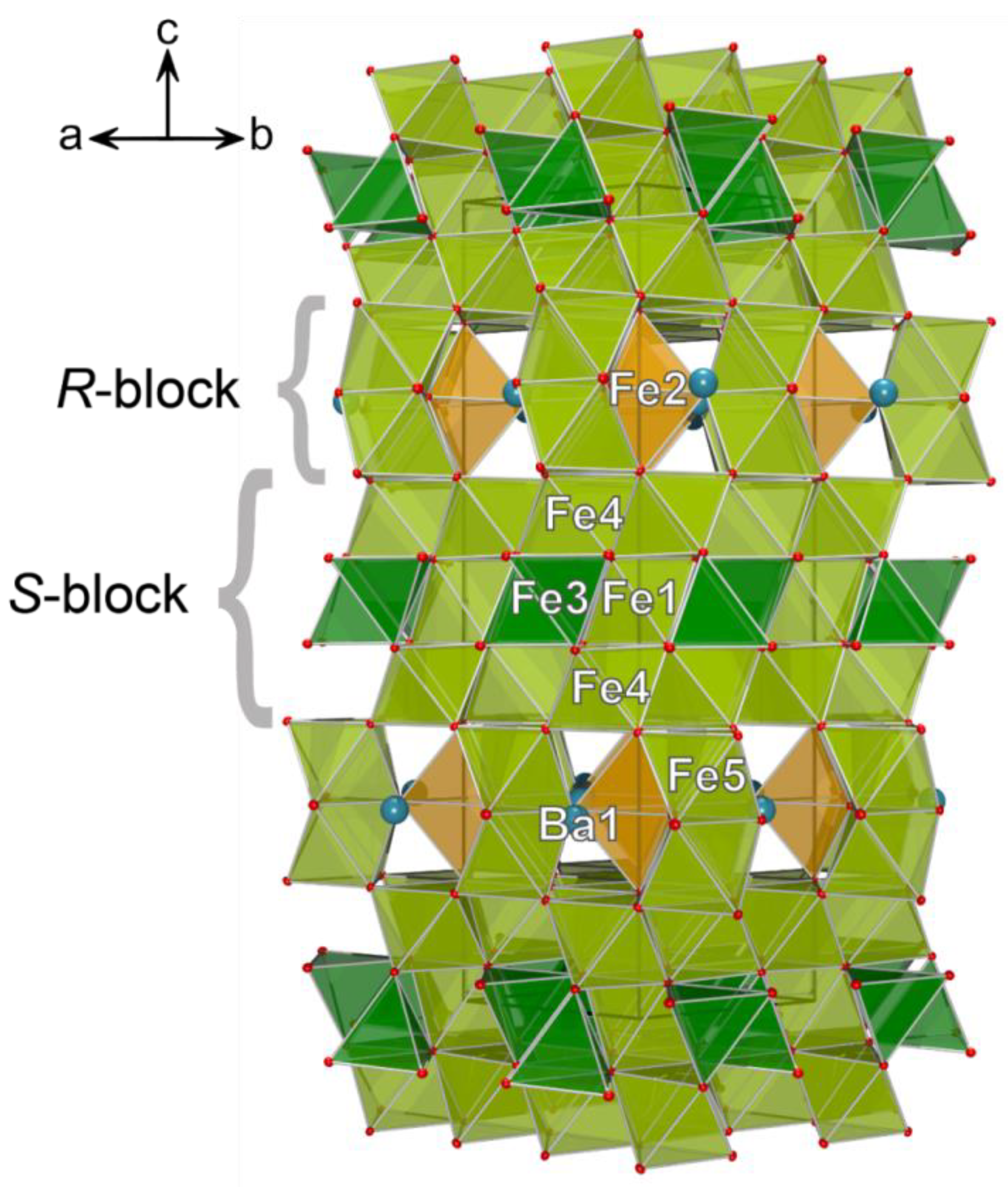
3.2. Single-Crystal X-ray Diffraction Data
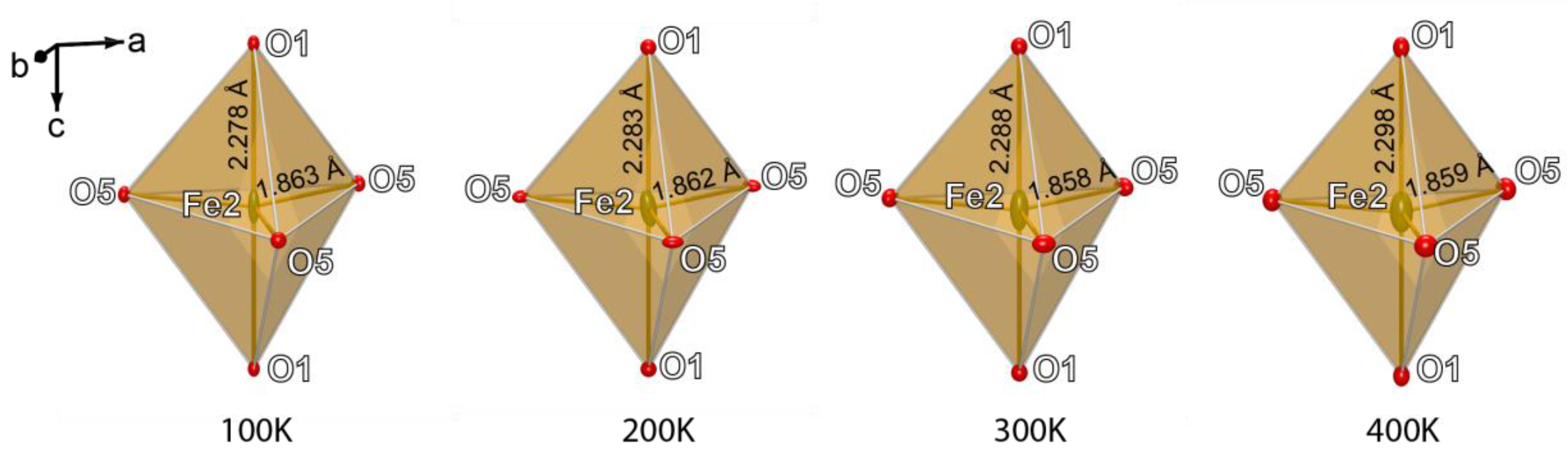
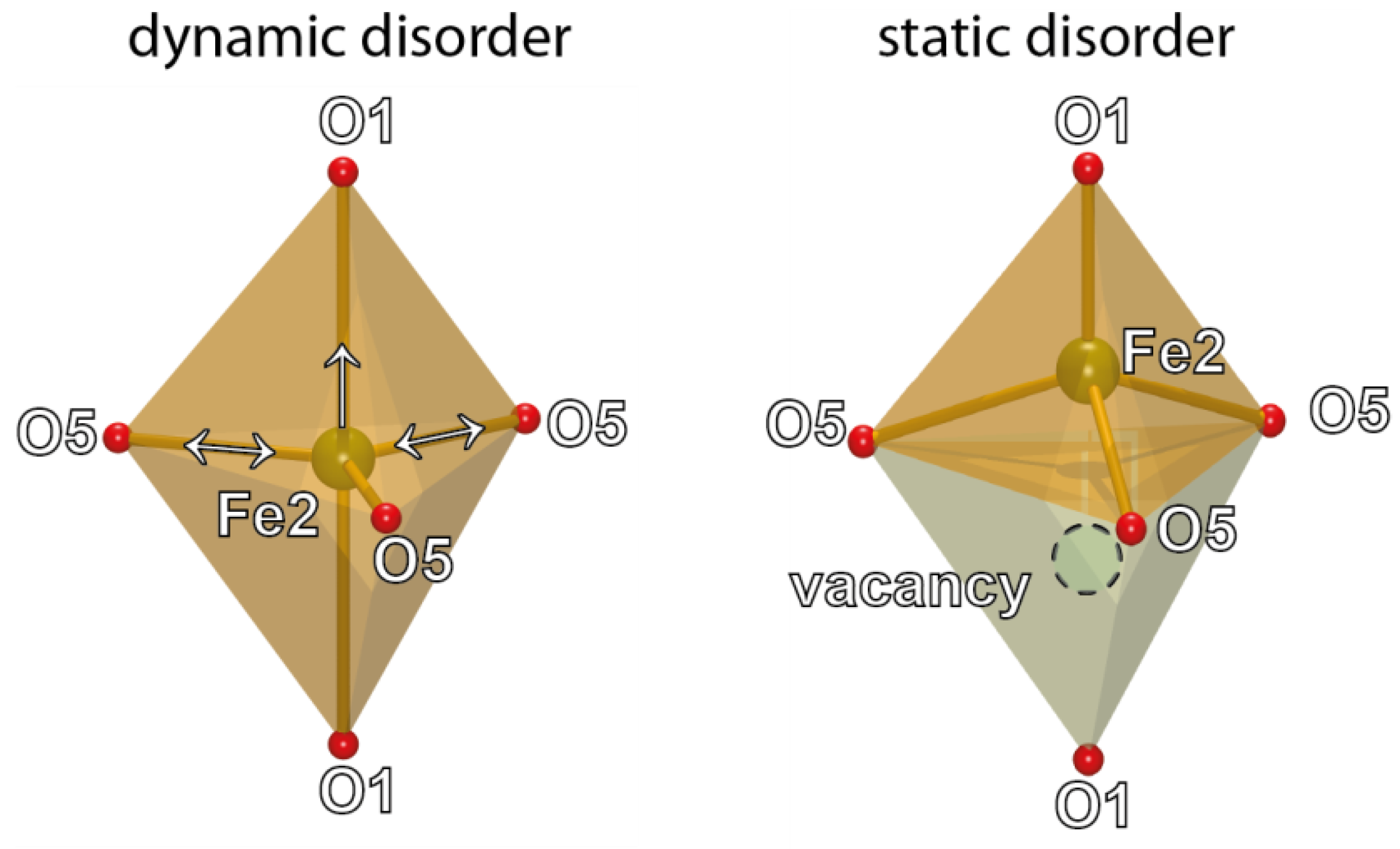
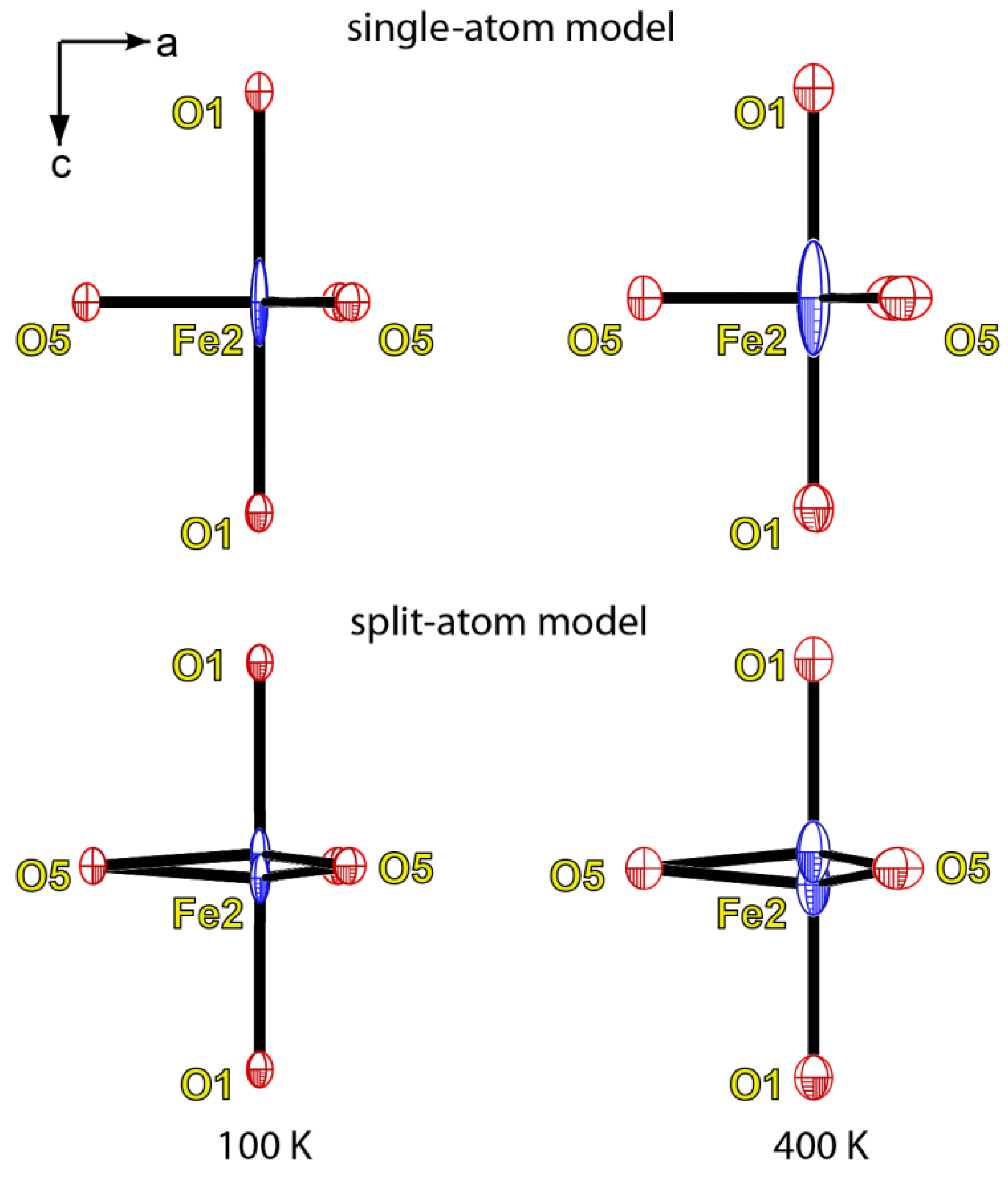
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murashko, M.N.; Chukanov, N.V.; Mukhanova, A.A.; Vapnik, E.; Britvin, S.N.; Polekhovsky, Y.S.; Ivakin, Y.D. Barioferrite BaFe12O19: A new mineral species of the magnetoplumbite group from the Haturim Formation in Israel. Geol. Ore Depos. 2011, 53, 558–563. [Google Scholar] [CrossRef]
- Obradors, X.; Collomb, A.; Pernet, M.; Samaras, D.; Joubert, J.C. X-ray analysis of the structural and dynamic properties of BaFe12O19 hexagonal ferrite at room temperature. J. Solid State Chem. 1985, 56, 171–181. [Google Scholar] [CrossRef]
- Bertaut, E.F.; Deschamps, A.; Pauthenet, R. Etude de la substitution de Fe par Al, Ga et Cr dans l’hexaferrite de baryum, BaO (Fe2O3)6. J. Phys. Radium 1959, 404–408. [Google Scholar] [CrossRef]
- Aleshko-Ozhevskii, O.P.; Faek, M.K.; Yamzin, I.I. A neutron diffraction study of the structure of magnetoplumbite. Sov. Phys. Crystallogr. 1969, 14, 367–369. [Google Scholar]
- Vinnik, D.A.; Zherebtsov, D.A.; Mashkovtseva, L.S.; Nemrava, S.; Perov, N.S.; Semisalova, A.S.; Krivtsov, I.V.; Isaenko, L.I.; Mikhailov, G.G.; Niewa, R. Ti-Substituted BaFe12O19 single crystal growth and characterization. Cryst. Growth Des. 2014, 14, 5834–5839. [Google Scholar] [CrossRef]
- Lengauer, C.L.; Tillmanns, E.; Hentschel, G. Batiferrite, Ba[Ti2Fe10]O19, a new ferrimagnetic magnetoplumbite-type mineral from the Quaternary volcanic rocks of the western Eifel area, Germany. Miner. Petrol. 2001, 71, 1–19. [Google Scholar] [CrossRef]
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Žáček, V.; Skála, R.; Chlupáčová, M.; Dvořák, Z. Ca–Fe3+-rich, Si-undersaturated buchite from Zelenky, North-Bohemian Brown Coal Basin, Czech Republic. Eur. J. Mineral. 2005, 17, 623–634. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Pakhomova, A.; Armbruster, T.; Vapnik, Y.; Włodyka, R.; Dzierżanowski, P.; Murashko, M. New minerals with a modular structure derived from hatrurite from the pyrometamorphic Hatrurim Complex. Part II. Zadovite, BaCa6[(SiO4)(PO4)](PO4)2F and aradite, BaCa6[(SiO4)(VO4)](VO4)2F, from paralavas of the Hatrurim Basin, Negev Desert, Israel. Mineral. Mag. 2015, 79, 1073–1087. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Vapnik, Y.; Prusik, K.; Stasiak, M.; Dzierżanowski, P.; Murashko, M.; Krivovichev, S. Gurimite, Ba3(VO4)2 and hexacelsian, BaAl2Si2O8—Two new minerals from schorlomite-rich paralava of the Hatrurim Complex, Negev Desert, Israel. Mineral. Mag. 2017, 81, 1009–1019. [Google Scholar] [CrossRef]
