Amethyst Occurrences in Tertiary Volcanic Rocks of Greece: Mineralogical, Fluid Inclusion and Oxygen Isotope Constraints on Their Genesis
Abstract
:1. Introduction
2. Meterials and Methods
3. Geological Setting
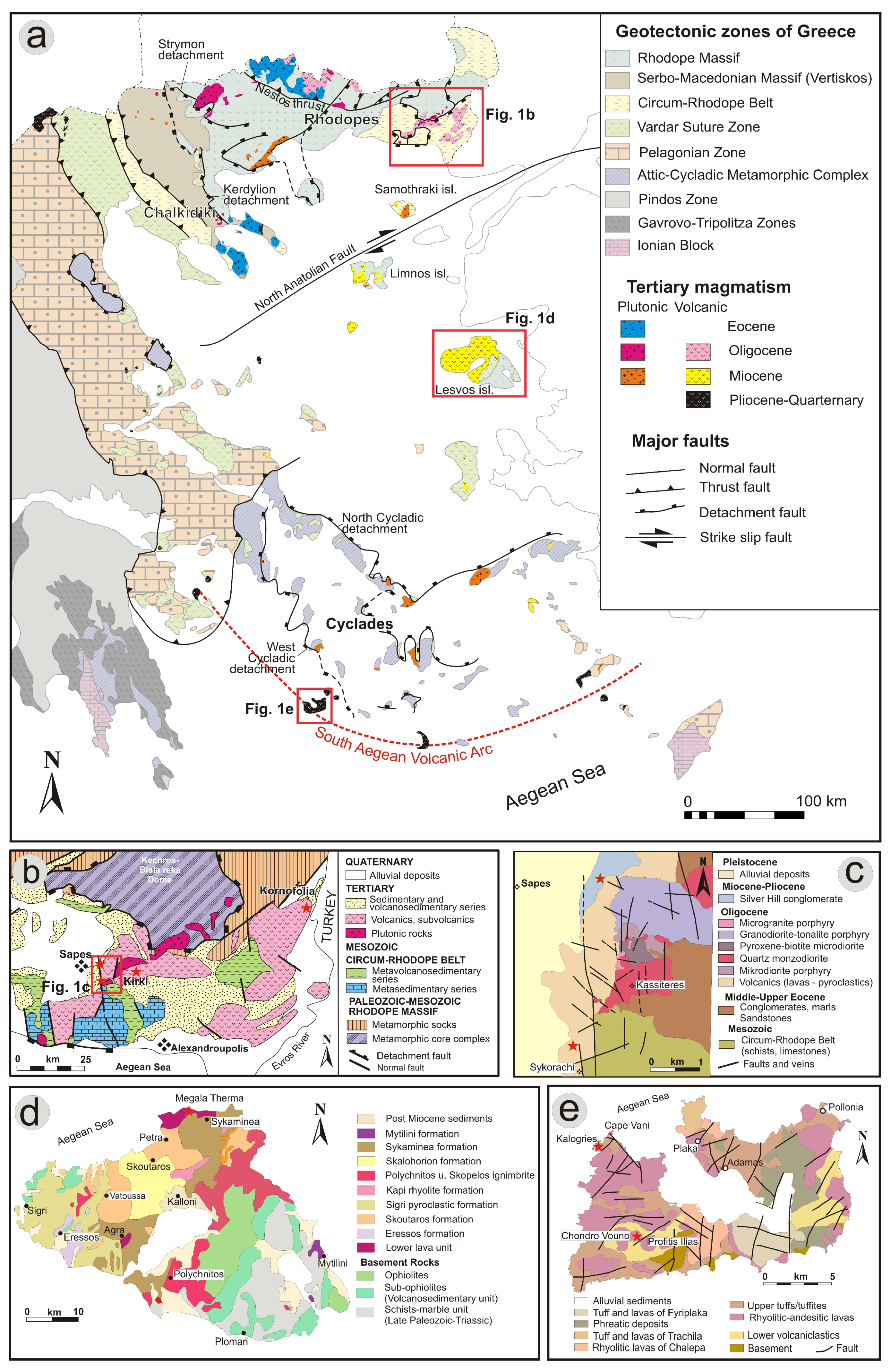
3.1. Regional Geology
3.2. Local Geology
3.2.1. Northeastern Greece (Sapes, Kirki and Kornofolia)
3.2.2. Lesvos Island
3.2.3. Milos Island
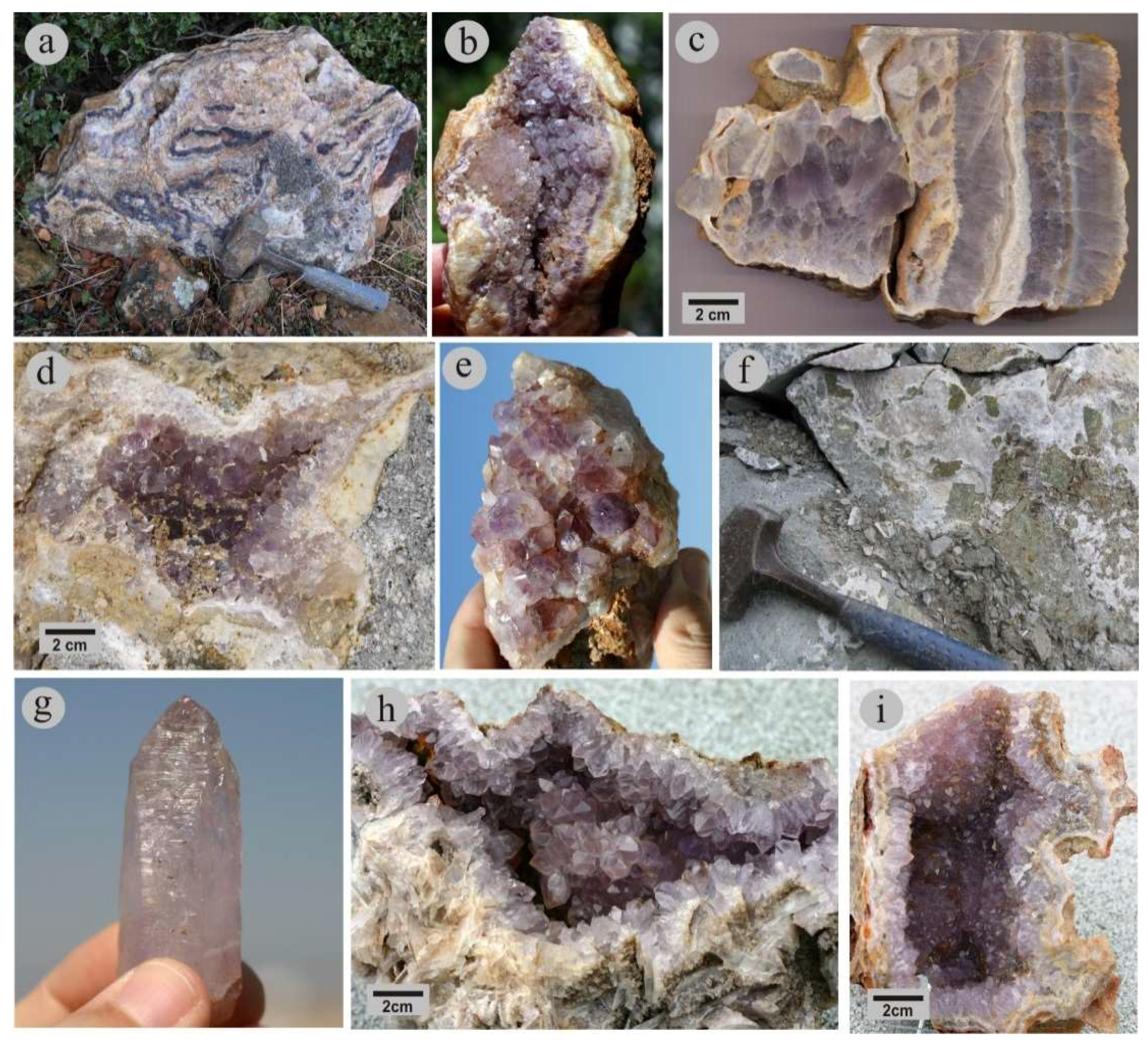
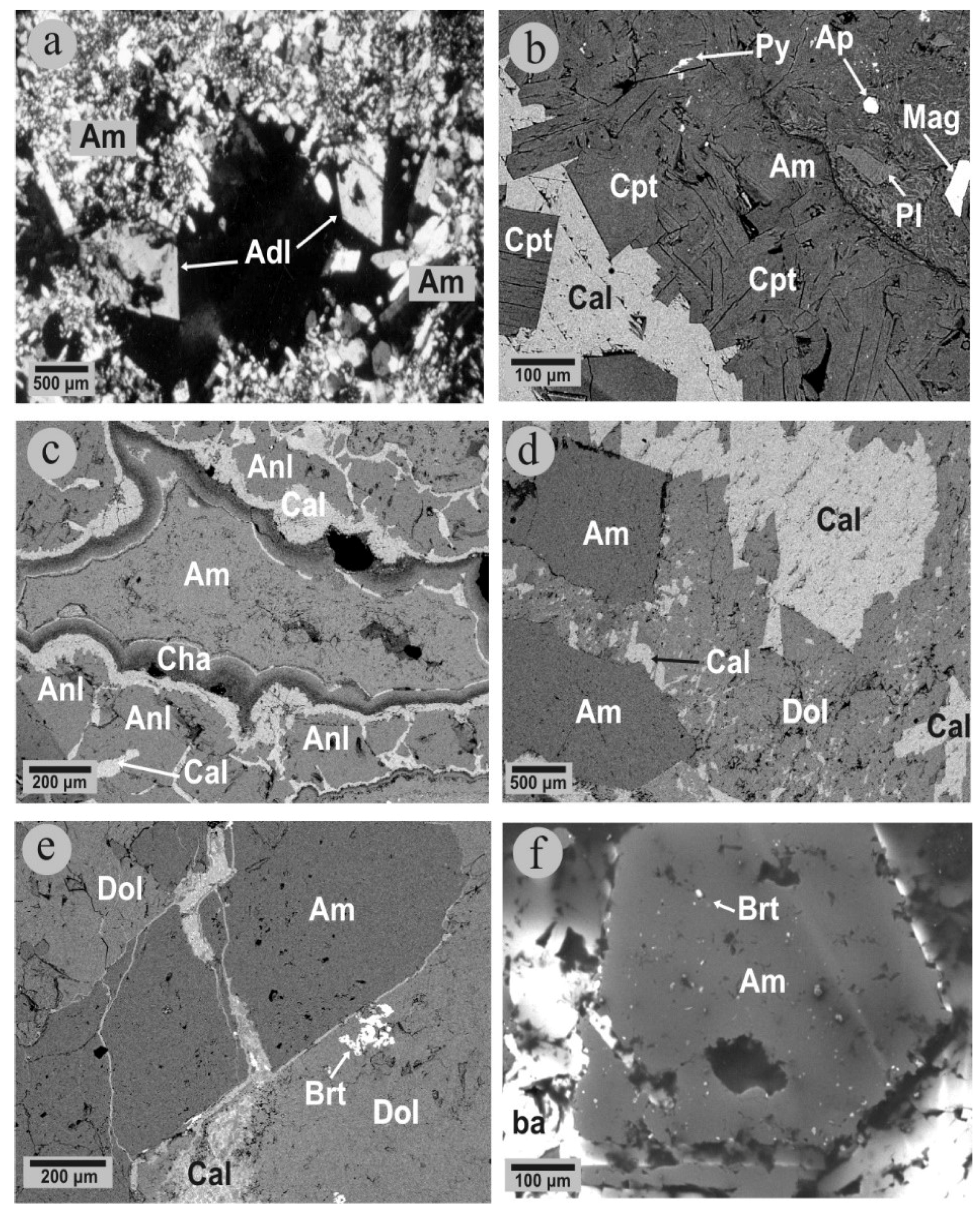
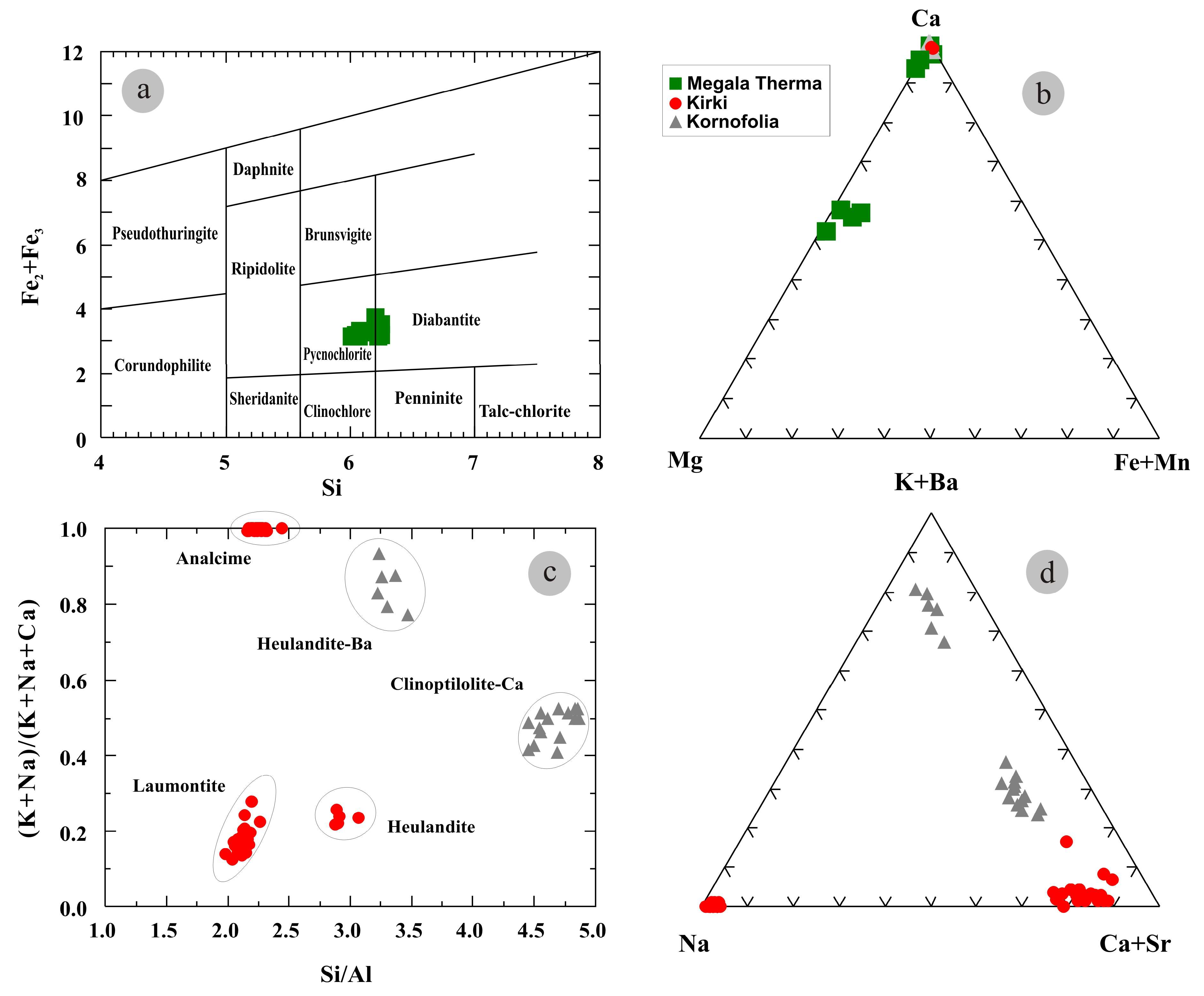
4. Mineralogy and Mineral Chemistry
5. Fluid Inclusions
5.1. Morphology and Types of Fluid Inclusions
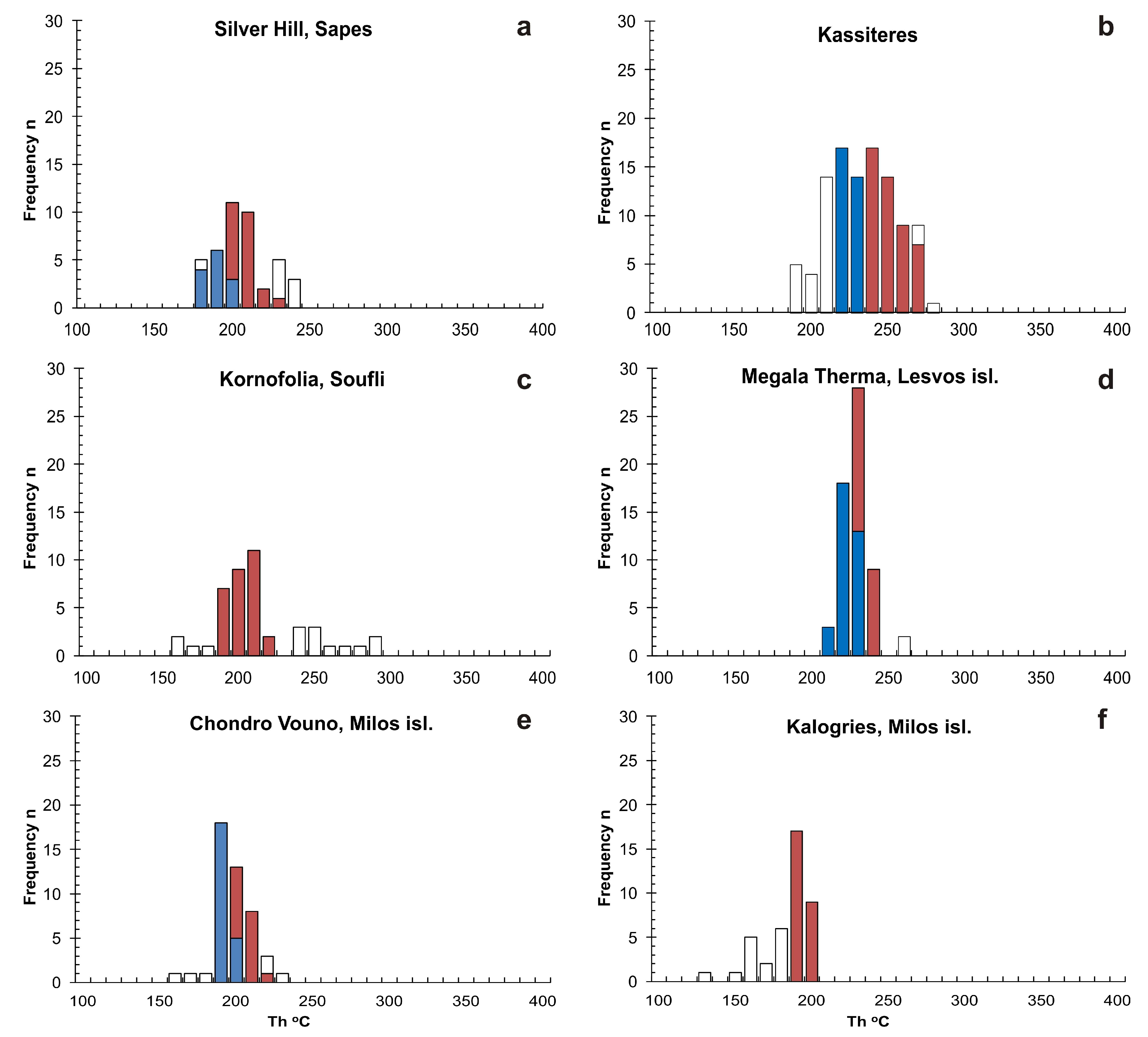
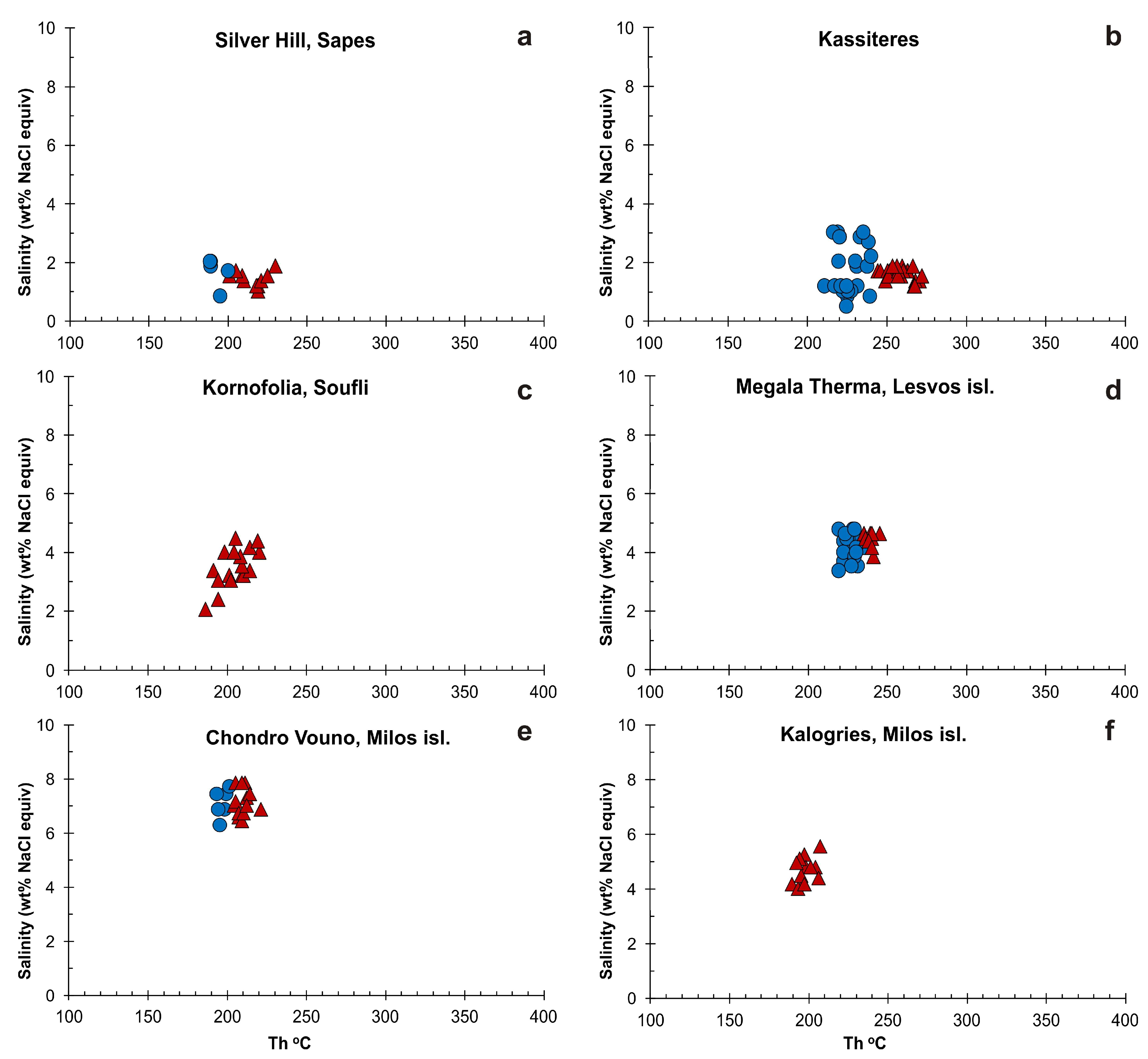
5.2. Microthermometry
6. Amethyst Oxygen Isotopes
7. Discussion
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lieber, W. Amethyst: Geschichte, Eigenschaften, Fundorte; Christian Weise Verlag: München, Germany, 1994; 188p. [Google Scholar]
- Rossman, G.R. Colored varieties of the silica minerals. Rev. Mineral. 1994, 29, 433–467. [Google Scholar]
- Cox, R.T. Optical absorption of the d4 ion Fe4+ in pleochroic amethyst quartz. J. Phys. C Solid State Phys. 1977, 10, 4631–4643. [Google Scholar] [CrossRef]
- Fritsch, E.; Rossman, G.R. An update of colors in gems part 2: Colors involving multiple atoms and color centers. Gems Gemol. 1988, 24, 3–15. [Google Scholar] [CrossRef]
- Cohen, A.J. Amethyst color in quartz, the result of radiation protection involving iron. Am. Mineral. 1985, 70, 1180–1185. [Google Scholar]
- Scholz, R.; Chaves, M.L.S.C.; Krambrock, K.; Pinheiro, V.B.; Barreto, S.B.; de Menezes, M.G. Brazilian quartz deposits with special emphasis on gemstone quartz and its color treatment. In Quartz: Deposits, Mineralogy and Analytics; Gӧtze, J., Mӧckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 139–159. [Google Scholar]
- Wilson, B.S. Colored gemstones from Canada. In Geology of Gem Deposits; Groat, L.A., Ed.; Mineralogical Association of Canada Short Course; Mineralogical Association of Canada: Québec, QC, Canada, 2007; Volume 37, pp. 255–270. [Google Scholar]
- Kievlenko, E.Y. Geology of Gems; Ocean Pictures Ltd.: Romsey, UK, 2003; 468p. [Google Scholar]
- Gӧtze, J. Chemistry, textures and physical properties of quartz—Geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Gӧtze, J.; Mӧckel, R. Quartz: Deposits, Mineralogy and Analytics; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Voudouris, P.; Katerinopoulos, A. New occurrences of mineral megacrysts in Tertiary magmatic-hydrothermal and epithermal environments in Greece. Doc. Nat. 2004, 151, 1–21. [Google Scholar]
- Melfos, V. Study of fluid inclusions in amethysts from areas of Macedonia and Thrace: Sapes, Soufli, Nevrokopi. In Proceedings of the 2nd Congress of the Economic Geology Committee, Mineralogy & Petrology (GSG), Thessaloniki, Greece, 7–9 October 2005; pp. 219–228. (In Greek). [Google Scholar]
- Maneta, V.; Voudouris, P. Quartz megacrysts in Greece: Mineralogy and environment of formation. Bull. Geol. Soc. Greece 2010, 43, 685–696. [Google Scholar] [CrossRef]
- Voudouris, P.; Katerinopoulos, A.; Melfos, V. Alpine-type fissure minerals in Greece. Doc. Nat. 2004, 151, 23–45. [Google Scholar]
- Voudouris, P.; Psimis, I.; Mavrogonatos, C.; Kanellopoulos, C.; Kati, M.; Chlekou, E. Amethyst occurrences in Tertiary volcanic rocks of Greece: Mineralogical and genetic implications. Bull. Geol. Soc. Greece 2013, 47, 477–486. [Google Scholar] [CrossRef]
- Driesner, T.; Heinrich, C.A. The system H2O-NaCl. I. Correlations for molar volume, enthalpy, and isobaric heat capacity from 0 to 1000 degrees C, 1 to 5000 bar, and 0 to 1 X-NaCl. Geochim. Cosmochim. Acta 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O-NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Mattey, D.P. LaserPrep: An Automatic Laser-Fluorination System for Micromass ‘Optima’ or ‘Prism’ Mass Spectrometers. Micromass Appl. Note 1997, 207, 8. [Google Scholar]
