Sulfide Breccias from the Semenov-3 Hydrothermal Field, Mid-Atlantic Ridge: Authigenic Mineral Formation and Trace Element Pattern
Abstract
:1. Introduction
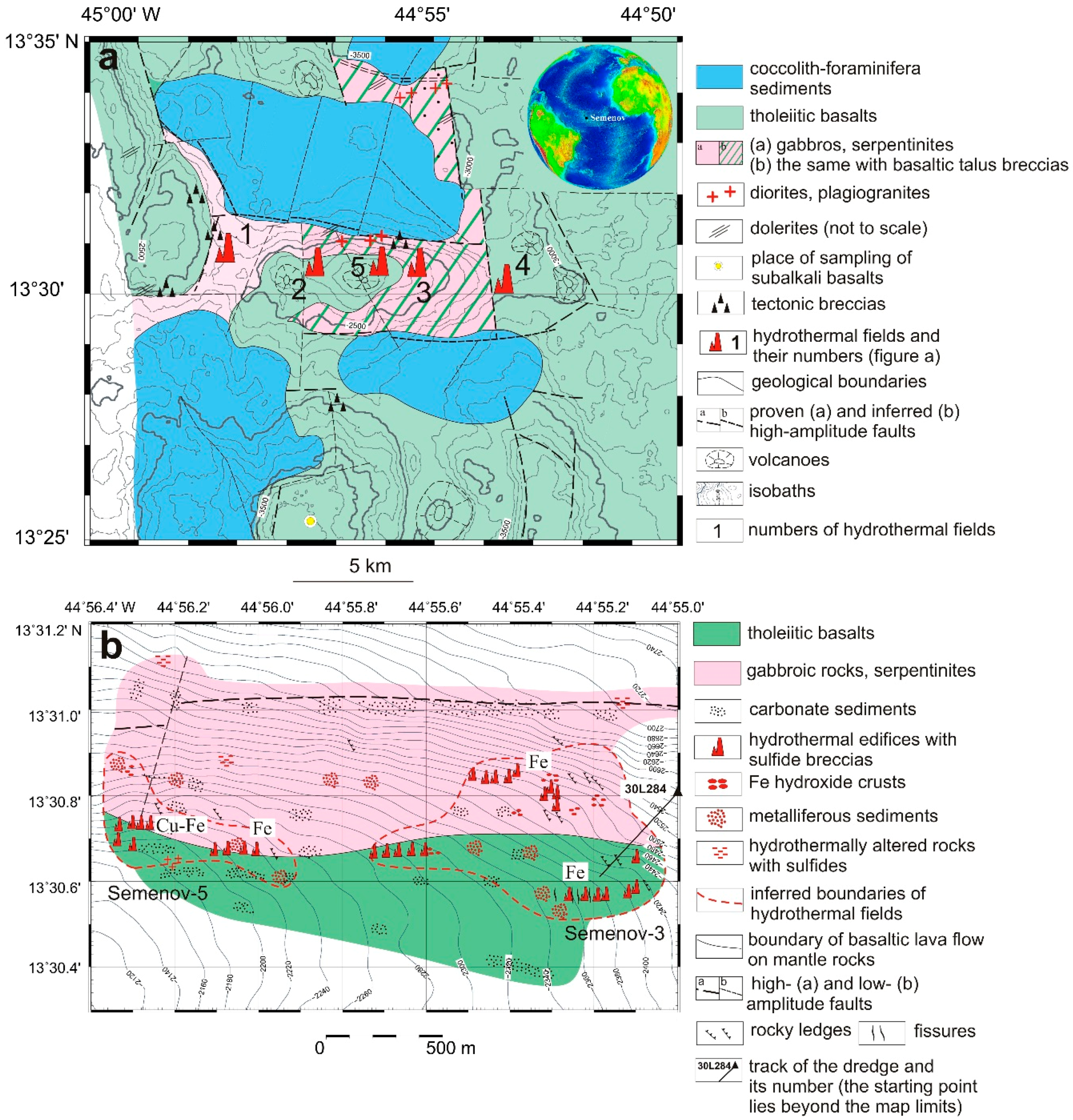
2. Geological Background
3. Samples and Analytical Techniques
4. Results
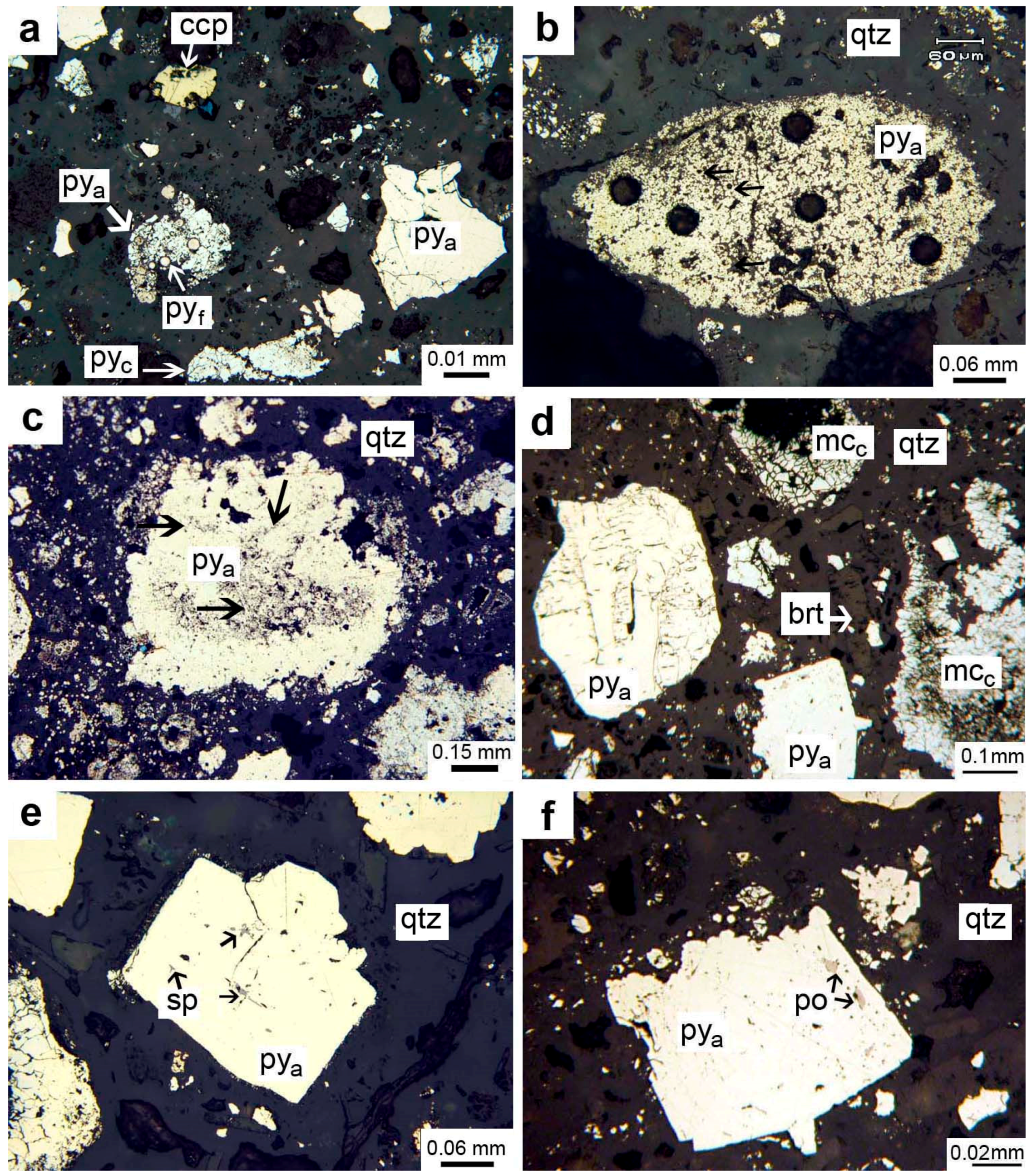
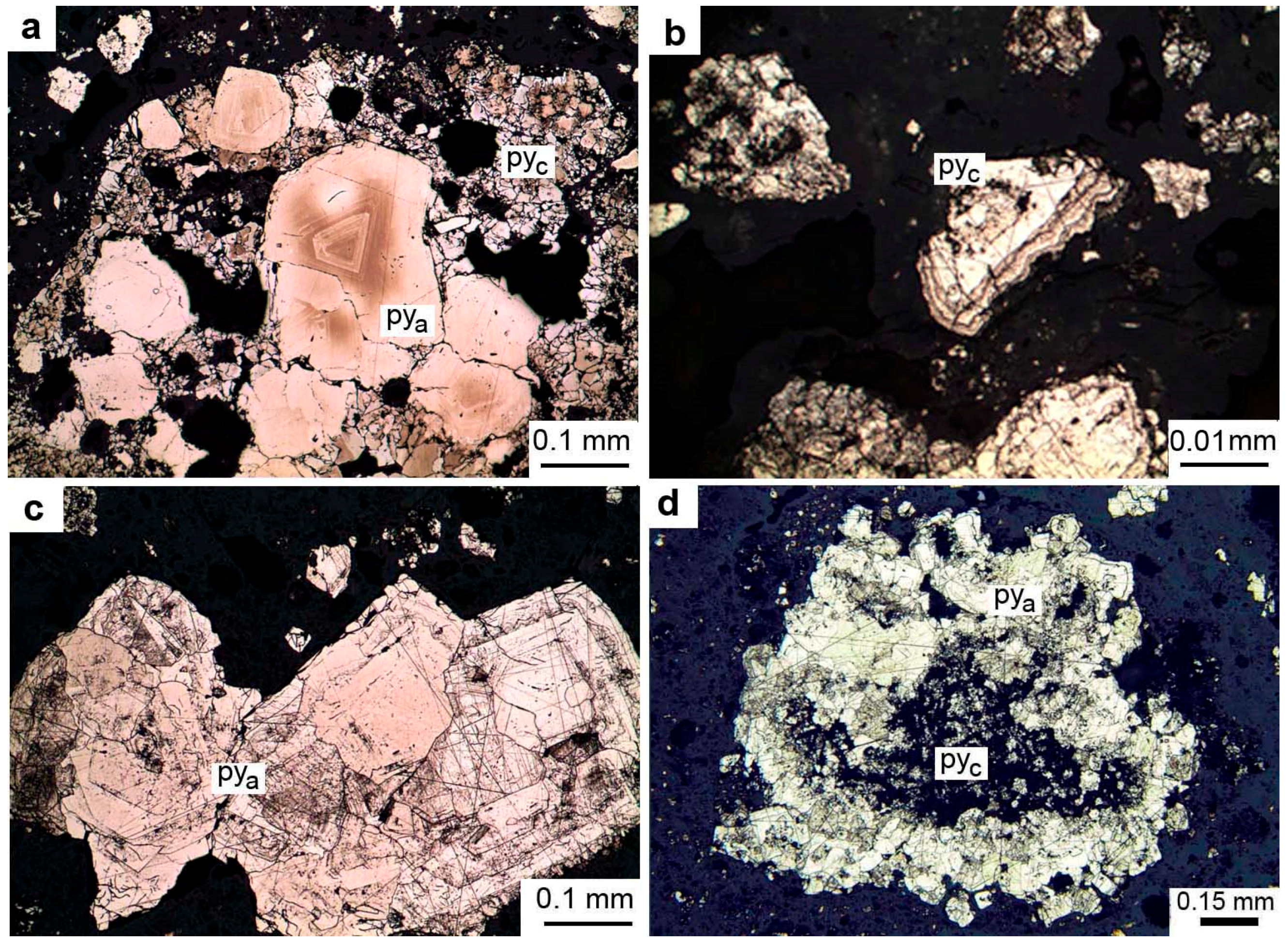
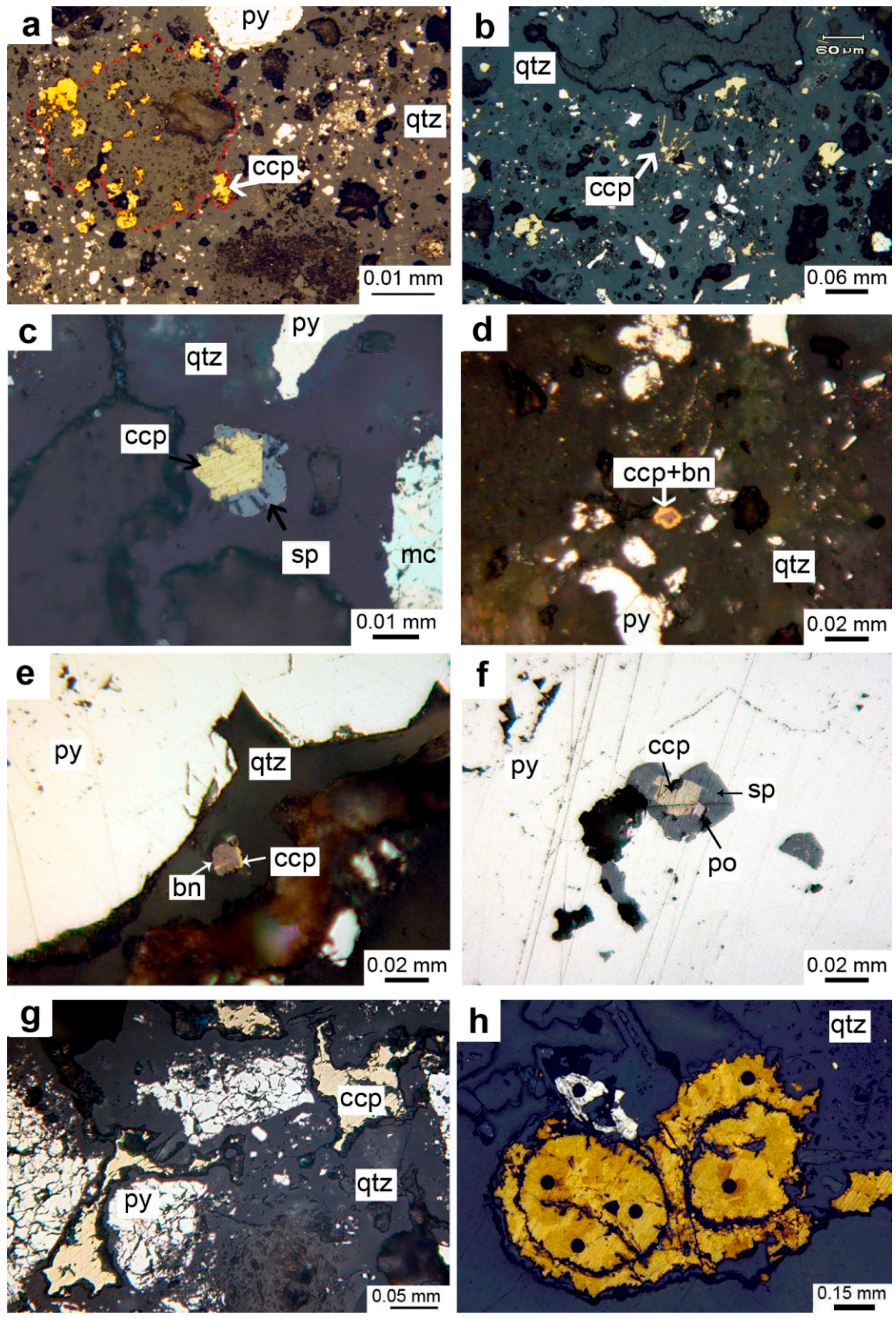
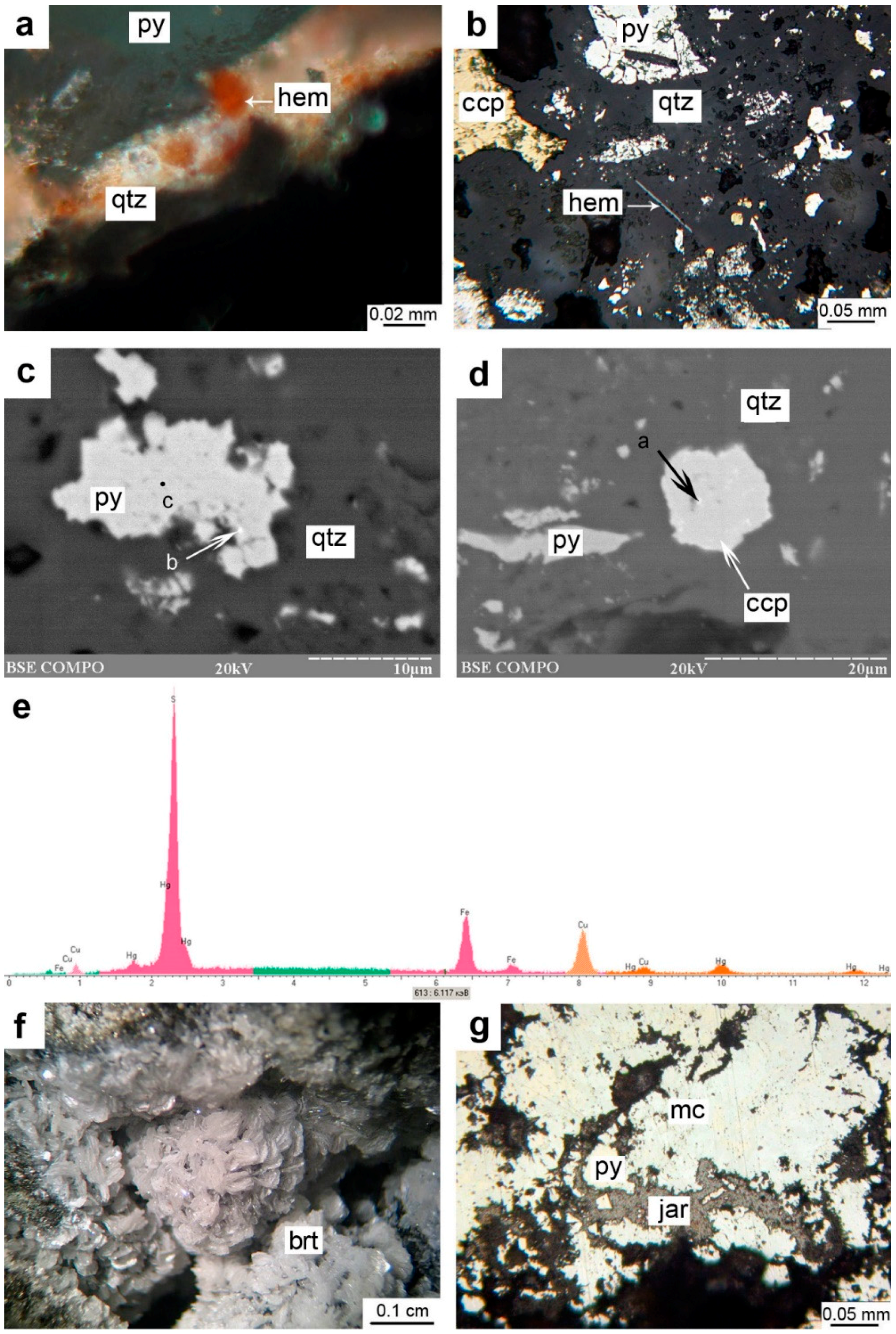
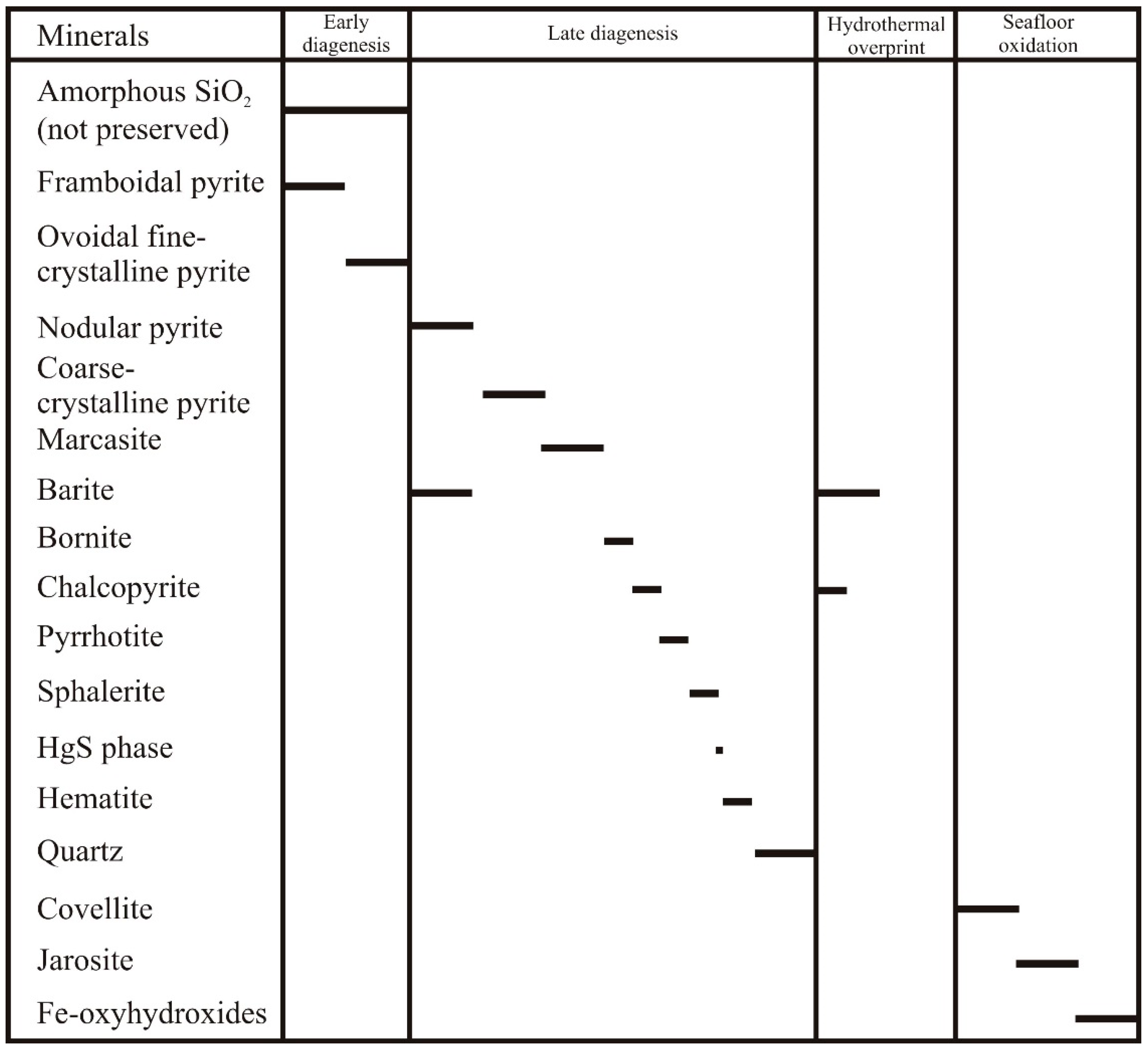
4.1. Textures, Structures, and Mineralogy of Sulfide Breccias
4.2. Bulk Chemistry
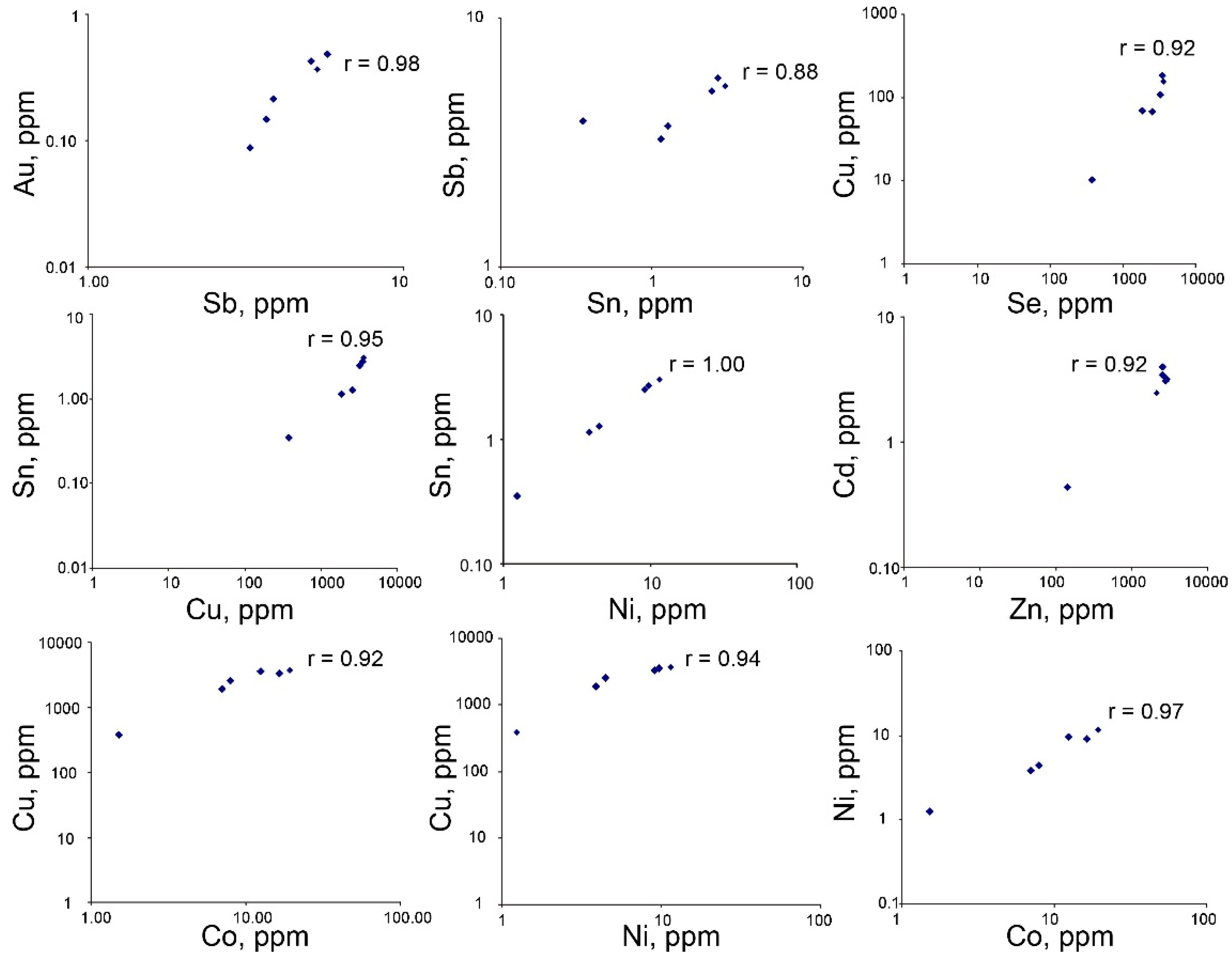
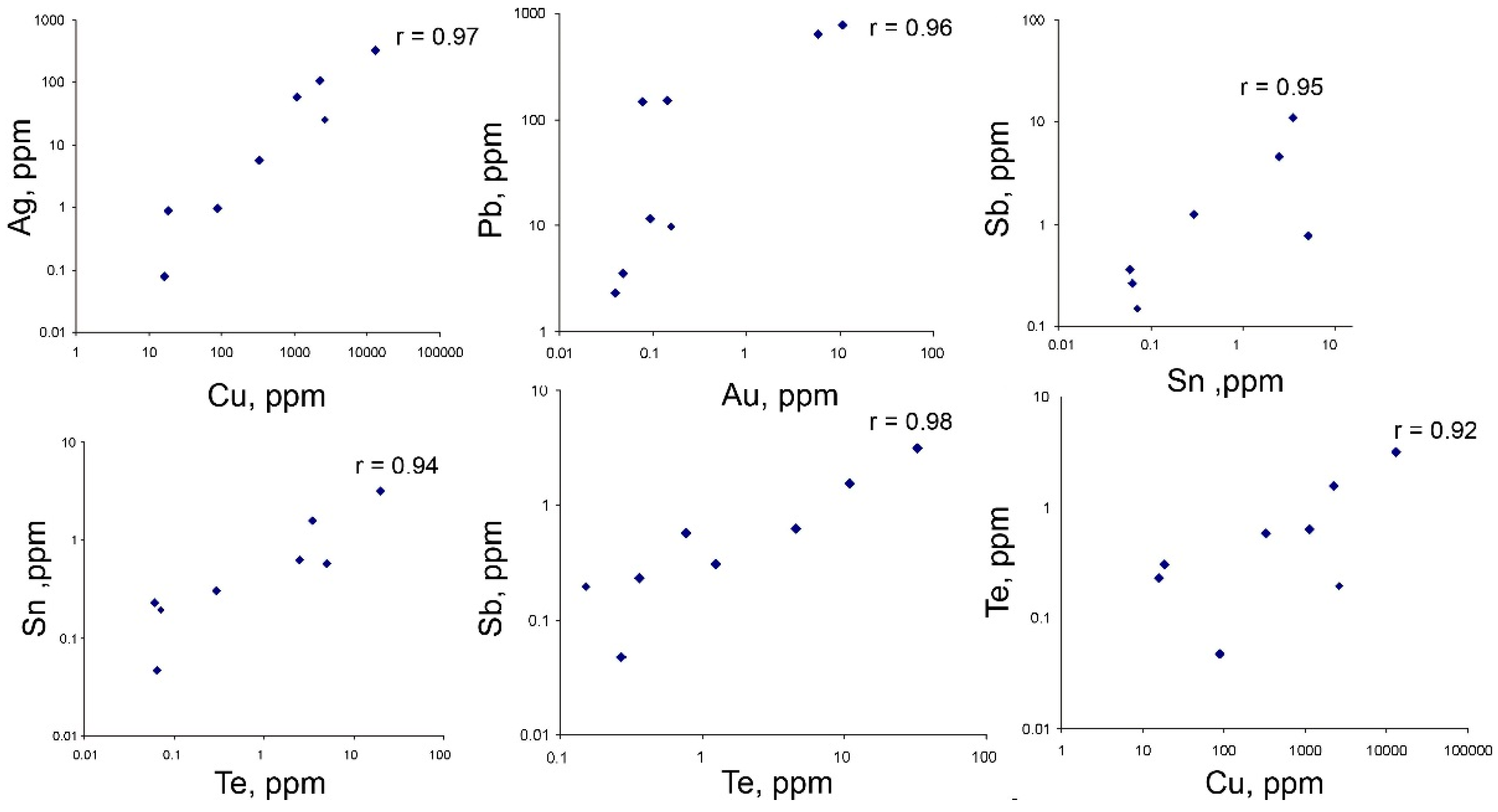
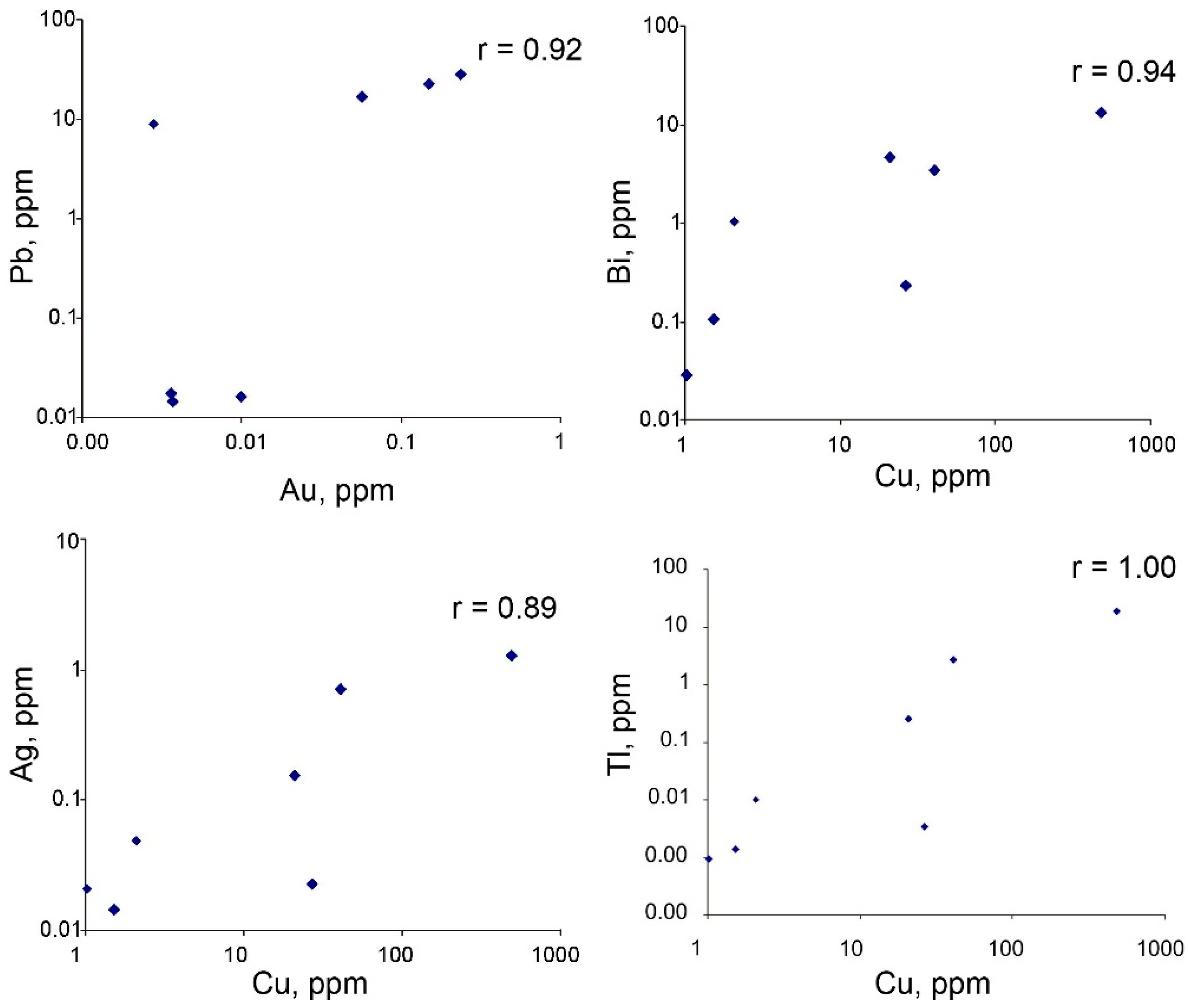
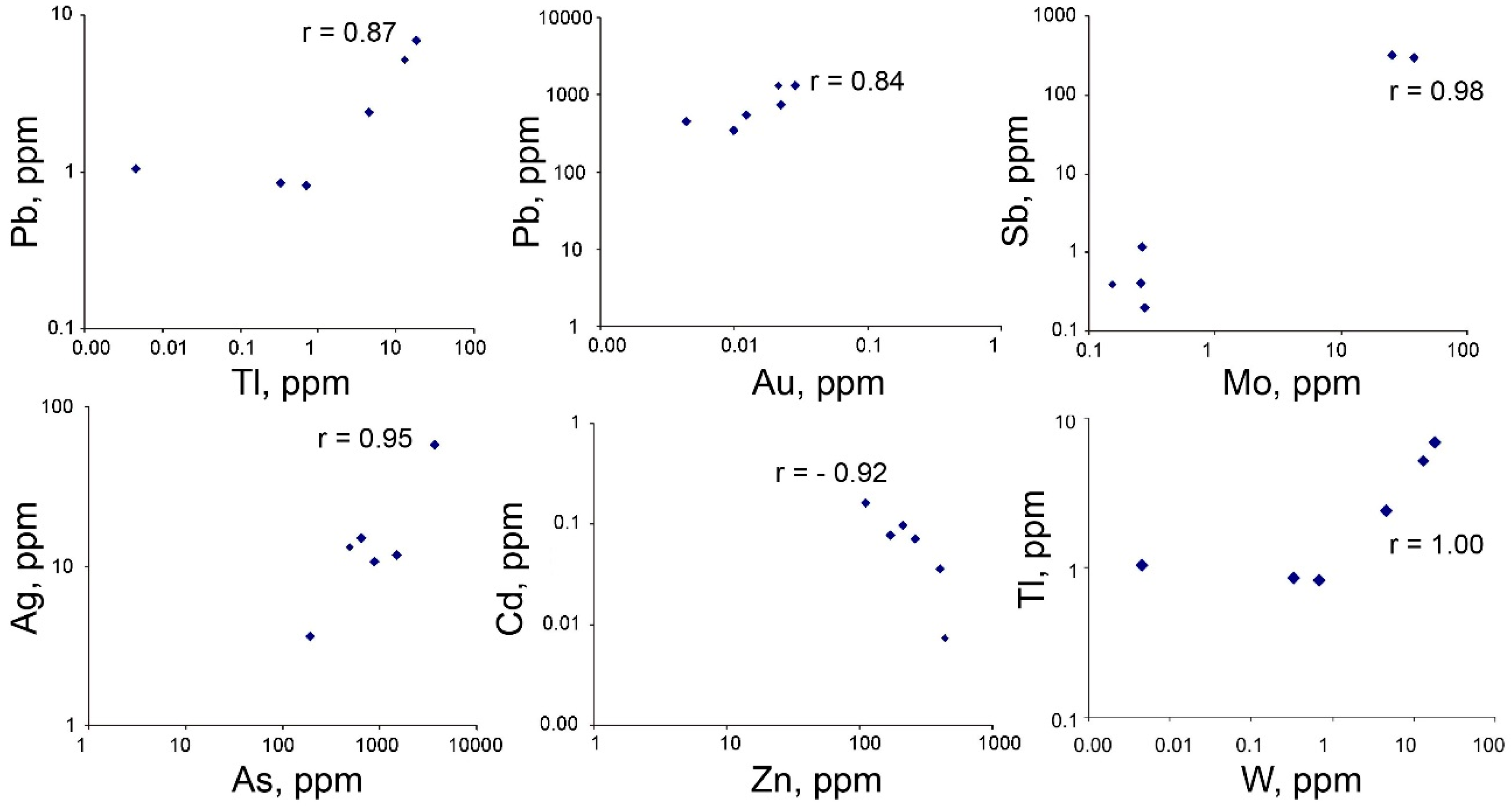
4.3. Trace Element Composition of Sulfides
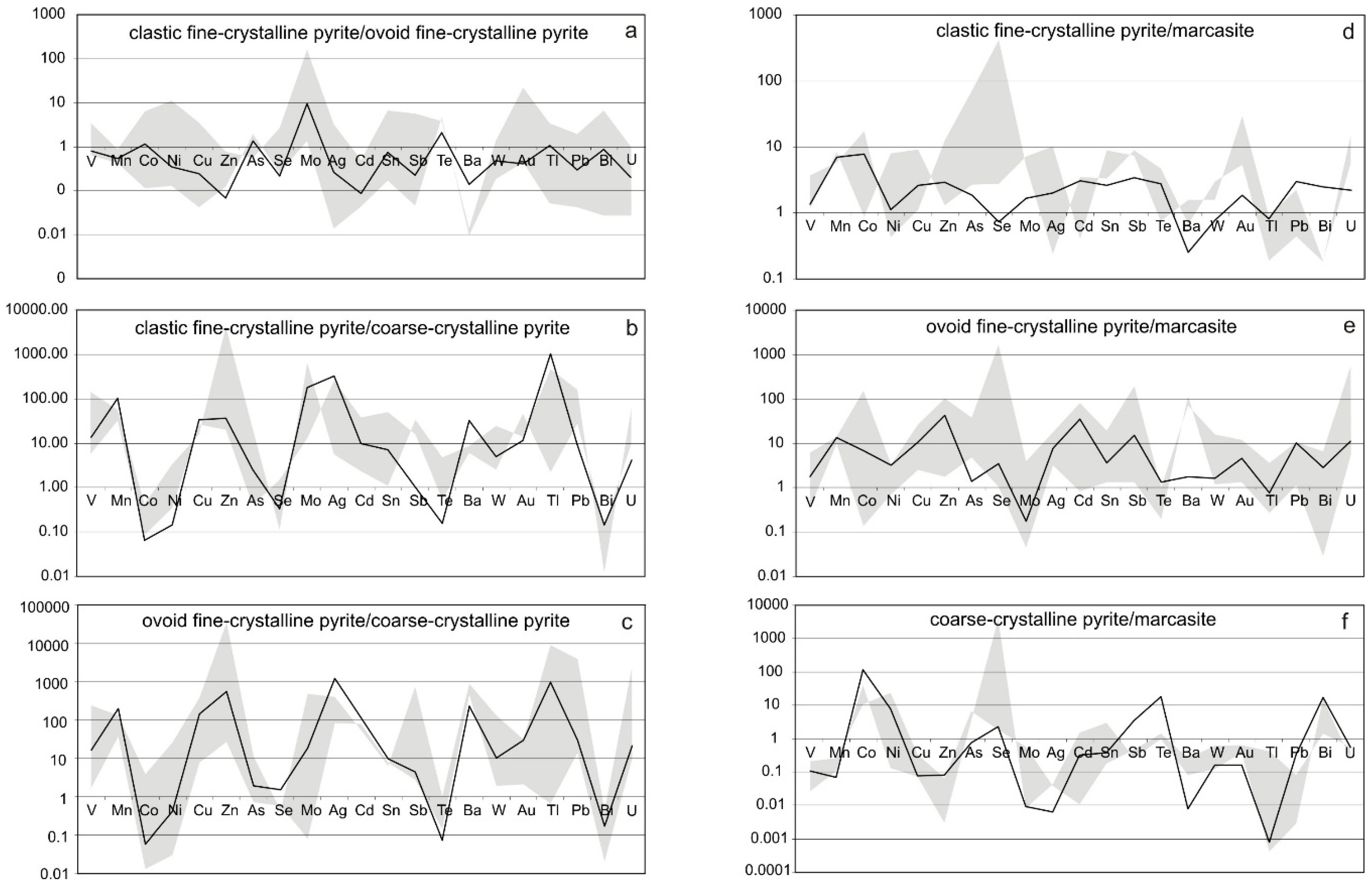
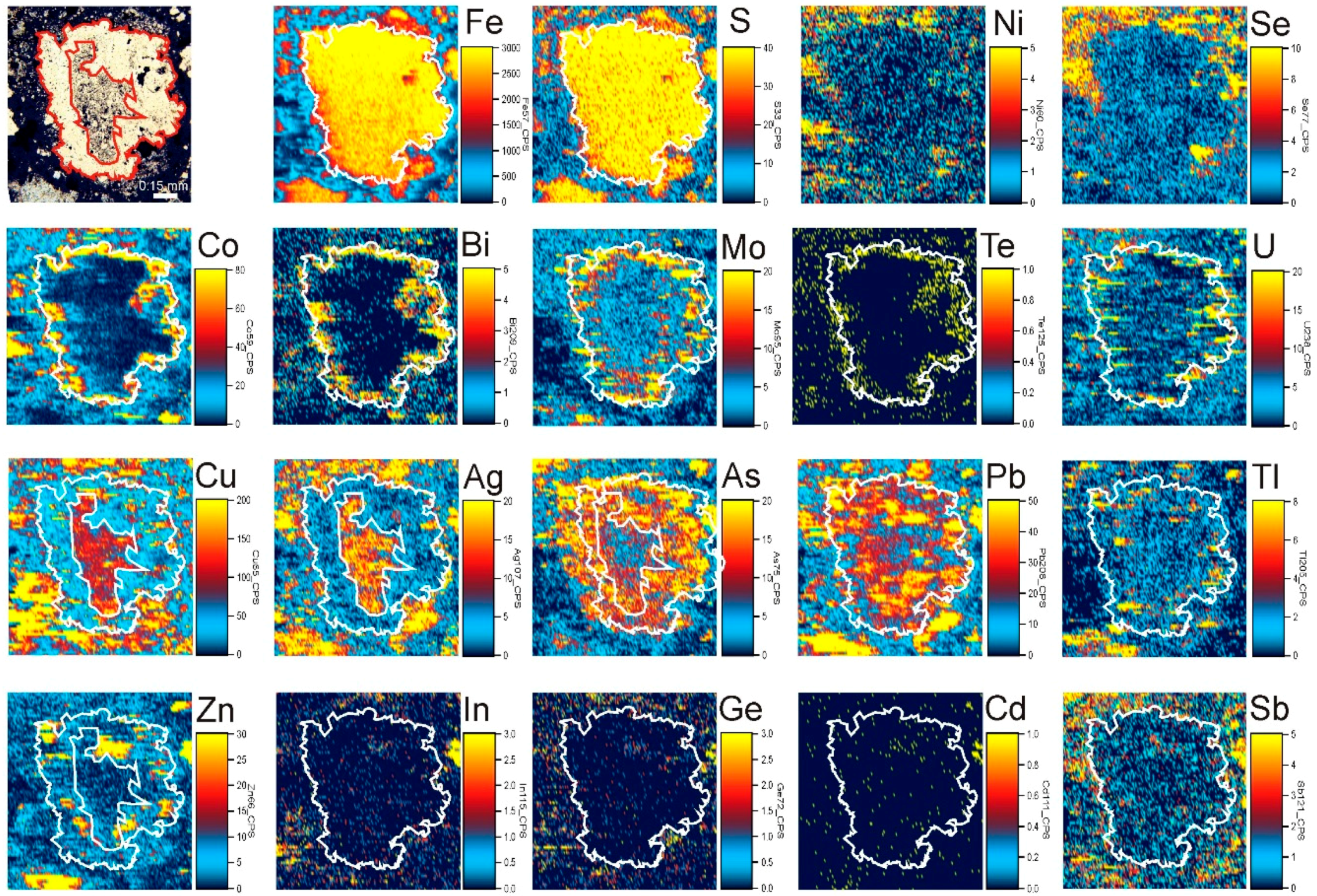
4.4. Trace Element Zoning in Nodular Pyrite
5. Discussion
5.1. Textures, Structures, and Mineralogy of Sulfide Breccias
5.2. Mineral Geochemistry
5.3. Trace Element Zoning of the Pyrite Nodule
5.4. Possible Exploration Guidelines
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Butler, I.B.; Nesbitt, R.V. Trace element distribution in the chalcopyrite wall of a black smoker chimney: Insights from laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Earth Planet. Sci. Lett. 1999, 167, 335–345. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy volcanic-hosted massive sulfide deposit (Southern Urals, Russia) using laser ablation-inductively coupled plasma mass spectrometry (LA-ICP-MS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.; Danyushevsky, L.; Herrington, R.J.; Ayupova, N.R.; dZaykov, V.V.; Lein, A.Y.; Tseluyko, A.S.; Melekestseva, I.Y.; et al. Chimneys in Paleozoic massive sulfide mounds of the Urals VMS deposits: Mineral and trace element comparison with modern black, grey, white and clear smokers. Ore Geol. Rev. 2017, 85, 64–106. [Google Scholar] [CrossRef]
- Melekestseva, I.Y.; Tret’yakov, G.A.; Nimis, P.; Yuminov, A.M.; Maslennikov, V.V.; Maslennikova, S.P.; Kotlyarov, V.A.; Beltenev, V.E.; Danyushevsky, L.V.; Large, R. Barite-rich massive sulfides from the Semenov-1 hydrothermal field (Mid-Atlantic Ridge, 13°30.87′ N): Evidence for phase separation and magmatic input. Mar. Geol. 2014, 349, 37–54. [Google Scholar] [CrossRef]
- Melekestseva, I.Y.; Maslennikov, V.V.; Maslennikova, S.P.; Danyushevsky, L.; Large, R. Covellite from Semenov-2 hydrothermal field (13°31.13′ N, Mid-Atlantic Ridge): Enrichment in trace elements according to LA-ICP-MS analysis. Dokl. Earth Sci. 2017, 473, 291–295. [Google Scholar] [CrossRef]