- Galuskina, I.O.; Galuskin, E.V.; Pakhomova, A.S.; Widmer, R.; Armbruster, T.; Krüger, B.; Grew, E.S.; Vapnik, Y.; Dzierażanowski, P.; Murashko, M. Khesinite, Ca4Mg2Fe3+10O4[(Fe3+ 10Si2)O36], a new rhönite-group (sapphirine supergroup) mineral from the Negev Desert, Israel—Natural analogue of the SFCA phase. Eur. J. Mineral. 2017, 101–116. [Google Scholar] [CrossRef]
- Picard, L. Geological Research in the Judean Desert; Goldberg’s Press: Jerusalem, Israel, 1931; p. 108. [Google Scholar]
- Kolodny, Y.; Gross, S. Thermal metamorphism by combustion of organic matter: Isotopic and petrological evidence. J. Geol. 1974, 82, 489–506. [Google Scholar] [CrossRef]
- Burg, A.; Starinsky, A.; Bartov, Y.; Kolodny, Y. Geology of the Hatrurim Formation (“Mottled Zone”) in the Hatruruim basin. Isr. J. Earth Sci. 1991, 40, 107–124. [Google Scholar]
- Burg, A.; Kolodny, Y.; Lyakhovsky, V. Hatrurim-2000: The “Mottled Zone” revisited, forty years later. Isr. J. Earth Sci. 2000, 48, 209–223. [Google Scholar]
- Townes, W.D.; Fang, J.H.; Perrotta, A.J. The crystal structure and refinement of ferrimagnetic barium ferrite, BaFe12O19. Z. Kristallogr. Cryst. Mater. 1967, 125, 437–449. [Google Scholar] [CrossRef]
- Aminoff, G. Über ein neues oxydisches mineral aus långban. (Magnetoplumbit.). Geologiska Föreningen i Stockholm Förhandlingar 1925, 47, 283–289. [Google Scholar] [CrossRef]
- Moore, P.B.; Gupta, P.K.S.; Le Page, Y. Magnetoplumbite, Pb2+Fe3+12O19: Refinement and lone-pair splitting. Am. Mineral. 1989, 74, 1186–1194. [Google Scholar]
- Shen, S.-P.; Chai, Y.-S.; Cong, J.-Z.; Sun, P.-J.; Lu, J.; Yan, L.-Q.; Wang, S.-G.; Sun, Y. Magnetic-ion-induced displacive electric polarization in FeO5 bipyramidal units of (Ba,Sr)Fe12O19 hexaferrites. Phys. Rev. B 2014, 90, 180404. [Google Scholar] [CrossRef]
- Bentor, Y.K. Lexique Stratigraphique International: Asie fascicule 10 c 2 Israel; Centre National de la Recherche Scientifique: Paris, France, 1960; Volume 3. [Google Scholar]
- Gross, S. The mineralogy of the Hatrurim Formation, Israel. Geol. Surv. Isr. Bull. 1977, 70, 1–80. [Google Scholar]
- Vapnik, Y.; Sharygin, V.V.; Sokol, E.V.; Shagam, R. Paralavas in a combustion metamorphic complex: Hatrurim Basin, Israel. In Geology of Coal Fires: Case Studies from Around the World; Geological Society of America: Boulder, CO, USA, 2007; Volume 18, pp. 133–153. ISBN 978-0-8137-4118-5. [Google Scholar]
- Novikov, I.; Vapnik, Y.; Safonova, I. Mud volcano origin of the Mottled Zone, South Levant. Geosci. Front. 2013, 4, 597–619. [Google Scholar] [CrossRef]
- Minster, T.; Yoffe, O.; Nathan, Y.; Flexer, A. Geochemistry, mineralogy, and paleoenvironments of deposition of the Oil Shale Member in the Negev. Isr. J. Earth Sci. 1997, 46, 41–59. [Google Scholar]
- Sokol, E.; Novikov, I.; Zateeva, S.; Vapnik, Y.; Shagam, R.; Kozmenko, O. Combustion metamorphism in the Nabi Musa dome: New implications for a mud volcanic origin of the Mottled Zone, Dead Sea area. Basin Res. 2010, 22, 414–438. [Google Scholar] [CrossRef]