- Jolivet, L.; Brun, J.P. Cenozoic geodynamic evolution of the Aegean region. Int. J. Earth Sci. 2010, 99, 109–138. [Google Scholar] [CrossRef]
- Kydonakis, K.; Brun, J.-P.; Sokoutis, D.; Gueydan, F. Kinematics of Cretaceous subduction and exhumation in the western Rhodope (Chalkidiki block). Tectonophysics 2015, 665, 218–235. [Google Scholar] [CrossRef]
- Menant, A.; Jolivet, L.; Tuduri, J.; Loiselet, C.; Bertrand, G.; Guillou-Frottier, L. 3D subduction dynamics: A first-order parameter of the transition from copper- to gold-rich deposits in the eastern Mediterranean region. Ore Geol. Rev. 2018, 94, 118–135. [Google Scholar] [CrossRef]
- Fytikas, M.; Innocenti, F.; Manneti, P.; Mazzuoli, R.; Peccerillo, A.; Villari, L. Tertiary to Quaternary evolution of volcanism in the Aegean region. In The Geological Evolution of the Eastern Mediterranean; Dixon, J.E., Robertson, A.H.F., Eds.; Geological Society Special Publication: Oxford, UK, 1984; Volume 17, pp. 687–699. [Google Scholar]
- Innocenti, F.; Kolios, N.; Manetti, O.; Mazzuoli, R.; Peccerillo, G.; Rita, F.; Villari, L. Evolution and geodynamic significance of the Tertiary orogenic volcanism in northeastern Greece. Bull. Volcanol. 1984, 47, 25–37. [Google Scholar] [CrossRef]
- Christofides, G.; Pecskay, Z.; Soldatos, T.; Eleftheriadis, G.; Koroneos, A. The Tertiary Evros volcanic rocks (Greece): Petrology, K/Ar geochronology and volcanism evolution. Geol. Carpath. 2004, 55, 397–409. [Google Scholar]
- Marchev, P.; Kaiser-Rohrmeier, M.; Heinrich, C.; Ovtcharova, M.; von Quadt, A.; Raicheva, R. Hydrothermal ore deposits related to post-orogenic extensional magmatism and core complex formation: The Rhodope Massif of Bulgaria and Greece. Ore Geol. Rev. 2005, 27, 53–89. [Google Scholar] [CrossRef]
- Melfos, V.; Voudouris, P. Cenozoic metallogeny of Greece and potential for precious, critical and rare metals exploration. Ore Geol. Rev. 2017, 59, 1030–1057. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W. The Igneous Rocks of Greece: The Anatomy of an Orogen; Gebruder Borntraeger: Berlin, Germany, 2002; Volume 30. [Google Scholar]
- Ottens, B.; Voudouris, P. Griechenland: Mineralien-Fundorte-Lagerstätten; Christian Weise Verlag: München, Germany, 2018; in press. [Google Scholar]
- Fytikas, M.; Innocenti, F.; Kolios, N.; Manneti, P.; Mazzuoli, R.; Poli, G.; Rita, F.; Villari, L. Volcanology and petrology of volcanic products from the island of Milos and neighboring islets. J. Volcanol. Geotherm. Res. 1986, 28, 297–317. [Google Scholar] [CrossRef]
- Stewart, A.L.; McPhie, J. Facies architecture and Late Pliocene–Pleistocene evolution of a felsic volcanic island, Milos, Greece. Bull. Volcanol. 2006, 68, 703–726. [Google Scholar] [CrossRef]
- Alfieris, D.; Voudouris, P.; Spry, P.G. Shallow submarine epithermal Pb-Zn-Cu-Au-Ag-Te mineralization on western Milos Island, Aegean Volcanic Arc, Greece: Mineralogical, Geological and Geochemical constraints. Ore Geol. Rev. 2013, 53, 159–180. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, northern Turkey. Contr. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Le Bas, M.J.; Le Maitre, R.W.; Streckeisen, A.; Zanettin, B. A chemical classification of volcanic rocks based on total alkali-silica diagram. J. Petrol. 1986, 27, 745–750. [Google Scholar] [CrossRef]
- Irvine, T.N.; Baragar, W.R.W. A guide to chemical classification of the common volcanic rocks. Can. J. Earth Sci. 1971, 8, 523–548. [Google Scholar]
- Miyashiro, A. Volcanic Rock Series in Island Arcs and Active Continental Margins. Am. J. Sci. 1974, 274, 321–355. [Google Scholar] [CrossRef]
- Shand, S.J. The Eruptive Rocks, 2nd ed.; John Wiley: New York, NY, USA, 1943. [Google Scholar]
- Liakopoulos, A. Hydrothermalisme et Minéralisations Métallifères de l’ île de Milos (Cyclades, Grèce). Ph.D. Thesis, Université Pierre et Marie Curie, Paris, France, 1987. [Google Scholar]
- Voudouris, P. Mineralogical, Geochemical and Fluid Inclusion Studies on Epithermal Vein Type Gold/Silver Mineralizations at Kassiteres/Sapes, (NE-Greece). Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1993. [Google Scholar]