- Melekestseva, I.Y.; Maslennikov, V.V.; Tret’yakov, G.A.; Nimis, P.; Beltenev, V.E.; Rozhdestvenskaya, I.I.; Maslennikova, S.P.; Belogub, E.V.; Danyushevsky, L.; Large, R.; et al. Gold- and silver-rich massive sulfides from the Semenov-2 hydrothermal field, 13°31.13′ N, Mid-Atlantic Ridge: A case of magmatic contribution? Econ. Geol. 2017, 112, 741–773. [Google Scholar] [CrossRef]
- Wohlgemuth-Ueberwasser, C.C.; Viljonen, F.; Petersen, S.; Vrster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- Keith, M.; Häckel, F.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R. Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol. Rev. 2016, 72, 728–745. [Google Scholar] [CrossRef]
- Fallon, E.K.; Petersen, S.; Brooker, R.A.; Scott, T.B. Oxidative dissolution of hydrothermal mixed-sulphide ore: An assessment of current knowledge in relation to seafloor massive sulphide mining. Ore Geol. Rev. 2017, 86, 309–337. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Petersen, S.; Frische, M.; Qiu, Z.; Li, H.; Wu, Z.; Cui, R. Mineralogy and trace element geochemistry of sulfide minerals from the Wocan Hydrothermal Field on the slow-spreading Carlsberg Ridge, Indian Ocean. Ore Geol. Rev. 2017, 84, 1–19. [Google Scholar] [CrossRef]
- Goodfellow, W.D.; Franklin, J.M. Geology, mineralogy, and chemistry of sediment-hosted clastic massive sulfides in shallow cores, Middle Valley, Northern Juan de Fuca Ridge. Econ. Geol. 1993, 88, 2037–2068. [Google Scholar] [CrossRef]