- Sokol, E.V.; Kozmenko, O.A.; Kokh, S.N.; Vapnik, Y. Gas reservoirs in the Dead Sea area: Evidence from chemistry of combustion metamorphic rocks in Nabi Musa fossil mud volcano. Russ. Geol. Geophys. 2012, 3, 745–762. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Krüger, B.; Galuskina, I.O.; Krüger, H.; Vapnik, Y.; Wojdyla, J.A.; Murashko, M. New mineral with modular structure derived from hatruritefrom the pyrometamorphic rocks of the hatrurim complex: Ariegilatite, BaCa12(SiO4)4(PO4)2F2O, from Negev desert, Israel. Minerals 2018, 8, 109. [Google Scholar] [CrossRef]
- Sokol, E.V.; Seryotkin, Y.V.; Kokh, S.N.; Vapnik, Y.; Nigmatulina, E.N.; Goryainov, S.V.; Belogub, E.V.; Sharygin, V.V. Flamite, (Ca,Na,K)2(Si,P)O4, a new mineral from ultrahightemperature combustion metamorphic rocks, Hatrurim Basin, Negev Desert, Israel. Mineral. Mag. 2015, 79, 583–596. [Google Scholar] [CrossRef]
- Sharygin, V.V.; Lazic, B.; Armbruster, T.M.; Murashko, M.N.; Wirth, R.; Galuskina, I.O.; Galuskin, E.V.; Vapnik, Y.; Britvin, S.N.; Logvinova, A.M. Shulamitite Ca3TiFe3+AlO8—A new perovskite-related mineral from Hatrurim Basin, Israel. Eur. J. Mineral. 2013, 97–111. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Gfeller, F.; Krüger, B.; Kusz, J.; Vapnik, Y.; Dulski, M.; Dzierżanowski, P. Silicocarnotite, Ca5[(SiO4)(PO4)](PO4), a new “old” mineral from the Negev Desert, Israel, and the ternesite–silicocarnotite solid solution: Indicators of high-temperature alteration of pyrometamorphic rocks of the Hatrurim Complex, Southern Levant. Eur. J. Mineral. 2016, 28, 105–123. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Gfeller, F.; Galuskina, I.O.; Armbruster, T.; Krzątała, A.; Vapnik, Y.; Kusz, J.; Dulski, M.; Gardocki, M.; Gurbanov, A.G.; et al. New minerals with a modular structure derived from hatrurite from the pyrometamorphic rocks. Part III. Gazeevite, BaCa6(SiO4)2(SO4)2O, from Israel and the Palestine Autonomy, South Levant, and from South Ossetia, Greater Caucasus. Mineral. Mag. 2017, 81, 499–513. [Google Scholar] [CrossRef]
- Pouchou, J.L.; Pichoir, F. Quantitative Analysis of Homogeneous or Stratified Microvolumes Applying the Model “PAP”. In Electron Probe Quantitation; Springer: Boston, MA, USA, 1991; pp. 31–75. ISBN 978-1-4899-2619-7. [Google Scholar]
- Britvin, S.N.; Dolivo-Dobrovolsky, D.V.; Krzhizhanovskaya, M.G. Software for processing the X-ray powder diffraction data obtained from the curved image plate detector of Rigaku RAXIS Rapid II diffractometer. Proc. Russ. Mineral. Soc. 2017, 146, 104–107. [Google Scholar]
- Galuskina, I.O.; Vapnik, Y.; Lazic, B.; Armbruster, T.; Murashko, M.; Galuskin, E.V. Harmunite CaFe2O4: A new mineral from the Jabel Harmun, West Bank, Palestinian Autonomy, Israel. Am. Mineral. 2014, 99, 965–975. [Google Scholar] [CrossRef]
- Kreisel, J.; Lucazeau, G.; Vincent, H. Raman Spectra and Vibrational Analysis of BaFe12O19Hexagonal Ferrite. J. Solid State Chem. 1998, 137, 127–137. [Google Scholar] [CrossRef]