- Voudouris, P. The minerals of Eastern Macedonia and Western Thrace: Geological framework and environment of formation. Bull. Geol. Soc. Greece 2005, 37, 62–77. [Google Scholar]
- Shawh, A.J.; Constantinides, D.C. The Sappes gold project. Bull. Geol. Soc. Greece 2001, 34, 1073–1080. [Google Scholar] [CrossRef]
- Voudouris, P.; Velitzelos, D.; Velitzelos, E.; Thewald, U. Petrified wood occurrences in western Thrace and Limnos island: Mineralogy, geochemistry and depositional environment. Bull. Geol. Soc. Greece 2007, 40, 238–250. [Google Scholar] [CrossRef]
- Voudouris, P.; Alfieris, D. New porphyry-Cu ± Mo occurrences in northeastern Aegean/Greece: Ore mineralogy and transition to epithermal environment. In Mineral Deposit Research: Meeting the Global Challenge; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin, Germany, 2005; pp. 473–476. [Google Scholar]
- Alfieris, D. Geological, Geochemical and Mineralogical Studies of Shallow Submarine Epithermal Mineralization in an Emergent Volcanic Edifice, at Western Milos Island, Greece. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 2006. [Google Scholar]
- Coffey, J. The Stratigraphy and Palaeontology of Cape Vani, Milos, Greece; Honours Research Report; School of Earth Sciences, University of Melbourne: Parkville, VIC, Australia, 2005. [Google Scholar]
- Hey, M.H. A new review of the chlorites. Mineral. Mag. 1954, 30, 277–292. [Google Scholar] [CrossRef]
- Coombs, D.S.; Alberti, A.; Armbruster, T.; Artioli, G.; Colella, C.; Galli, E.; Grice, J.D.; Liebau, F.; Mandarino, J.A.; Minato, H.; et al. Recommended nomenclature for zeolite minerals: Report of the Subcommittee on Zeolites of the Mineralogical Association, Commission on New Minerals and Mineral Names. Eur. J. Mineral. 1998, 10, 1037–1081. [Google Scholar] [CrossRef]
- Larsen, A.O.; Nordrum, F.S.; Döbelin, N.; Armbruster, T.; Petersen, O.V.; Erambert, M. Heulandite-Ba, a new zeolite species from Norway. Eur. J. Mineral. 2005, 17, 143–153. [Google Scholar] [CrossRef]
- Voudouris, P.; Maneta, V. Quartz in Greece; CreateSpace Publ. and Amazon.com, Inc.: Seattle, WA, USA, 2017. (In Greek) [Google Scholar]
- Roedder, E. Fluid inclusions. In Reviews in Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1984; Volume 12, p. 644. [Google Scholar]
- Van den Kerkhof, A.M.; Hein, U.F. Fluid inclusion petrography. Lithos 2001, 55, 27–47. [Google Scholar] [CrossRef]
- Goldstein, R.H.; Reynolds, T.J. Systematics of Fluid Inclusions in Diagenetic Minerals; SEPM Short Course; Society for Sedimentary Geology: Broken Arrow, OK, USA, 1994; Volume 31. [Google Scholar]
- Audétat, A.; Günther, D. Mobility and H2O loss from fluid inclusions in natural quartz crystals. Contrib. Mineral. Petrol. 1999, 137, 1–14. [Google Scholar] [CrossRef]
- Tarantola, A.; Diamond, L.W.; Stünitz, H. Modification of fluid inclusions in quartz by deviatoric stress I: Experimentally induced changes in inclusion shapes and microstructures. Contrib. Mineral. Petrol. 2010, 160, 825–843. [Google Scholar] [CrossRef]
- Tarantola, A.; Diamond, L.W.; Stünitz, H.; Thust, A.; Pec, M. Modification of fluid inclusions in quartz by deviatoric stress III: Influence of principal stresses on inclusion density and orientation. Contrib. Mineral. Petrol. 2012, 164, 537–550. [Google Scholar] [CrossRef]
- Diamond, L.W.; Tarantola, A. Interpretation of fluid inclusions in quartz deformed by weak ductile shearing: Reconstruction of differential stress magnitudes and pre-deformation fluid properties. Earth Planet. Sci. Lett. 2015, 417, 107–119. [Google Scholar] [CrossRef]
- Stünitz, H.; Thust, A.; Heilbronner, R.; Behrens, H.; Kilian, R.; Tarantola, A.; Fitz Gerald, J.D. Water redistribution in experimentally deformed natural milky quartz single crystals-Implications for H2O-weakening processes. J. Geophys. Res. Solid Earth 2017, 122, 866–894. [Google Scholar] [CrossRef]