- Ohmoto, H. Formation of volcanogenic massive sulfide deposits: The Kuroko perspective. Ore Geol. Rev. 1996, 16, 135–177. [Google Scholar] [CrossRef]
- Prokin, V.A.; Buslaev, F.P. Massive copper-zinc sulphide deposits in the Urals. Ore Geol. Rev. 1998, 14, 1–69. [Google Scholar] [CrossRef]
- Herrington, R.; Maslennikov, V.; Zaykov, V.; Seravkin, I.; Kosarev, A.; Buschmann, B.; Orgeval, J.-J.; Holland, N.; Tesalina, S.; Nimis, P.; et al. Classification of VMS deposits: Lesson from the South Uralides. Ore Geol. Rev. 2005, 27, 203–237. [Google Scholar] [CrossRef]
- Maslennikov, V.V. Morphogenetic analysis of massive sulfide bodies as a reflection of volcanic regimes. Litosfera 2012, 5, 96–113. (In Russian) [Google Scholar]
- Tornos, F.; Peter, J.M.; Allen, R.; Conde, C. Controls on the siting and style of volcanogenic massive sulphide deposits. Ore Geol. Rev. 2015, 68, 142–163. [Google Scholar] [CrossRef]
- Fairbridge, R.W. Phases of diagenesis and authigenesis. In Diagenesis in Sediments; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1967; pp. 19–89. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Ayupova, N.R.; Artemyev, D.A.; Tseluyko, A.S. Microtopochemistry of marcasite–pyrite nodule in illite–hematite gossanites of the Lahanos massive sulfide deposit (Pontides, Turkey) by LA-ICP-MS data. Mineralogiya 2017, 3, 48–70. (In Russian) [Google Scholar]