- Kumar, S.; Supriya, S.; Pandey, R.; Pradhan, L.K.; Singh, R.K.; Kar, M. Effect of lattice strain on structural and magnetic properties of Ca substituted barium hexaferrite. J. Magn. Magn. Mater. 2018, 458, 30–38. [Google Scholar] [CrossRef]
- Kreisel, J.; Lucazeau, G.; Vincent, H. Raman study of substituted barium ferrite single crystals, BaFe12−2xMexCoxO19 (Me = Ir, Ti). J. Raman Spectrosc. 1999, 30, 115–120. [Google Scholar] [CrossRef]
- Silva Júnior, F.M.; Paschoal, C.W.A. Spin-phonon coupling in BaFe12O19 M-type hexaferrite. J. Appl. Phys. 2015, 116, 244110. [Google Scholar] [CrossRef]
- Ganapathi, L.; Gopalakrishnan, J.; Rao, C.N.R. Barium hexaferrite (M-phase) exhibiting superstructure. Mater. Res. Bull. 1984, 19. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Agilent Technologies. CrysAlisCCD and CrysAlis Red; Oxford Diffraction Ltd.: Oxford, UK, 2014. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Nagashima, M.; Armbruster, T.; Hainschwang, T. A temperature-dependent structure study of gem-quality hibonite from Myanmar. Mineral. Mag. 2010, 74, 871–885. [Google Scholar] [CrossRef]
- Siegrist, T.; Vanderah, T.A. Combining magnets and dielectrics: Crystal chemistry in the BaO-Fe2O3-TiO2 System. Eur. J. Inorg. Chem. 2003, 1483–1501. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- An, S.Y.; Shim, I.-B.; Kim, C.S. Mössbauer and magnetic properties of Co-Ti substituted barium hexaferrite nanoparticles. J. Appl. Phys. 2002, 91, 8465–8467. [Google Scholar] [CrossRef]
- Hofmeister, A.M.; Wopenka, B.; Locock, A.J. Spectroscopy and structure of hibonite, grossite, and CaAl2O4: Implications for astronomical environments. Geochim. Cosmochim. Acta 2004, 68, 4485–4503. [Google Scholar] [CrossRef]
- Bermanec, V.; Holtstam, D.; Sturman, D.; Criddle, A.J.; Back, M.E.; Scavnicar, S. Nezilovite, a new member of the magnetoplumbite group, and the crystal chemistry of magnetoplumbite and hibonite. Can. Mineral. 1996, 34, 1287–1297. [Google Scholar]
- Ardit, M.; Borcănescu, S.; Cruciani, G.; Dondi, M.; Lazău, I.; Păcurariu, C.; Zanelli, C. Ni–Ti codoped hibonite ceramic pigments by combustion synthesis: Crystal structure and optical properties. J. Am. Ceram. Soc. 2016, 99, 1749–1760. [Google Scholar] [CrossRef]
- Giannini, M.; Ballaran, T.B.; Langenhorst, F. Crystal chemistry of synthetic Ti–Mg-bearing hibonites: A single-crystal X-ray study. Am. Mineral. 2014, 99, 2060–2067. [Google Scholar] [CrossRef]
- Kitagawa, Y.; Hiraoka, Y.; Honda, T.; Ishikura, T.; Nakamura, H.; Kimura, T. Low-field magnetoelectric effect at room temperature. Nat. Mater. 2010, 9, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Graetsch, H.; Gebert, W. Positional and thermal disorder in the trigonal bipyramid of magnetoplumbite structure type SrGa12O19. Z. Kristallogr. 1994, 209, 338–342. [Google Scholar] [CrossRef]