- Bakker, R.J.; Jansen, J.B.H. Preferential water leakage from fluid inclusions by means of mobile dislocations. Nature 1990, 345, 58–60. [Google Scholar] [CrossRef]
- Bakker, R.J.; Jansen, J.B.H. A mechanism for preferential H2O leakage from fluid inclusions in quartz, based on TEM observations. Contrib. Mineral. Petrol. 1994, 116, 7–20. [Google Scholar] [CrossRef]
- Goldstein, R.H. Fluid inclusions: Analysis and interpretation. In Fluid Inclusions: Analysis and Interpretation; Samson, I.M., Anderson, A.J., Marshall, D.D., Eds.; SEPM Short Course; Society for Sedimentary Geology: Broken Arrow, OK, USA, 2003; Volume 32, pp. 9–53. [Google Scholar]
- Shepherd, T.; Rankin, A.; Alderton, D. A Practical Guide to Fluid Inclusion Studies; Blackie and Son: Glasgow, UK, 1985. [Google Scholar]
- Bodnar, R.J. Introduction to fluid inclusions. In Fluid Inclusions: Analysis and Interpretation; Samson, I.M., Anderson, A.J., Marshall, D.D., Eds.; SEPM Short Course; Society for Sedimentary Geology: Broken Arrow, OK, USA, 2003; Volume 32, pp. 1–8. [Google Scholar]
- Sterner, S.M.; Bodnar, R.J. Synthetic fluid inclusions-VII. Re-equilibration of fluid inclusions in quartz during laboratory-simulated metamorphic burial and uplift. J. Metamorph. Geol. 1989, 7, 243–260. [Google Scholar] [CrossRef]
- Sterner, S.M.; Hall, D.L.; Keppler, H. Compositional re-equilibration of fluid inclusions in quartz. Contrib. Mineral. Petrol. 1995, 119, 1–15. [Google Scholar] [CrossRef]
- Sharp, Z.D.; Gibbons, J.A.; Maltsev, O.; Atudorei, V.; Pack, A.; Sengupta, S.; Shock, E.L.; Knauth, L.P. A calibration of the triple oxygen isotope fractionation in the SiO2–H2O system and applications to natural samples. Geochim. Cosmochim. Acta 2016, 186, 105–119. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Reynolds, T.J.; Kuehn, C.A. Fluid-Inclusion systematics in epithermal systems. Rev. Econ. Geol. 1985, 2, 73–97. [Google Scholar]
- Taylor, H.P., Jr. Oxygen and hydrogen isotope relationships in hydrothermal mineral deposits. In Geochemistry of Hydrothermal Ore Deposits, 2nd ed.; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1979; pp. 236–318. [Google Scholar]
- Giggenbach, W.F. The origin and evolution of fluids in magmatic-hydrothermal systems. In Geochemistry of Hydrothermal Ore Deposits, 3nd ed.; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1997; pp. 737–796. [Google Scholar]
- Ettig, F. Sachsischer Amethyst. Aufschluss 1952, 3, 52–53. [Google Scholar]
- Bringe, H.H.; Grubessi, O. Amethyst und Yugawaralith von Osilo. Aufschluss 1982, 33, 41–44. [Google Scholar]
- Breskovska, V.; Tarkian, M. Mineralogy and Fluid Inclusion Study of Polymetallic Veins in the Madjarovo Ore Field, Eastern Rhodope, Bulgaria. Mineral. Petrol. 1993, 49, 103–118. [Google Scholar] [CrossRef]
- Henn, J.; Lieber, W. Amethyst von Brandberg, Namibia. Lapis 1993, 18, 44–48. [Google Scholar]
- Robinson, R.W.; Norman, D.I. Mineralogy and Fluid Inclusion Study of the Southern Amethyst Vein System, Creede Mining District, Colorado. Econ. Geol. 1984, 79, 439–447. [Google Scholar] [CrossRef]
- Wallace, T. Die violetten Schätze von Guerrero und Veracruz, Mexico. Extra Lapis 2015, 49, 80–99. [Google Scholar]
- Gilg, H.A.; Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gatter, I.; Strieder, A.J. Genesis of amethyst geodes in basaltic rocks of the Serra Geral Formation (Ametista do Sul, Rio Grande do Sul, Brazil): A fluid inclusion, REE, oxygen, carbon, and Sr isotope study on basalt, quartz, and calcite. Miner. Depos. 2003, 38, 1009–1025. [Google Scholar] [CrossRef]
- Gilg, H.A.; Krüger, Y.; Taubald, H.; van den Kerkhof, A.M.; Frenz, M.; Morteani, M. Mineralisation of amethyst-bearing geodes in Ametista do Sul (Brazil) from low-temperature sedimentary brines: Evidence from monophase liquid inclusions and stable isotopes. Miner. Depos. 2014, 49, 861–877. [Google Scholar] [CrossRef]
- Proust, D.; Fontaine, C. Amethyst-bearing lava flows in the Paraná Basin (Rio Grande do Sul, Brazil): Cooling, vesiculation and formation of the geodic cavities. Geol. Mag. 2007, 144, 53–65. [Google Scholar] [CrossRef]