- Safina, N.P.; Maslennikov, V.V.; Artemyev, D.A.; Arkhireeva, N.S. Microtopochemistry and geochemical features of a pyrite nodule of carbonaceous siltstones of the Saf′yanovka massive sulfide deposit (Central Urals). Mineralogiya 2017, 3, 22–36. (In Russian) [Google Scholar]
- Hannington, M.; Jamieson, J.; Monecke, T.; Petersen, S.; Beaulieu, S. The abundance of seafloor massive sulfide deposits. Geology 2011, 39, 1155–1158. [Google Scholar] [CrossRef]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based deposits. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Monecke, T.; Petersen, S.; Hannington, M.D.; Grant, H.; Samson, I. The minor element endowment of modern sea-floor massive sulfides and comparison with deposits hosted in ancient volcanic successions. Econ. Geol. 2016, 18, 245–306. [Google Scholar]
- Van Dover, C.L.; Arnaud-Haond, S.; Gianni, M.; Helmreich, S.; Huber, J.A.; Jaeckel, A.L.; Metaxas, A.; Pendleton, L.H.; Petersen, S.; Ramirez-Llodra, E.; et al. Scientific rationale and international obligations for protection of active hydrothermal vent ecosystems from deep-sea mining. Mar. Policy 2018, 90, 20–28. [Google Scholar] [CrossRef]
- Nautilus Minerals. Available online: http://www.nautilusminerals.com/irm/content/png.aspx?RID=258 (accessed on 14 May 2018).