- Galuskin, E.V.; Galuskina, I.O.; Widmer, R.; Armbruster, T. First natural hexaferrite with mixed β‴-ferrite (β-alumina) and magnetoplumbite structure from Jabel Harmun, Palestinian Autonomy. Eur J. Mineral. 2018. [Google Scholar] [CrossRef]


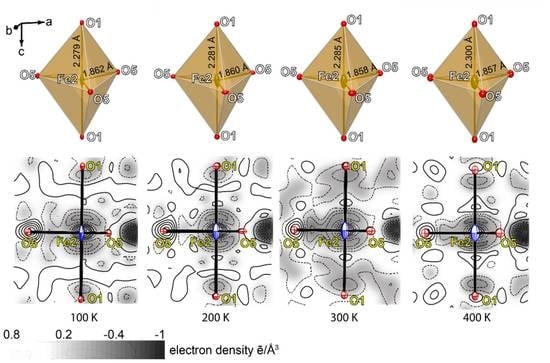
| Identification Code | 100 K | 200 K | 300 K | 400 K |
|---|---|---|---|---|
| Temperature (K) | 100(2) | 200(2) | 300(2) | 400(2) |
| Crystal system, Z | Hexagonal | |||
| Space group | P63/mmc | |||
| a (Å) | 5.8920(2) | 5.8901(2) | 5.8901(2) | 5.8992(2) |
| c (Å) | 23.1092(7) | 23.1171(7) | 23.1235(6) | 23.1891(9) |
| Volume (Å3) | 694.78(5) | 694.57(4) | 694.75(4) | 698.87(6) |
| ρcalc (g/cm3) | 5.183 | 5.184 | 5.183 | 5.152 |
| μ (mm−1) | 14.131 | 14.136 | 14.132 | 14.049 |
| F(000) | 1017.0 | 1017.0 | 1017.0 | 1017.0 |
| Crystal size (mm3) | 0.20 × 0.09 × 0.07 | |||
| Radiation | Mo Kα (λ = 0.71073) | |||
| 2θ range (°) | 7.054–64.038 | 7.05–63.804 | 7.048–61.54 | 7.028–64.436 |
| hkl range | −8 ≤ h ≤ 8, −6 ≤ k ≤ 6, −33 ≤ l ≤ 33 | −8 ≤ h ≤ 8, −8 ≤ k ≤ 8, −32 ≤ l ≤ 32 | −7 ≤ h ≤ 7, −8 ≤ k ≤ 8, −32 ≤ l ≤ 28 | −8 ≤ h ≤ 8, −7 ≤ k ≤ 8, −32 ≤ l ≤ 32 |
| Reflections collected | 3907 | 6363 | 6065 | 6804 |
| Independent reflections | 501 [Rint = 0.0176, Rsigma = 0.0106] | 505 [Rint = 0.0300, Rsigma = 0.0148] | 461 [Rint = 0.0281, Rsigma = 0.0121] | 519 [Rint = 0.0279, Rsigma = 0.0118] |
| Data/restraints/parameters | 501/0/42 | 508/0/42 | 461/0/42 | 519/0/42 |
| GooF | 1.229 | 1.270 | 1.288 | 1.268 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0142, wR2 = 0.0364 | R1 = 0.0207, wR2 = 0.0493 | R1 = 0.0161, wR2 = 0.0425 | R1 = 0.0173, wR2 = 0.0433 |
| Final R indexes [all data] | R1 = 0.0164, wR2 = 0.0372 | R1 = 0.0234, wR2 = 0.0506 | R1 = 0.0179, wR2 = 0.0431 | R1 = 0.0193, wR2 = 0.0440 |
| Largest difference peak/hole (e Å−3) | 0/53/−0.56 | 1.13/−0.99 | 0.43/−0.89 | 0.79/−1.16 |
| Component (wt %) | Mean (n = 17) | Range | Standard Deviation. | Calculated on 19 O | |
|---|---|---|---|---|---|
| TiO2 | 3.05 | 2.90–3.27 | 0.09 | Ba | 0.85 |
| SiO2 | 0.05 | 0.00–0.10 | 0.02 | Ca | 0.12 |
| Fe2O3 | 80.47 | 78.89–81.53 | 0.72 | Sr | 0.03 |
| Al2O3 | 2.22 | 2.11–2.38 | 0.06 | Asite | 1 |
| BaO | 12.32 | 11.56–13.41 | 0.46 | Fe3+ | 10.72 |
| MgO | 0.58 | 0.51–0.68 | 0.05 | Al | 0.46 |
| CaO | 1.03 | 0.83–1.32 | 0.17 | Ti | 0.41 |
| MnO | 0.18 | 0.11–0.23 | 0.04 | Mg | 0.15 |
| SrO | 0.32 | 0.14–0.43 | 0.07 | Cu2+ | 0.09 |
| CuO | 0.65 | 0.48–0.84 | 0.11 | Ca | 0.08 |
| ZnO | 0.33 | 0.19–0.47 | 0.08 | Zn | 0.04 |
| 101.2 | 100.09–101.69 | Mn2+ | 0.03 | ||
| Si | 0.01 | ||||
| Msites | 11.99 | ||||
| Temperature: | 100 K | 200 K | ||||||||
| Site | population | x/a | y/b | z/c | Ueq | Population | x/a | y/b | z/c | Ueq |
| Ba1 | Ba0.89Ca0.011 | 2/3 | 1/3 | 1/4 | 0.0038(1) | Ba0.89Ca0.011 | 2/3 | 1/3 | 1/4 | 0.0063(1) |