- Proust, D.; Fontaine, C. Amethyst geodes in the basaltic flow from Triz quarry at Ametista do Sul (Rio Grande do Sul, Brazil): Magmatic source of silica for the amethyst crystallizations. Geol. Mag. 2007, 144, 731–740. [Google Scholar] [CrossRef]
- Commin-Fischer, A.; Berger, G.; Polvé, M.; Dubois, M.; Sardini, P.; Beaufort, D.; Formoso, M. Petrography and chemistry of SiO2 filling phases in the amethyst geodes from the Serra Geral Formation deposit, Rio Grande do Sul, Brazil. J. South Am. Earth Sci. 2010, 29, 751–760. [Google Scholar] [CrossRef]
- Hartmann, L.A.; da Cunha Duarte, L.; Massonne, H.J.; Michelin, C.; Rosenstengel, L.M.; Bergmann, M.; Theye, T.; Pertille, J.; Arena, K.R.; Duarte, S.K. Sequential opening and filling of cavities forming vesicles, amygdales and giant amethyst geodes in lavas from the southern Paraná volcanic province, Brazil and Uruguay. Int. Geol. Rev. 2012, 54, 1–14. [Google Scholar] [CrossRef]
- Hartmann, L.A.; Antunes, L.M.; Rosenstengel, L.M. Stratigraphy of amethyst geode-bearing lavas and fault-block structures of the Entre Rios mining district, Paraná volcanic province, southern Brazil. Ann. Braz. Acad. Sci. 2014, 86, 187–198. [Google Scholar] [CrossRef]
- Duarte, L.C.; Hartmann, L.A.; Vasconcellos, M.A.Z.; Medeiros, J.T.N.; Theye, T. Epigenetic formation of amethyst-bearing geodes from Los Catalanes gemological district, Artigas, Uruguay, southern Paraná Magmatic Province. J. Volcanol. Geotherm. Res. 2009, 184, 427–436. [Google Scholar] [CrossRef]
- Morteani, G.; Kostitsyn, Y.; Preinfalk, C.; Gilg, H.A. The genesis of the amethyst geodes at Artigas (Uruguay) and the paleohydrology of the Guarani aquifer: Structural, geochemical, oxygen, carbon, strontium isotope and fluid inclusion study. Int. J. Earth Sci. 2010, 99, 927–947. [Google Scholar] [CrossRef]
- Duarte, L.C.; Hartmann, L.A.; Ronchi, L.H.; Berner, Z.; Theye, T.; Massonne, H.J. Stable isotope and mineralogical investigation of the genesis of amethyst geodes in the Los Catalanes gemological district, Uruguay, southernmost Parana volcanic province. Miner. Depos. 2011, 46, 239–255. [Google Scholar] [CrossRef]
- Wilkinson, J.J. Fluid inclusions in hydrothermal ore deposits. Lithos 2001, 55, 229–272. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Henley, R.W. The importance of CO2 on freezing point measurements of fluid inclusions; evidence from active geothermal systems and implications for epithermal ore deposition. Econ. Geol. 1985, 80, 1379–1406. [Google Scholar] [CrossRef]
- Simmons, S.F.; Browne, P.R.L. Hydrothermal minerals and precious metals in the Broadlands-Ohaaki geothermal system: Implications for understanding low-sulfidation epithermal environments. Econ. Geol. 2001, 95, 971–999. [Google Scholar] [CrossRef]
- Simmons, S.F.; White, N.W.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. Econ. Geol. 2005, 100, 485–522. [Google Scholar]
- Hedenquist, J.W. Boiling and dilution in the shallow portion of the Waiotapu geothermal system, New Zealand. Geochim. Cosmochim. Acta 1991, 55, 2753–2765. [Google Scholar] [CrossRef]
- Moncada, D.; Mutchler, S.; Nieto, A.; Reynolds, T.J.; Rimstidt, J.D.; Bodnar, R.J. Mineral textures and fluid inclusion petrography of the epithermal Ag-Au deposits at Guanajuato, Mexico: Application to exploration. J. Geochem. Explor. 2012, 114, 20–35. [Google Scholar] [CrossRef]
- Kilias, S.P.; Naden, J.; Cheliotis, I.; Shepherd, T.J.; Constandinidou, H.; Crossing, J.; Simos, I. Epithermal gold mineralisation in the active Aegean volcanic arc: The Profitis Ilias deposit, Milos island, Greece. Miner. Depos. 2001, 36, 32–44. [Google Scholar] [CrossRef]
- Kilias, S.P.; Detsi, K.; Godelitsas, A.; Typas, M.; Naden, J.; Marantos, Y. Evidence of Mn-oxide biomineralization, Vani Mn deposit, Milos, Greece. In Digging Deeper, Proceedings of the Ninth Biennial Meeting of the Society for Geology Applied to Mineral Deposits, Dublin, Ireland 20–23 August 2007; Irish Association of Economic Geologists: Dublin, Ireland, 2007; pp. 1069–1072. [Google Scholar]