- Nautilus Minerals. Available online: http://www.nautilusminerals.com/IRM/ShowCategory.aspx?CategoryId=311&FilterStyle=B&archive=true&masterpage=31&year=2014&RID=291 (accessed on 14 May 2018).
- Marchig, V.; Blum, N.; Roonwal, G. Massive sulfide chimneys from the East Pacific Rise at 7°24′ S and 16°43′ S. Mar. Georesour. Geotechnol. 1997, 15, 49–66. [Google Scholar] [CrossRef]
- Embley, R.W.; Jonasson, I.R.; Perfit, M.R.; Franklin, J.M.; Tivey, M.A.; Malahoff, A.; Smith, M.F.; Francis, T.J.G. Submersible investigation of an extinct hydrothermal system on the Galapagos Ridge; sulfide mounds, stockwork zone, and differentiated lavas. Can. Mineral. 1988, 26, 517–539. [Google Scholar]
- Fouquet, Y.; Wafik, A.; Cambon, P.; Mevel, C.; Meyer, G.; Gente, P. Tectonic setting and mineralogical and geochemical zonation in the Snake Pit sulfide deposit (Mid-Atlantic Ridge at 23° N). Econ. Geol. 1993, 88, 2018–2036. [Google Scholar] [CrossRef]
- Rona, P.A.; Hannington, M.D.; Raman, C.V.; Thompson, G.; Tivey, M.K.; Humphris, S.E.; Lalou, C.; Petersen, S. Active and relict sea-floor hydrothermal mineralization at the TAG hydrothermal field, Mid-Atlantic Ridge. Econ. Geol. 1993, 88, 1989–2117. [Google Scholar] [CrossRef]
- Duckworth, R.C.; Knott, R.; Fallick, A.E.; Ricard, D.; Murton, B.J.; Van Dover, C. Mineralogy and sulphur isotope geochemistry of the Broken Spur sulphides, 29° N, Mid-Atlantic Ridge. In Hydrothermal Vents and Processes; Parson, L.M., Walker, C.L., Dixon, D.R., Eds.; Geological Society London Special Publication: London, UK, 1995; pp. 175–190. [Google Scholar] [CrossRef]
- Petersen, S.; Herzig, P.M.; Hannington, M.D. Third dimension of a presently forming VMS deposit: TAG hydrothermal field, Mid-Atlantic Ridge, 26° N. Miner. Depos. 2000, 35, 233–259. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Lein, A.Y.; Sagalevich, A.M.; Dorofeev, S.A.; Ul’yanova, N.V. Hydrothermal sulfide deposits of the Lucky Strike vent field, Mid-Atlantic Ridge. Geochem. Int. 2006, 44, 403–418. [Google Scholar] [CrossRef]
- Bogdanov, Y.A.; Lein, A.Y.; Maslennikov, V.V.; Li, S.; Ul’yanov, A.A. Mineralogical–geochemical features of sulfide ores from the Broken Spur hydrothermal vent field. Oceanology 2008, 48, 679–700. [Google Scholar] [CrossRef]
- Tao, C.; Li, H.; Huang, W.; Han, X.; Wu, G.; Su, X.; Zhou, N.; Lin, J.; He, Y.; Zhou, J. Mineralogical and geochemical features of sulfide chimneys from the 49°39′ E hydrothermal field on the Southwest Indian Ridge and their geological inferences. Chin. Sci. Bull. 2011, 56, 2828−2838. [Google Scholar] [CrossRef]
- Cherkashov, G.A.; Ivanov, V.N.; Beltenev, V.E.; Lazareva, L.I.; Rozhdestvenskaya, I.I.; Samovarov, M.L.; Poroshina, I.M.; Sergeev, M.B.; Stepanova, T.V.; Dobretsova, I.G.; et al. Sulfide ores of the northern equatorial part of the Mid-Atlantic Ridge. Oceanology 2013, 53, 680–693. [Google Scholar] [CrossRef]
- Wang, Y.; Han, X.; Petersen, S.; Jin, X.; Qiu, Z.; Zhu, J. Mineralogy and geochemistry of hydrothermal precipitates from Kairei hydrothermal field, Central Indian Ridge. Mar. Geol. 2014, 354, 69–80. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Zhai, S.; Yu, Z.; Cai, Z. Geochemical features of sulfides from the Deyin-1 hydrothermal field at the Southern Mid-Atlantic Ridge near 15° S. J. Oceanol. Univ. China 2017, 16, 1043–1054. [Google Scholar] [CrossRef]
- Beltenev, V.; Ivanov, V.; Rozhdestvenskaya, I.; Cherkashov, G.; Stepanova, T.; Shilov, V.; Pertsev, A.; Davydov, M.; Egorov, I.; Melekestseva, I.; et al. A new hydrothermal field at l3°30′ N on the Mid-Atlantic Ridge. InterRidge News 2007, 16, 9–10. [Google Scholar]
- MacLeod, C.J.; Searle, R.C.; Murton, B.J.; Casey, J.F.; Mallows, C.; Unsworth, S.C.; Achenbach, K.L.; Harris, M. Life cycle of oceanic core complexes. Earth Planet. Sci. Lett. 2009, 287, 333–344. [Google Scholar] [CrossRef]
- Beltenev, V.; Ivanov, V.; Rozhdestvenskaya, I.; Cherkashov, G.; Stepanova, T.; Shilov, V.; Davydov, M.; Laiba, A.; Kaylio, V.; Narkevsky, E.; et al. New data about hydrothermal fields on the Mid-Atlantic Ridge between 11°–14° N: 32nd cruise of R/V Professor Logatchev. InterRidge News 2009, 18, 14–18. [Google Scholar]
- Pertsev, A.N.; Bortnikov, N.S.; Vlasov, E.A.; Beltenev, V.E.; Dobretsova, I.G.; Ageeva, O.A. Recent sulfide deposits of the Semenov ore region (Mid-Atlantic Ridge, 13°30’ N): Types of associated rocks of the oceanic core complex and their hydrothermal alteration. Geol. Ore Depos. 2012, 54, 334–346. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Maksimov, F.; Zheleznov, A.; Cherkashov, G.; Bel’tenev, V.; Lazareva, L. 230Th/U chronology of ore formation within the Semyenov hydrothermal district (13°31’ N) at the Mid-AtlanticRidge. Geochronometria 2011, 38, 72–76. [Google Scholar] [CrossRef]
- Longerich, H.P.; Jackson, S.E.; Gunte, D. Laser ablation inductively coupled plasma mass spectrometric transient signal data acquisition and analyte concentration calculation. J. Anal. At. Spectrom. 1996, 11, 899–904. [Google Scholar] [CrossRef]
- Danyushevsky, L.V.; Robinson, R.; Gilbert, S.; Norman, M.; Large, R.; McGoldrick, P.; Shelley, J.M.G. Routine quantitative multielement analysis of sulfide minerals by laser ablation ICP-MS: Standard development and consideration of matrix effects. Geochem. Explor. Environ. Anal. 2011, 11, 51–60. [Google Scholar] [CrossRef]
- Wilson, S.A.; Ridley, W.I.; Koenig, A.E. Development of sulphide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Safina, N.P.; Melekestseva, I.Y.; Nimis, P.; Ankusheva, N.N.; Yuminov, A.M.; Kotlyarov, V.A.; Sadykov, S.A. Barite from the Saf’yanovka VMS deposit (Central Urals) and Semenov-1 and Semenov-3 hydrothermal sulfide fields (Mid-Atlantic Ridge): A comparative analysis of formation conditions. Miner. Depos. 2016, 54, 491–507. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Zaykov, V.V. Massive Sulfide Paleohydrothermal Fields of Marginal Oceanic Structures of the Urals: Classification, Ore Facies, Evolution Model; IMin UB RAS: Miass, Russia, 1998; 92p. (In Russian) [Google Scholar]
- Maslennikov, V.V. Sedimentogenesis, Halmyrolysis and Ecology of Massive Sulfide-Bearing Paleohydrothermal Fields; Geotur: Miass, Russia, 1999; 348p. (In Russian) [Google Scholar]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V.; Herrington, R.J.; Stanley, C.J. Tellurium-bearing minerals in zoned sulfide chimneys from Cu-Zn massive sulfide deposits of the Urals, Russia. Mineral. Petrol. 2013, 107, 67–99. [Google Scholar] [CrossRef]
- Love, L.G. Early diagenetic iron sulphide in Recent sediments of the Wash (England). Sedimentology 1967, 9, 327–352. [Google Scholar] [CrossRef]
- Love, L.G.; Amstutz, G.C. Review of microscopic pyrite from the Devonian Chattanooga shale and Rammelsberg Banderz. Fortschr. Mineral. 1966, 43, 273–309. [Google Scholar]