| Fe1 | Fe0.69Al0.31 | 0 | 0 | 0 | 0.0020(3) | Fe0.69Al0.31 | 0 | 0 | 0 | 0.0038(2) |
| Fe2 | Fe0.96Ti0.04 | 0 | 0 | 1/4 | 0.0116(2) | Fe0.96Ti0.04 | 0 | 0 | 1/4 | 0.0154(2) |
| Fe3 | Fe0.94Mg0.06 | 1/3 | 2/3 | 0.02730(3) | 0.0025(1) | Fe0.94Mg0.06 | 1/3 | 2/3 | 0.02729(3) | 0.0043(2) |
| Fe4 | Fe0.94Ti0.06 | 0.33617(8) | 0.16809(4) | 0.10792(2) | 0.0026(1) | Fe0.94Ti0.06 | 0.33622(7) | 0.16811(4) | 0.10789(2) | 0.0044(1) |
| Fe5 | Fe | 1/3 | 2/3 | 0.18977(3) | 0.0031(1) | Fe | 1/3 | 2/3 | 0.18974(3) | 0.0047(2) |
| O1 | O | 0 | 0 | 0.1514(1) | 0.0043(5) | O | 0 | 0 | 0.15126(14) | 0.0057(6) |
| O2 | O | 2/3 | 1/3 | 0.0555(1) | 0.0037(5) | O | 2/3 | 1/3 | 0.05564(15) | 0.0054(6) |
| O3 | O | 0.5022(2) | 0.0043(3) | 0.1496(1) | 0.0035(3) | O | 0.5022(2) | 0.0044(4) | 0.14967(8) | 0.0056(4) |
| O4 | O | 0.1543(2) | 0.3086(3) | 0.05198(5) | 0.0050(3) | O | 0.15399(19) | 0.3080(4) | 0.05197(9) | 0.0062(4) |
| O5 | O | 0.1825(2) | 0.3651(5) | 1/4 | 0.0051(4) | O | 0.1825(3) | 0.3649(5) | 1/4 | 0.0066(5) |
| Temperature: | 300 K | 400 K | ||||||||
| Site | population | x/a | y/b | z/c | Ueq | population | x/a | y/b | z/c | Ueq |
| Ba1 | Ba0.89Ca0.011 | 2/3 | 1/3 | 1/4 | 0.0082(1) | Ba0.89Ca0.011 | 2/3 | 1/3 | 1/4 | 0.0104(1) |
| Fe1 | Fe0.69Al0.31 | 0 | 0 | 0 | 0.0049(2) | Fe0.69Al0.31 | 0 | 0 | 0 | 0.0063(2) |
| Fe2 | Fe0.96Ti0.04 | 0 | 0 | 1/4 | 0.0184(3) | Fe0.96Ti0.04 | 0 | 0 | 1/4 | 0.0222(3) |
| Fe3 | Fe0.94Mg0.06 | 1/3 | 2/3 | 0.0272(3) | 0.0052(2) | Fe0.94Mg0.06 | 1/3 | 2/3 | 0.02729(3) | 0.0066(1) |
| Fe4 | Fe0.94Ti0.06 | 0.33623(7) | 0.16811(4) | 0.10789(2) | 0.00563(14) | Fe0.94Ti0.06 | 0.33624(7) | 0.16812(4) | 0.10789(2) | 0.00720(11) |
| Fe5 | Fe | 1/3 | 2/3 | 0.18975(3) | 0.0055(1) | Fe | 1/3 | 2/3 | 0.18972(3) | 0.00744(14) |
| O1 | O | 0 | 0 | 0.15107(13) | 0.0063(6) | O | 0 | 0 | 0.1509(1) | 0.0079(6) |
| O2 | O | 2/3 | 1/3 | 0.0557(1) | 0.0063(6) | O | 2/3 | 1/3 | 0.0557(1) | 0.0076(6) |
| O3 | O | 0.5022(2) | 0.0044(3) | 0.14958(7) | 0.0064(4) | O | 0.5024(2) | 0.0048(4) | 0.14951(8) | 0.0083(3) |
| O4 | O | 0.1541(2) | 0.3082(4) | 0.05206(8) | 0.0073(4) | O | 0.1542(2) | 0.3084(4) | 0.05187(8) | 0.0088(3) |
| O5 | O | 0.1821(3) | 0.3643(5) | 1/4 | 0.0080(5) | O | 0.1820(3) | 0.3639(5) | 1/4 | 0.0098(5) |
| Temperature Bond | 100 K Mean | Temperature Bond | 200 K Mean | Temperature Bond | 300 K Mean | Temperature Bond | 400 K Mean | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ba1‒O3 | x6 | 2.8624(16) | Ba1‒O3 | x6 | 2.8624(19) | Ba1‒O3 | x6 | 2.8646(18) | Ba1‒O3 | x6 | 2.8711(18) |
| Ba1‒O5 | x6 | 2.9505(2) | Ba1‒O5 | x6 | 2.9495(2) | Ba1‒O5 | x6 | 2.9493(16) | Ba1‒O5 | x6 | 2.9537(2) |
| <Ba1‒O> | 2.9061 | <Ba1‒O> | 2.906 | <Ba1‒O> | 2.907 | <Ba1‒O> | 2.913 | ||||
| Fe1‒O4 | x6 | 1.980(2) | Fe1‒O4 | x6 | 1.9782(19) | Fe1‒O4 | x6 | 1.9796(19) | Fe1‒O4 | x6 | 1.9822(18) |
| Fe2‒O1 | x2 | 2.278(3) | Fe2‒O1 | x2 | 2.283(3) | Fe2‒O1 | x2 | 2.288(3) | Fe2‒O1 | x2 | 2.298(3) |