- Naden, J.; Kilias, S.P.; Darbyshire, D.B.F. Active geothermal systems with entrained seawater as analogues for transitional continental magmato-hydrothermal and volcanic-hosted massive sulfide mineralization-the example of Milos Island, Greece. Geology 2005, 33, 541–544. [Google Scholar] [CrossRef]
- Smith, D.J.; Naden, J.; Miles, A.J.; Bennett, H.; Bicknell, S.H. Mass wasting events and their impact on the formation and preservation of submarine ore deposits. Ore Geol. Rev. 2018, 97, 143–151. [Google Scholar] [CrossRef]
- Vavelidis, M.; Melfos, V. Fluid inclusion evidence for the origin of the barite silver-gold-bearing Pb-Zn mineralization of the Triades area, Milos island, Greece. Bull. Geol. Soc. Greece 1998, 32, 137–144. [Google Scholar]
- Cathelineau, M. Cation size occupancy in chlorites and illites as a function of temperature. Clay Miner. 1988, 23, 471–485. [Google Scholar] [CrossRef]
- Fournier, R.O. The behaviour of silica in hydrothermal solutions. Geology and geochemistry of epithermal systems. Rev. Econ. Geol. 1985, 2, 45–61. [Google Scholar]

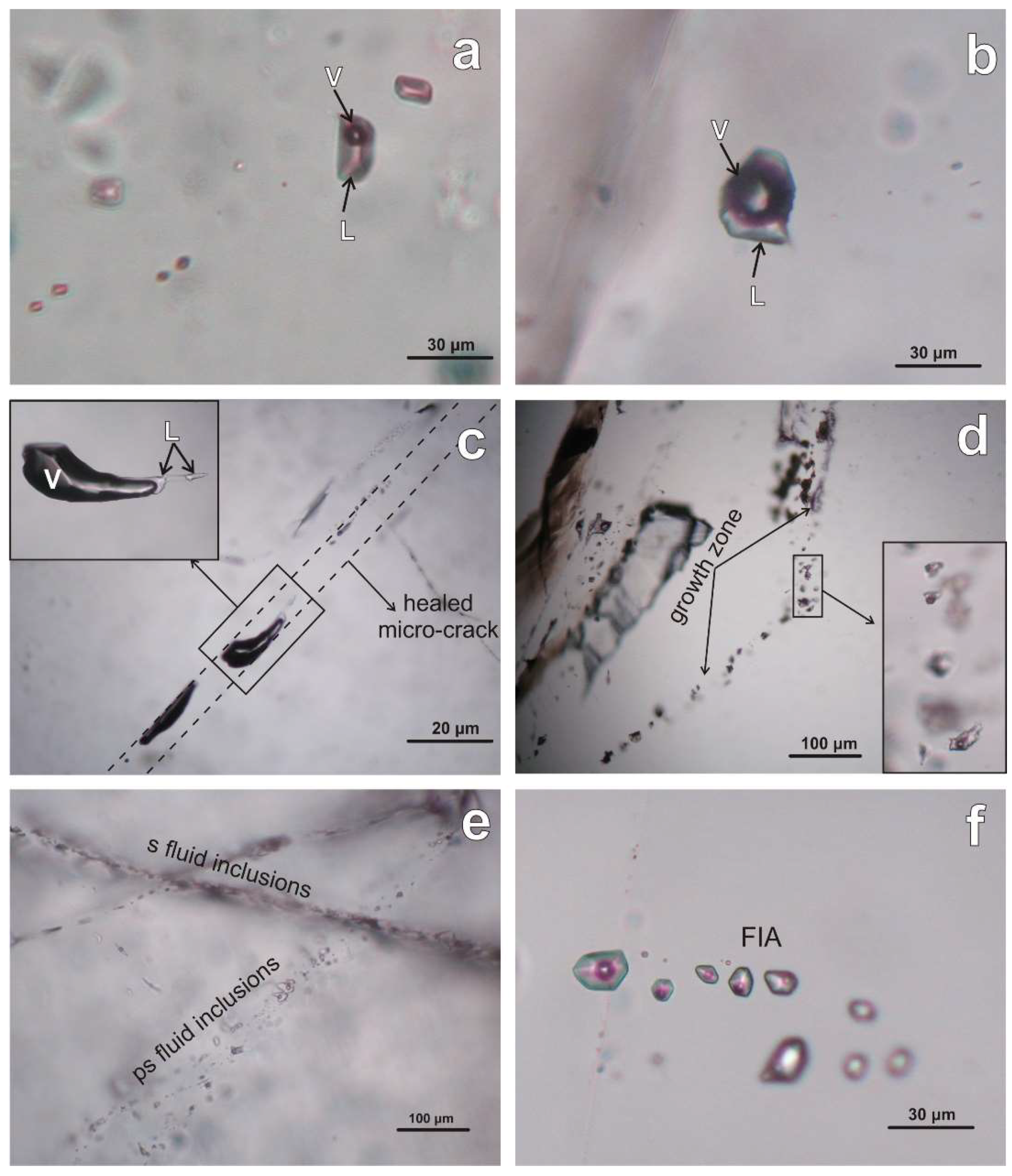

| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 69.34 | 68.99 | 69.26 | 66.40 | 61.85 | 64.09 | 62.47 | 65.23 | 54.45 | 54.49 | 61.46 | 61.41 | 67.23 | 65.19 | 56.20 | 57.21 | 61.96 |
| TiO2 | 0.46 | 0.46 | 0.50 | 0.56 | 0.59 | 0.56 | 0.54 | 0.78 | 0.75 | 0.73 | 0.62 | 0.48 | 0.48 | 0.51 | 0.85 | 1.01 | 0.66 |
| Al2O3 | 15.54 | 15.50 | 15.02 | 16.94 | 16.36 | 16.42 | 16.18 | 16.19 | 16.78 | 15.98 | 17.31 | 16.01 | 16.01 | 16.25 | 18.12 | 18.93 | 16.57 |
| Fe2O3 | 3.23 | 3.57 | 3.43 | 6.14 | 5.80 | 5.90 | 4.91 | 9.42 | 8.90 | 6.23 | 5.84 | 3.48 | 3.48 | 4.11 | 6.60 | 6.26 | 6.03 |
| MnO | 0.05 | 0.06 | 0.06 | 0.12 | 0.08 | 0.08 | 0.07 | 0.09 | 0.23 | 0.13 | 0.10 | 0.08 | 0.08 | 0.11 | 0.12 | 0.11 | 0.10 |
| MgO | 0.87 | 1.25 | 1.19 | 2.56 | 2.19 | 2.33 | 1.85 | 5.42 | 6.01 | 3.21 | 2.68 | 1.63 | 1.63 | 2.20 | 3.61 | 3.87 | 2.65 |
| CaO | 4.18 | 3.52 | 5.22 | 5.48 | 5.29 | 6.07 | 4.80 | 9.37 | 7.73 | 6.04 | 6.01 | 4.64 | 4.64 | 5.20 | 7.20 | 5.74 | 6.63 |
| Na2O | 3.12 | 3.12 | 2.56 | 3.43 | 3.63 | 3.34 | 3.35 | 2.63 | 2.51 | 2.91 | 2.92 | 2.91 | 2.91 | 2.85 | 2.94 | 2.42 | 3.33 |
| K2O | 2.94 | 2.89 | 2.51 | 2.68 | 2.11 | 2.64 | 2.89 | 1.18 | 1.93 | 3.00 | 2.02 | 2.51 | 2.51 | 2.82 | 3.55 | 3.67 | 1.61 |
| P2O5 | 0.13 | 0.14 | 0.15 | 0.11 | 0.10 | 0.10 | 0.11 | 0.14 | 0.22 | 0.14 | 0.13 | 0.13 | 0.13 | 0.12 | 0.42 | 0.48 | - |
| Total | 99.86 | 99.50 | 99.99 | 99.87 | 99.24 | 99.91 | 99.93 | 99.67 | 99.54 | 99.83 | 99.04 | 99.90 | 99.10 | 99.36 | 99.61 | 99.79 | 99.87 |
| Ba | 726 | 732 | 462 | 560 | 663 | 526 | 627 | 679 | 376 | 881 | 809 | 507 | 1175 | 705 | 1466 | 1077 | 383 |
| Ce | 61 | 65 | 43 | 74 | 52 | 58 | 62 | 43 | - | 32 | 38 | 87 | 55 | 64 | 139 | 120 | - |
| Cr | 6 | 5 | 8 | 14 | 12 | 12 | 12 | 10 | 89 | 135 | 40 | 20 | 10 | 19 | 16 | 34 | - |
| Ga | 14 | 17 | 17 | 16 | 18 | 14 | 18 | 14 | 19 | 14 | 15 | 10 | - | 14 | 22 | 21 | - |
| La | 28 | 39 | 26 | 58 | 23 | 35 | 39 | 27 | - | 16 | 29 | 38 | 13 | 46 | 88 | 57 | - |
| Nb | 6 | 5 | 2 | 7 | 5 | 6 | 6 | 7 | 4 | 5 | 8 | 6 | 8 | 8 | - | 2 | - |
| Nd | 28 | 27 | 20 | 25 | 24 | 23 | 24 | 17 | 9 | 14 | 15 | 4 | 16 | 25 | 55 | 50 | - |
| Pb | 23 | 30 | 52 | 25 | 20 | 19 | 22 | 21 | - | 27 | 24 | 20 | 30 | 17 | 28 | 24 | 14 |
| Rb | 86 | 75 | 50 | 61 | 55 | 49 | 56 | 76 | 34 | 35 | 202 | 75 | 128 | 88 | 131 | 132 | 34 |
| Sc | 11 | 10 | 14 | 22 | 11 | 24 | 21 | 16 | - | - | - | - | - | - | 16 | 24 | - |
| Sr | 273 | 281 | 345 | 264 | 321 | 318 | 296 | 281 | 393 | 309 | 320 | 297 | 353 | 341 | 942 | 748 | 215 |
| Th | 8 | 12 | 3 | 8 | 8 | 10 | 4 | 9 | 5 | 7 | 11 | 13 | 18 | 13 | 15 | 19 | - |
| U | 5 | 9 | 12 | 2 | 1 | 5 | 5 | 5 | - | - | - | 0 | - | - | 1 | 8 | - |
| V | 87 | 95 | 96 | 153 | 141 | 152 | 138 | 133 | 237 | 221 | 160 | 121 | 91 | 111 | 160 | 179 | 118 |
| Y | 22 | 25 | 22 | 25 | 32 | 26 | 22 | 24 | 27 | 25 | 27 | 28 | 20 | 22 | 24 | 25 | - |