- Posfai, M.; Dunin-Borkowski, R.E. Sulfides in biosystems. Rev. Mineral. Geochem. 2006, 61, 679–714. [Google Scholar] [CrossRef] [Green Version]
- Safina, N.P.; Maslennikov, V.V. Sequence of mineral formation in clastic ores of the Saf’yanovka volcanic-hosted copper massive sulfide deposit, the Central Urals. Geol. Ore Depos. 2009, 51, 633–643. [Google Scholar] [CrossRef]
- Safina, N.P.; Maslennikov, V.V. Ore Clastic Sediments from Yaman-Kasy and Saf’yanovka Massive Sulfide Deposits, the Urals; IMin UB RAS: Miass, Russia, 2009; 260p. (In Russian) [Google Scholar]
- Binns, R.A.; Scott, S.D. Actively forming polymetallic sulfide deposits associated with felsic volcanic rocks in the eastern Manus back-arc basin, Papua New Guinea. Econ. Geol. 1993, 88, 2226–2236. [Google Scholar] [CrossRef]
- De Ronde, C.E.J.; Massoth, G.J.; Butterfield, D.A.; Christenson, B.W.; Ishibashi, J.; Ditchburn, R.G.; Hannington, M.D.; Brathwaite, R.L.; Lupton, J.E.; Kamenetsky, V.S.; et al. Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand. Miner. Depos. 2011, 46, 541–584. [Google Scholar] [CrossRef]
- Berkenbosch, H.A.; de Ronde, C.E.J.; Gemmel, J.B.; McNeil, A.W.; Goemann, K. Mineralogy and formation of black smoker chimneys from Brothers submarine volcano, Kermadec arc. Econ. Geol. 2012, 107, 1613–1633. [Google Scholar] [CrossRef]
- Large, R.R.; Danyushevsky, L.; Hollit, C.; Maslennikov, V.; Meffre, S.; Gilbert, S.; Bull, S.; Scott, R.; Emsbo, P.; Thomas, H.; et al. Gold and trace element zonation in pyrite using a laser imaging technique: Implications for the timing of gold in orogenic and Carlin-style sediment-hosted deposits. Econ. Geol. 2009, 104, 635–668. [Google Scholar] [CrossRef]
- Deditius, A.P.; Utsunomiya, S.; Reich, M.; Kesler, S.E.; Ewing, R.C.; Hough, R.; Walshe, J. Trace metal nanoparticles in pyrite. Ore Geol. Rev. 2011, 42, 32–46. [Google Scholar] [CrossRef]
- Huston, D.L.; Sie, S.H.; Suter, G.F.; Cooke, D.R.; Both, R.A.Q. Trace elements in sulfide minerals from eastern Australian volcanic hosted massive sulfide deposits. Part I. Proton microprobe analyses of pyrite, chalcopyrite, and sphalerite. Part II. Selenium levels in pyrite comparison with δ34S values and implication for the source of sulfur in volcanogenic hydrothermal systems. Econ. Geol. 1995, 90, 1167–1196. [Google Scholar] [CrossRef]
- Fouquet, Y.; Cambon, P.; Etoubleau, J.; Charlou, J.-L.; Ondréas, H.; Barriga, F.J.A.S.; Cherkashov, G.; Semkova, T.; Poroshina, I.; Bohn, M.; et al. Geodiversity of hydrothermal processes along the Mid-Atlantic Ridge and ultramafic-hosted mineralization: A new type of oceanic Cu-Zn-Co-Au volcanogenic massive sulfide deposits. In Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges; Rona, P.A., Devey, C.W., Dyment, J., Murton, B.J., Eds.; American Geophysical Union: Washington, DC, USA, 2010; pp. 321–368. [Google Scholar]
- Hannington, M.D.; Bleeker, W.; Kjarsgaard, I. Sulfide mineralogy, geochemistry and ore genesis of the Kidd Creek deposit: Part I. North, Central and South orebodies. In The Giant Kidd Creek Volcanogenic Massive Sulfide Deposit, Western Abitibi Subprovince, Canada; Economic Geology Monograph; Society of Economic Geologists: Littleton, CO, USA, 1999; Volume 10, pp. 163–224. [Google Scholar]
- Halbach, P.E.; Tunnicliffe, V.; Hein, J.R. Energy and Mass Transfer in Marine Hydrothermal Systems; Dahlem University Press: Berlin, Germany, 2003; 365p. [Google Scholar]
- George, L.; Cook, N.J.; Ciobanu, C.L.; Wade, B.P. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef] [Green Version]
- Genna, D.; Gaboury, D. Deciphering the hydrothermal evolution of a VMS system by LA-ICP-MS using trace elements in pyrite: An example from the Bracemac-McLeod deposits, Aditibi, Canada, and implication for exploration. Econ. Geol. 2015, 110, 2087–2108. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Ayupova, N.R.; Herrington, R.J.; Danyushevskiy, L.V.; Large, R.R. Ferruginous and manganiferous haloes around massive sulphide deposits of the Urals. Ore Geol. Rev. 2012, 47, 5–41. [Google Scholar] [CrossRef]
- Tseluyko, A.S.; Maslennikov, V.V.; Artemyev, D.A. Microtopochemistry of pyrite nodules of siliceous siltstones from the Yubileynoe massive sulfide deposit (South Urals): LA-ICP-MS data. Litosfera 2018. in press (In Russian) [Google Scholar]
- Safina, N.P.; Maslennikov, V.V. Lithological-mineralogical zonality of sulfide cyclothems in the Yaman-Kasy and Saf’yanovka massive sulfide deposits (Urals). Dokl. Earth Sci. 2008, 419, 432–434. [Google Scholar] [CrossRef]
- Fallon, E.K.; Niehorster, E.; Brooker, R.A.; Scott, T.B. Experimental leaching of massive sulphide from TAG active hydrothermal mound and implications for seafloor mining. Mar. Pollut. Bull. 2018, 126, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Berlin, M.; Zalups, R.K.; Fowler, B.A. Mercury. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 675–729. [Google Scholar]
- Halbach, P.; Praceius, B.; Marten, A. Geology and mineralogy of massive sulphide ores from the Central Okinawa Trough, Japan. Econ. Geol. 1993, 88, 2210–2225. [Google Scholar] [CrossRef]
- Lodenius, M. Mercury in terrestrial ecosystems: A review. In Mercury Pollution: Integration and Synthesis; Watras, C.J., Huckabee, J.W., Eds.; Lewis Publishers: Boca Raton, FL, USA, 1994; pp. 343–354. [Google Scholar]
- Petersen, S.; Herzig, P.M.; Schwarz-Schampera, U.; Hannington, M.D.; Jonasson, I.R. Hydrothermal precipitates associated with bimodal volcanism in the Central Bransfield Strait, Antarctica. Miner. Depos. 2004, 39, 358–379. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Ayupova, N.R.; Maslennikova, S.P.; Tret’yakov, G.A.; Melekestseva, I.Y.; Safina, N.P.; Belogub, E.V.; Large, R.R.; Danyushevsky, L.V.; Tseluyko, A.S.; et al. Toxic Elements in Massive Sulfide-Forming Systems; UB RAS: Yekaterinburg, Russia, 2014; 340p. (In Russian) [Google Scholar]
- Kowalczuk, P.B.; Manaig, D.O.; Drivenes, K.; Snook, B.; Aasly, K.; Kleiv, R.A. Galvanic leaching of seafloor massive sulphides using MnO2 in H2SO4-NaCl media. Minerals 2018, 8, 235. [Google Scholar] [CrossRef]


| Latitude | Longitude | Depth | |||
|---|---|---|---|---|---|
| Start | Finish | Start | Finish | Start | Finish |
| 13°30.801′ | 13°30.601′ N | 44°54.800′ W | 44°55.195′ W | 2616 m | 2393 m |
| Sample Number | Fe | S | Cu | Zn | Pb | Si | Ca | Mn | Mg | Ba | Ni | Co | Mo | Cd | Sn | Ag | Au |
| wt % | ppm | ||||||||||||||||
| Polar Marine Geosurvey Expedition (PMGE) Data | |||||||||||||||||
| 284-B2 | 44.34 | 46.22 | 0.21 | 0.10 | 0.02 | 0.13 | 0.70 | 0.02 | 0.12 | 0.88 | 0.003 | 0.003 | 0.001 | 8.45 | 7.90 | 11.6 | 0.63 |
| 284-B3 | 37.73 | 39.99 | 0.15 | 0.03 | 0.01 | 7.35 | 0.60 | 0.01 | 0.12 | 0.53 | 0.003 | 0.013 | 0.001 | 6.55 | 16.62 | 10.3 | 0.29 |