| Fe2‒O5 | x3 | 1.863(2) | Fe2‒O5 | x3 | 1.862(3) | Fe2‒O5 | x3 | 1.858(3) | Fe2‒O5 | x3 | 1.859(3) |
| <Fe2‒O> | 2.028 | <Fe2‒O> | 2.030 | <Fe2‒O> | 2.041 | <Fe2‒O> | 2.034 | ||||
| Fe3‒O2 | 1.914(3) | Fe3‒O2 | 1.917(4) | Fe3‒O2 | 1.916(2) | Fe3‒O2 | 1.922(3) | ||||
| Fe3‒O4 | x3 | 1.914(2) | Fe3‒O4 | x3 | 1.917(2) | Fe3‒O4 | x3 | 1.915(4) | Fe3‒O4 | x3 | 1.9167(19) |
| <Fe3‒O> | 1.914 | <Fe3‒O> | 1.917 | <Fe3‒O> | 1.917 | <Fe3‒O> | 1.919 | ||||
| Fe4‒O1 | 1.9886(5) | Fe4‒O1 | 1.9867(17) | Fe4‒O1 | 1.9847(16) | Fe4‒O1 | 1.9866(17) | ||||
| Fe4‒O2 | 2.0757(17) | Fe4‒O2 | 2.074(2) | Fe4‒O2 | 2.0750(19) | Fe4‒O2 | 2.0783(19) | ||||
| Fe4‒O3 | x2 | 1.9399(10) | Fe4‒O3 | x2 | 1.9394(12) | Fe4‒O3 | x2 | 1.9385(12) | Fe4‒O3 | x2 | 1.9406(12) |
| Fe4‒O4 | x2 | 2.0956(12) | Fe4‒O4 | x2 | 2.0943(15) | Fe4‒O4 | x2 | 2.0949(13) | Fe4‒O4 | x2 | 2.1013(14) |
| <Fe4‒O> | 2.022 | <Fe4‒O> | 2.021 | <Fe4‒O> | 2.020 | <Fe4‒O> | 2.025 | ||||
| Fe5‒O3 | x3 | 1.9562(16) | Fe5‒O3 | x3 | 1.9560(19) | Fe5‒O3 | x3 | 1.9574(19) | Fe5‒O3 | x3 | 1.9629(18) |
| Fe5‒O5 | x3 | 2.0749(18) | Fe5‒O5 | x3 | 2.076(2) | Fe5‒O5 | x3 | 2.078(12) | Fe5‒O5 | x3 | 2.0847(19) |
| <Fe5‒O> | 2.016 | <Fe5‒O> | 2.016 | <Fe5‒O> | 2.018 | <Fe5‒O> | 2.024 | ||||
| 100 K | 200 K | |||||||||||
| Atom | U11 | U22 | U33 | U23 | U13 | U12 | U11 | U22 | U33 | U23 | U13 | U12 |
| Ba1 | 0.00371(12) | 0.00371(12) | 0.00389(17) | 0.000 | 0.000 | 0.00186(6) | 0.00695(15) | 0.00695(15) | 0.0050(2) | 0.000 | 0.000 | 0.00348(8) |
| Fe1 | 0.0024(2) | 0.0024(2) | 0.0010(4) | 0.000 | 0.000 | 0.00121(12) | 0.0051(3) | 0.0051(3) | 0.0014(4) | 0.000 | 0.000 | 0.00253(15) |
| Fe2 | 0.0012(2) | 0.0012(2) | 0.0323(6) | 0.000 | 0.000 | 0.00062(11) | 0.0032(3) | 0.0032(3) | 0.0400(7) | 0.000 | 0.000 | 0.00159(14) |
| Fe3 | 0.00195(17) | 0.00195(17) | 0.0037(3) | 0.000 | 0.000 | 0.00097(8) | 0.0042(2) | 0.0042(2) | 0.0043(3) | 0.000 | 0.000 | 0.00212(11) |
| Fe4 | 0.00195(16) | 0.00214(13) | 0.00356(16) | −0.00001(6) | −0.00003(11) | 0.00098(8) | 0.0042(2) | 0.00473(17) | 0.0041(2) | −0.00010(6) | −0.00021(12) | 0.00211(10) |
| Fe5 | 0.00272(17) | 0.00272(17) | 0.0038(3) | 0.000 | 0.000 | 0.00136(8) | 0.0052(2) | 0.0052(2) | 0.0039(3) | 0.000 | 0.000 | 0.00258(10) |
| O1 | 0.0032(7) | 0.0032(7) | 0.0064(12) | 0.000 | 0.000 | 0.0016(4) | 0.0054(9) | 0.0054(9) | 0.0064(14) | 0.000 | 0.000 | 0.0027(4) |
| O2 | 0.0030(7) | 0.0030(7) | 0.0051(12) | 0.000 | 0.000 | 0.0015(4) | 0.0061(9) | 0.0061(9) | 0.0042(14) | 0.000 | 0.000 | 0.0030(5) |
| O3 | 0.0022(5) | 0.0034(7) | 0.0054(7) | 0.0009(6) | 0.0004(3) | 0.0017(3) | 0.0051(6) | 0.0059(8) | 0.0059(9) | 0.0011(6) | 0.0005(3) | 0.0030(4) |
| O4 | 0.0049(5) | 0.0053(7) | 0.0050(7) | 0.0010(6) | 0.0005(3) | 0.0026(4) | 0.0068(6) | 0.0073(9) | 0.0047(9) | 0.0008(7) | 0.0004(3) | 0.0036(5) |