| Zr | 122 | 113 | 112 | 144 | 145 | 144 | 137 | 135 | 89 | 84 | 147 | 115 | 113 | 128 | 208 | 202 | - |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 66.15 | 65.63 | 65.77 | 30.41 | 31.50 | na | na | na | na | 66.36 | 67.56 | 53.27 | 53.48 | 56.38 | 55.12 | 51.36 |
| Al2O3 | 17.92 | 18.19 | 18.30 | 17.60 | 19.65 | na | na | na | na | 11.69 | 12.31 | 13.37 | 13.97 | 20.85 | 20.51 | 21.28 |
| FeO | 0.03 | bd | 0.01 | 20.48 | 19.81 | 0.08 | 1.93 | 0.12 | 0.36 | 0.11 | 0.10 | 1.01 | 0.39 | bd | 0.02 | bd |
| MgO | na | na | na | 17.15 | 17.83 | 0.16 | 18.06 | bd | 0.08 | bd | 0.08 | 0.29 | 0.02 | 0.08 | bd | bd |
| MnO | na | na | na | 0.37 | 0.39 | bd | bd | 0.82 | 0.45 | bd | 0.09 | 0.05 | bd | 0.04 | bd | bd |
| CaO | bd | 0.01 | bd | 0.71 | 0.45 | 50.03 | 29.53 | 49.00 | 49.24 | 3.95 | 4.38 | 0.55 | 0.50 | 0.04 | 0.11 | 10.37 |
| Na2O | 0.04 | 0.08 | 0.26 | 0.63 | 0.65 | na | na | na | na | 0.90 | 0.68 | 0.66 | 0.90 | 10.97 | 11.52 | 0.59 |
| K2O | 16.13 | 16.54 | 15.99 | 0.04 | 0.19 | na | na | na | na | 1.97 | 1.64 | 1.88 | 4.18 | 0.05 | bd | 0.89 |
| SrO | na | na | na | 0.86 | 0.11 | bd | 0.18 | 0.01 | bd | 2.33 | 1.59 | 0.79 | 1.87 | 0.43 | 0.58 | 1.10 |
| BaO | 0.03 | 0.20 | 0.55 | na | na | na | na | bd | bd | 0.52 | 0.29 | 15.97 | 14.66 | bd | bd | 0.14 |
| Total | 100.33 | 100.65 | 100.88 | 87.64 | 90.60 | 50.27 | 49.7 | 49.95 | 50.13 | 87.84 | 88.72 | 87.87 | 90.56 | 88.80 | 87.86 | 85.73 |
| Atoms | 8(O) | 8(O) | 8(O) | 28(O) | 28(O) | 1(Ctn) | 2(Ctn) | 1(Ctn) | 1(Ctn) | 72(O) | 72(O) | 72(O) | 72(O) | 96(O) | 96(O) | 48(O) |
| Si | 3.03 | 3.02 | 3.01 | 6.23 | 6.20 | - | - | - | - | 29.61 | 29.52 | 27.27 | 26.82 | 33.72 | 33.60 | 16.14 |
| Al | 0.97 | 0.98 | 0.99 | 4.24 | 4.55 | - | - | - | - | 6.12 | 6.30 | 8.10 | 8.28 | 14.76 | 14.76 | 7.86 |
| Fe3+ | 0.00 | 0.00 | 0.00 | 0.24 | 0.32 | 0.00 | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.45 | 0.18 | 0.00 | 0.00 | 0.00 |
| Fe2+ | 0.00 | 0.00 | 0.00 | 3.24 | 2.90 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mg | - | - | - | 5.22 | 5.22 | 0.01 | 0.90 | 0.00 | 0.00 | 0.00 | 0.09 | 0.18 | 0.00 | 0.00 | 0.00 | 0.00 |
| Mn | - | - | - | 0.07 | 0.07 | 0.00 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Ca | 0.00 | 0.00 | 0.00 | 0.11 | 0.11 | 0.99 | 1.05 | 0.99 | 0.98 | 1.89 | 2.07 | 0.27 | 0.27 | 0.00 | 0.12 | 3.48 |
| Na | 0.00 | 0.01 | 0.02 | 0.25 | 0.25 | - | - | - | - | 0.81 | 0.54 | 0.63 | 0.90 | 12.72 | 13.56 | 0.36 |
| K | 0.94 | 0.97 | 0.94 | 0.04 | 0.04 | - | - | - | - | 1.08 | 0.90 | 1.26 | 2.70 | 0.00 | 0.00 | 0.36 |
| Sr | - | - | - | 0.11 | 0.00 | 0.00 | 0.00 | - | - | 0.63 | 0.36 | 0.27 | 0.54 | 0.12 | 0.24 | 0.18 |
| Ba | 0.00 | 0.00 | 0.01 | - | - | - | - | - | - | 0.09 | 0.09 | 3.24 | 2.88 | 0.00 | 0.00 | 0.00 |
| Sample | Location | FI Types | FIA | Th (°C) | Tm(ice) (°C) | Salinity (wt % NaCl equiv) |
|---|---|---|---|---|---|---|
| ASH, KP627 | Silver Hill, Sapes | High Th (°C) | ||||
| L + V → L | FIA 1 | 201–219 (n = 5) | −0.9 to −0.6 (n = 5) | 1.1 to 1.6 | ||
| L + V → L | FIA 2 | 205–212 (n = 4) | n.a. | - | ||
| L + V → L | FIA 3 | 205–215 (n = 3) | −1.0 (n = 1) | 1.7 | ||
| L + V → L | FIA 4 | 207–218 (n = 5) | n.a. | - | ||
| L + V → L | FIA 5 | 218–230 (n = 4) | −1.1 to −0.7 (n = 5) | 1.2 to 1.9 | ||
| Low Th (°C) | ||||||
| L + V → L | FIA 6 | 189–190 (n = 5) | −1.2 (n = 3) | 2.1 | ||
| L + V → L | FIA 7 | 189–200 (n = 3) | −1.1 to −0.5 (n = 3) | 0.9 to 1.9 | ||
| L + V → L | FIA 8 | 190–203 (n = 5) | n.a. | - | ||
| 288, 347, 900, 494, 886, 888, 495, 812, 363, 818 | Kassiteres | High Th (°C) | ||||
| L + V → L | FIA 1 | 238–243 (n = 3) | −1.6 to −1.3 (n = 2) | 2.2 to 2.7 | ||
| L + V → L | FIA 2 | 244–263 (n = 13) | −1.1 to −0.8 (n = 11) | 1.4 to 1.9 | ||
| L + V → L | FIA 3 | 252–258 (n = 9) | −1.1 to −0.7 (n = 6) | 1.2 to 1.9 | ||
| L + V → L | FIA 4 | 267–275 (n = 9) | −0.9 to −0.7 (n = 6) | 1.2 to 1.6 | ||
| Low Th (°C) | ||||||
| L + V → L | FIA 5 | 211–227 (n = 5) | −1.2 to −0.7 (n = 5) | 1.2 to 2.1 | ||