| O5 | 0.0052(8) | 0.0033(10) | 0.0062(10) | 0.000 | 0.000 | 0.0016(5) | 0.0092(10) | 0.0055(13) | 0.0039(12) | 0.000 | 0.000 | 0.0028(6) |
| 300 K | 400 K | |||||||||||
| Atom | U11 | U22 | U33 | U23 | U13 | U12 | U11 | U22 | U33 | U23 | U13 | U12 |
| Ba1 | 0.00855(16) | 0.00855(16) | 0.0075(2) | 0.000 | 0.000 | 0.00428(8) | 0.01059(14) | 0.01059(14) | 0.01000(19) | 0.000 | 0.000 | 0.00529(7) |
| Fe1 | 0.0060(3) | 0.0060(3) | 0.0026(4) | 0.000 | 0.000 | 0.00300(15) | 0.0074(3) | 0.0074(3) | 0.0041(4) | 0.000 | 0.000 | 0.00371(14) |
| Fe2 | 0.0036(3) | 0.0036(3) | 0.0480(8) | 0.000 | 0.000 | 0.00179(14) | 0.0044(3) | 0.0044(3) | 0.0579(8) | 0.000 | 0.000 | 0.00219(13) |
| Fe3 | 0.0050(2) | 0.0050(2) | 0.0056(3) | 0.000 | 0.000 | 0.00251(11) | 0.00611(19) | 0.00611(19) | 0.0076(3) | 0.000 | 0.000 | 0.00305(9) |
| Fe4 | 0.0048(2) | 0.00538(17) | 0.00608(19) | −0.00019(7) | −0.00037(13) | 0.00239(10) | 0.00589(18) | 0.00685(14) | 0.00853(18) | −0.00031(6) | −0.00062(12) | 0.00295(9) |
| Fe5 | 0.0062(2) | 0.0062(2) | 0.0056(3) | 0.000 | 0.000 | 0.00312(11) | 0.00755(18) | 0.00755(18) | 0.0072(3) | 0.000 | 0.000 | 0.00377(9) |
| O1 | 0.0054(9) | 0.0054(9) | 0.0083(13) | 0.000 | 0.000 | 0.0027(5) | 0.0068(8) | 0.0068(8) | 0.0100(13) | 0.000 | 0.000 | 0.0034(4) |
| O2 | 0.0066(9) | 0.0066(9) | 0.0057(14) | 0.000 | 0.000 | 0.0033(5) | 0.0075(8) | 0.0075(8) | 0.0079(13) | 0.000 | 0.000 | 0.0038(4) |
| O3 | 0.0060(6) | 0.0060(9) | 0.0074(8) | 0.0020(7) | 0.0010(3) | 0.0030(4) | 0.0068(5) | 0.0078(8) | 0.0107(8) | 0.0025(6) | 0.0012(3) | 0.0039(4) |
| O4 | 0.0077(6) | 0.0078(9) | 0.0065(8) | 0.0005(7) | 0.0003(3) | 0.0039(5) | 0.0091(6) | 0.0093(8) | 0.0081(8) | 0.0006(6) | 0.0003(3) | 0.0047(4) |
| O5 | 0.0104(10) | 0.0054(13) | 0.0066(11) | 0.000 | 0.000 | 0.0027(6) | 0.0125(9) | 0.0062(12) | 0.0085(11) | 0.000 | 0.000 | 0.0031(6) |
| Polyhedron | Vol. (Å3) 100 K | Vol. (Å3) 200 K | Vol. (Å3) 300 K | Vol. (Å3) 400 K |
|---|---|---|---|---|
| Ba1 | 57.060 | 57.045 | 57.124 | 57.463 |
| Fe1 | 10.318 | 10.273 | 10.304 | 10.340 |
| Fe2 | 6.847 | 6.849 | 6.841 | 6.877 |
| Fe3 | 3.591 | 3.606 | 3.596 | 3.615 |
| Fe4 | 10.908 | 10.886 | 10.882 | 10.941 |
| Fe5 | 10.680 | 10.687 | 10.720 | 10.815 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzątała, A.; Panikorovskii, T.L.; Galuskina, I.O.; Galuskin, E.V. Dynamic Disorder of Fe3+ Ions in the Crystal Structure of Natural Barioferrite. Minerals 2018, 8, 340. https://doi.org/10.3390/min8080340
Krzątała A, Panikorovskii TL, Galuskina IO, Galuskin EV. Dynamic Disorder of Fe3+ Ions in the Crystal Structure of Natural Barioferrite. Minerals. 2018; 8(8):340. https://doi.org/10.3390/min8080340
Chicago/Turabian StyleKrzątała, Arkadiusz, Taras L. Panikorovskii, Irina O. Galuskina, and Evgeny V. Galuskin. 2018. "Dynamic Disorder of Fe3+ Ions in the Crystal Structure of Natural Barioferrite" Minerals 8, no. 8: 340. https://doi.org/10.3390/min8080340