| L + V → L | FIA 6 | 218–227 (n = 6) | −0.6 (n = 3) | 1.1 | ||
| L + V → L | FIA 7 | 218–234 (n = 7) | −1.8 to −1.7 (n = 5) | 2.9 to 3.1 | ||
| L + V → L | FIA 8 | 225–234 (n = 5) | −1.7 to −0.3 (n = 3) | 0.5 to 2.9 | ||
| L + V → L | FIA 9 | 229–239 (n = 6) | −1.2 to −0.5 (n = 5) | 0.9 to 2.1 | ||
| SF2, AS1 | Kornofolia, Soufli | L + V → L | FIA 1 | 191–205 (n = 5) | −2.7 to −1.2 (n = 5) | 2.1 to 4.5 |
| L + V → L | FIA 2 | 191–212 (n = 6) | n.a. | - | ||
| L + V → L | FIA 3 | 194–219 (n = 8) | −2.4 to −1.4 (n = 8) | 2.4 to 4.0 | ||
| L + V → L | FIA 4 | 206–220 (n = 6) | −2.4 to −1.9 (n = 3) | 3.2 to 4.0 | ||
| L + V → L | FIA 5 | 211–223 (n = 5) | n.a. | - | ||
| LS1, LS3 | Megala Therma, Lesvos isl. | High Th (°C) | ||||
| L + V → L | FIA 1 | 235–245 (n = 7) | −2.8 to −2.7 (n = 5) | 4.5 to 4.7 | ||
| L + V → L | FIA 2 | 236–246 (n = 9) | −2.6 to −2.5 (n = 2) | 4.2 to 4.4 | ||
| L + V → L | FIA 3 | 236–246 (n = 5) | −2.7 to −2.5 (n = 3) | 4.2 to 4.5 | ||
| L + V → L | FIA 4 | 238–241 (n = 3) | n.a. | - | ||
| Low Th (°C) | ||||||
| L + V → L | FIA 5 | 219–229 (n = 6) | −2.9 to −2.2 (n = 6) | 3.7 to 4.8 | ||
| L + V → L | FIA 6 | 219–235 (n = 8) | −2.4 to −2.1 (n = 4) | 3.6 to 4.0 | ||
| L + V → L | FIA 7 | 219–230 (n= 6) | −2.9 to −2.0 (n = 6) | 3.4 to 4.8 | ||
| L + V → L | FIA 8 | 226–234 (n = 5) | n.a. | - | ||
| L + V → L | FIA 9 | 227–232 (n = 9) | −2.9 to −2.1 (n = 9) | 3.6 to 4.8 | ||
| M1 | Chondro Vouno, Milos isl. | High Th (°C) | ||||
| L + V → L | FIA 1 | 204–211 (n = 4) | −5.0 to −4.2 (n = 4) | 6.7 to 7.9 | ||
| L + V → L | FIA 2 | 205–219 (n = 5) | −5.0 to −4.1 (n = 2) | 6.6 to 7.9 | ||
| L + V → L | FIA 3 | 205–221 (n = 5) | −4.7 to −4.0 (n = 4) | 6.5 to 7.5 | ||
| L + V → L | FIA 4 | 209–212 (n = 3) | −5.0 to −4.2 (n = 3) | 6.7 to 7.9 | ||
| Low Th (°C) | ||||||
| L + V → L | FIA 5 | 193–198 (n = 6) | −4.7 to −3.9 | 6.3 to 7.5 | ||
| L + V → L | FIA 6 | 195–201 (n = 4) | −4.9 to −4.7 (n = 2) | 7.5 to 7.7 | ||
| L + V → L | FIA 7 | 196–197 (n = 3) | n.a. | - | ||
| L + V → L | FIA 8 | 196–203 (n = 8) | n.a. | - | ||
| M2, ML2 | Kalogries, Milos isl. | L + V → L | FIA 1 | 190–201 (n = 5) | −3.1 to −2.9 (n = 3) | 4.8 to 5.1 |
| L + V → L | FIA 2 | 193–197 (n = 3) | n.a. | - | ||
| L + V → L | FIA 3 | 193–202 (n = 6) | −2.9 to −2.4 (n = 2) | 4.0 to 4.8 | ||
| L + V → L | FIA 4 | 193–204 (n = 3) | −2.5 (n = 1) | 4.2 | ||
| L + V → L | FIA 5 | 193–206 (n = 5) | −2.9 to −2.5 (n = 4) | 4.2 to 4.8 | ||
| L + V → L | FIA 6 | 196–207 (n = 4) | −3.4 to −2.5 (n = 4) | 4.2 to 5.6 |
| Sample | Locality | δ18ΟQtz | Th (°C) Range | δ18ΟFl min | δ18ΟFl max |
|---|---|---|---|---|---|
| KS1 | Kassiteres | 10.5 | 211–275 | −0.5 | 2.6 |
| SH2 | Sapes | 8.2 | 189–230 | −4.3 | −1.7 |
| KIR1 | Kirki | 19.1 | - | - | - |
| SF2 | Kornofolia | 20.5 | 194–223 | 8.4 | 10.2 |
| LS2 | Lesvos | 3.3 | 219–246 | −7.3 | −5.8 |
| M1 | Milos | 14.1 | 193–219 | 1.9 | 3.5 |
| M2 | Milos | 13.4 | 190–207 | 1.0 | 2.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voudouris, P.; Melfos, V.; Mavrogonatos, C.; Tarantola, A.; Gӧtze, J.; Alfieris, D.; Maneta, V.; Psimis, I. Amethyst Occurrences in Tertiary Volcanic Rocks of Greece: Mineralogical, Fluid Inclusion and Oxygen Isotope Constraints on Their Genesis. Minerals 2018, 8, 324. https://doi.org/10.3390/min8080324
Voudouris P, Melfos V, Mavrogonatos C, Tarantola A, Gӧtze J, Alfieris D, Maneta V, Psimis I. Amethyst Occurrences in Tertiary Volcanic Rocks of Greece: Mineralogical, Fluid Inclusion and Oxygen Isotope Constraints on Their Genesis. Minerals. 2018; 8(8):324. https://doi.org/10.3390/min8080324
Chicago/Turabian StyleVoudouris, Panagiotis, Vasilios Melfos, Constantinos Mavrogonatos, Alexandre Tarantola, Jens Gӧtze, Dimitrios Alfieris, Victoria Maneta, and Ioannis Psimis. 2018. "Amethyst Occurrences in Tertiary Volcanic Rocks of Greece: Mineralogical, Fluid Inclusion and Oxygen Isotope Constraints on Their Genesis" Minerals 8, no. 8: 324. https://doi.org/10.3390/min8080324