| 284-B4 | 38.38 | 36.50 | 0.15 | 0.15 | 0.01 | 4.91 | 0.70 | 0.01 | 0.12 | 0.88 | 0.002 | 0.007 | <0.001 | 10.55 | 29.93 | 6.3 | 0.53 |
| 284-B5 | 25.81 | 28.31 | 0.20 | 0.04 | 0.01 | 13.75 | 0.99 | 0.01 | 0.11 | 2.59 | 0.003 | 0.006 | <0.001 | 5.45 | 48.58 | 10.0 | 0.36 |
| 284-B7 | 32.28 | 33.68 | 0.12 | 0.06 | 0.01 | 6.27 | 0.70 | 0.01 | 0.10 | 4.12 | 0.003 | 0.002 | <0.001 | 6.90 | 10.38 | 17.4 | 0.28 |
| median | 37.73 | 36.50 | 0.15 | 0.06 | 0.01 | 6.27 | 0.70 | 0.01 | 0.12 | 0.88 | 0.003 | 0.006 | 0.001 | 6.90 | 16.62 | 10.3 | 0.36 |
| IMin Data | SiO2 | CaO | MnO | MgO | |||||||||||||
| wt % | ppm | wt % | ppm | ||||||||||||||
| 284-a | 47.47 | 22.98 | 2119 | 375 | 47.50 | 22.74 | 0.02 | 0.01 | 0.04 | n.a. | 47.50 | 23.75 | n.a. | 8.00 | n.a. | 12.7 | 0.48 |
| 284-b | 35.82 | 21.21 | 2706 | 419 | 31.50 | 37.60 | 0.01 | 0.01 | 0.05 | n.a. | 56.75 | 55.75 | n.a. | 6.50 | n.a. | 20.4 | 0.34 |
| 284-c | 49.37 | 22.62 | 665 | 265 | 52.75 | 22.62 | 0.01 | 0.01 | 0.03 | n.a. | 34.75 | 51.00 | n.a. | 9.50 | n.a. | 9.3 | 0.39 |
| 284-d | 45.00 | 23.96 | 1600 | 351 | 57.75 | 26.18 | 0.01 | 0.01 | 0.02 | n.a. | 47.75 | 35.00 | n.a. | 7.25 | n.a. | 11.8 | 0.32 |
| 284-e | 63.06 | 21.57 | 328 | 135 | 28.25 | 3.00 | 0.01 | 0.01 | 0.01 | n.a. | 95.75 | 51.25 | n.a. | 11.25 | n.a. | 5.8 | 0.20 |
| median | 47.47 | 22.62 | 1600 | 351 | 47.5 | 22.74 | 0.01 | 0.01 | 0.03 | 47.75 | 51 | 8 | 11.8 | 0.34 | |||
| Sample Number | V | Cr | Mn | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Sr | Mo | Cd | Sn | Sb | Te | Ba | Tl | Pb | Bi | Th | U |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 284-a | 3.30 | 19 | 83 | 24 | 13 | 751 | 203 | 0.39 | 0.11 | 148 | 38 | 77 | 30 | 0.47 | 1.99 | 2.09 | 0.08 | 1182 | 6.31 | 72 | 0.12 | 0.06 | 1.34 |
| 284-b | 4.21 | 46 | 45 | 38 | 12 | 1745 | 257 | 0.39 | 0.06 | 123 | 44 | 116 | 37 | 0.94 | 3.34 | 2.45 | 0.76 | 1340 | 5.69 | 85 | 0.43 | 0.01 | 0.72 |
| Number of Analysis | Fe | Cu | S | Co | Ni | As | Se | Au | Total |
|---|---|---|---|---|---|---|---|---|---|
| Pyrite | |||||||||
| 284-3-5-1 | 47.75 | <0.07 | 53.18 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 101.00 |
| 284-3-5-3 | 47.85 | <0.07 | 53.12 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 101.07 |
| 284-3-7-13 | 47.21 | <0.07 | 53.47 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 100.75 |
| Chalcopyrite | |||||||||
| 284-3-7-1 | 29.92 | 33.05 | 35.08 | <0.06 | <0.06 | <0.12 | 0.08 | <0.20 | 98.15 |
| 284-3-7-8 | 30.09 | 33.26 | 34.79 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 98.14 |
| 284-3-7-9 | 30.10 | 33.77 | 34.98 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 98.91 |
| 284-3-7-10 | 29.65 | 33.47 | 34.60 | <0.06 | <0.06 | <0.12 | <0.07 | 0.43 | 98.15 |
| 284-3-7-23 | 30.36 | 33.95 | 34.69 | <0.06 | <0.06 | <0.12 | 0.30 | <0.20 | 99.35 |
| 284-3-7-22 | 30.05 | 34.21 | 34.90 | <0.06 | <0.06 | <0.12 | <0.07 | <0.20 | 99.20 |
| 51V | 55Mn | 59Co | 60Ni | 65Cu | 66Zn | 75As | 77Se | 95Mo | 107Ag | 111Cd | 118Sn | 121Sb | 125Te | 182W | 197Au | 205Tl | 208Pb | 209Bi | 238U | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Clastic Fine-Crystalline Pyrite (8) | ||||||||||||||||||||
| m | 0.92d | 95 | 12 | 2.36 | 715 | 169 | 186 | 19 | 35 | 15 | 0.27 | 1.38 | 1.00 | 0.44 | 0.24 | 0.12 | 10 | 79 | 0.15 | 0.38 |
| av | 1.78 | 136 | 37 | 27 | 2417 | 528 | 236 | 148 | 139 | 66 | 0.66 | 3.95 | 6.33 | 0.83 | 0.40 | 2.14 | 12 | 216 | 0.52 | 0.60 |
| min | 0.54 | 5.09 | 0.17 | 0.16 | 16 | 17 | 63 | 2.65 | 2.17 | 0.08 | 0.02 | 0.06 | 0.15 | 0.05 | 0.06 | 0.04 | 0.44 | 2.35 | 0.00 | 0.02 |
| max | 5.99 | 422 | 123 | 128 | 12913 | 2191 | 463 | 481 | 696 | 333 | 1.96 | 20 | 32 | 3.15 | 1.33 | 11 | 41 | 772 | 1.82 | 2.23 |
| Ovoid Fine-Crystalline Pyrite (6) | ||||||||||||||||||||
| m | 1.17 | 180 | 10 | 6.82 | 2913 | 2524 | 140 | 90 | 3.68 | 59 | 3.12 | 1.88 | 4.47 | 0.21 | 0.49 | 0.29 | 9.69 | 264 | 0.18 | 1.87 |
| av | 1.20 | 243 | 11 | 6.68 | 2544 | 2161 | 240 | 100 | 3.22 | 56 | 2.76 | 1.83 | 4.49 | 0.36 | 0.53 | 0.29 | 10 | 259 | 0.16 | 1.74 |
| min | 0.86 | 12 | 1.52 | 1.25 | 379 | 144 | 33 | 10 | 1.64 | 5.77 | 0.44 | 0.35 | 3.26 | 0.01 | 0.32 | 0.09 | 8.53 | 56 | 0.01 | 0.85 |
| max | 1.73 | 490 | 19 | 12 | 3662 | 2867 | 834 | 184 | 4.37 | 105 | 3.99 | 3.03 | 5.74 | 0.83 | 1.00 | 0.49 | 12 | 396 | 0.28 | 2.24 |
| Coarse-Crystalline Pyrite (7) | ||||||||||||||||||||
| m | 0.07 | 0.92 | 177 | 16 | 21 | 4.61 | 75 | 59 | 0.19 | 0.05 | 0.03 | 0.19 | 1.00 | 2.86 | 0.05 | 0.01 | 0.01 | 8.79 | 1.05 | 0.09 |
| av | 0.34 | 3.73 | 339 | 67 | 82 | 21 | 251 | 120 | 10 | 0.32 | 0.03 | 0.21 | 0.86 | 2.40 | 0.12 | 0.07 | 3.07 | 11 | 3.23 | 0.08 |
| min | 0.00 | 0.09 | 0.40 | 0.05 | 1.02 | 0.00 | 2.96 | 23.67 | 0.00 | 0.01 | 0.01 | 0.05 | 0.00 | 0.01 | 0.00 | 0.00 | 0.00 | 0.01 | 0.03 | 0.00 |
| max | 1.03 | 13 | 1445 | 375 | 484 | 109 | 1140 | 318 | 54 | 1.28 | 0.05 | 0.40 | 2.06 | 5.81 | 0.52 | 0.23 | 19 | 28 | 13 | 0.22 |
| Marcasite (9) | ||||||||||||||||||||
| m | 0.68 | 14 | 1.50 | 2.09 | 276 | 58 | 100 | 26 | 21 | 7.76 | 0.09 | 0.52 | 0.29 | 0.16 | 0.31 | 0.06 | 13 | 26 | 0.06 | 0.17 |
| av | 1.29 | 22 | 18 | 3.49 | 474 | 265 | 99 | 45 | 31 | 12 | 0.65 | 0.68 | 1.02 | 0.61 | 0.35 | 0.10 | 15 | 67 | 1.10 | 0.16 |
| min | 0.14 | 0.87 | 0.01 | 0.37 | 14 | 1.38 | 0.86 | 0.01 | 0.42 | 0.33 | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 2.31 | 5.26 | 0.00 | 0.00 |
| max | 5.01 | 51 | 140 | 16 | 1422 | 1649 | 172 | 173 | 96 | 33 | 4.82 | 2.25 | 4.36 | 4.20 | 0.84 | 0.37 | 45 | 345 | 9.55 | 0.40 |
| Reniform Chalcopyrite (5) | ||||||||||||||||||||
| m | 8.86 | 0.85 | 0.08 | 0.16 | 350904 | 210 | 895 | 2.78 | 0.27 | 13 | 0.08 | 0.32 | 1.16 | 0.12 | 0.69 | 0.02 | 1.04 | 738 | 0.00 | 0.00 |
| av | 7.59 | 0.75 | 0.11 | 0.18 | 350799 | 268 | 1445 | 11 | 13 | 22 | 0.08 | 0.32 | 125 | 0.11 | 6.45 | 0.02 | 2.95 | 879 | 0.01 | 0.00 |
| 51V | 55Mn | 59Co | 60Ni | 65Cu | 66Zn | 75As | 77Se | 95Mo | 107Ag | 111Cd | 118Sn | 121Sb | 125Te | 182W | 197Au | 205Tl | 208Pb | 209Bi | 238U | |
| min | 2.01 | 0.22 | 0.05 | 0.07 | 348967 | 110 | 494 | 1.54 | 0.15 | 11 | 0.01 | 0.26 | 0.39 | 0.01 | 0.00 | 0.00 | 0.82 | 460 | 0.00 | 0.00 |
| max | 12 | 1.29 | 0.19 | 0.27 | 352425 | 444 | 3675 | 40 | 38 | 58 | 0.16 | 0.37 | 326 | 0.18 | 18 | 0.03 | 6.83 | 1330 | 0.01 | 0.00 |
| Clastic Crystalline Chalcopyrite (3) | ||||||||||||||||||||
| m | 21 | 6.11 | 8.40 | 1.95 | 323647 | 264 | 375 | 639 | 31 | 110 | 0.07 | 8.75 | 17 | 0.45 | 4.55 | 0.10 | 2.41 | 349 | 0.10 | 0.17 |
| av | 18 | 8.22 | 5.94 | 1.81 | 325500 | 225 | 449 | 617 | 23 | 407 | 0.13 | 9.97 | 11 | 0.84 | 15 | 0.17 | 3.22 | 396 | 0.16 | 0.13 |
| min | 12 | 0.63 | 0.07 | 0.01 | 299448 | 141 | 195 | 11 | 0.28 | 3.63 | 0.07 | 0.37 | 0.19 | 0.23 | 3.60 | 0.01 | 1.65 | 256 | 0.00 | 0.00 |
| max | 21 | 18 | 9.34 | 3.46 | 344139 | 271 | 778 | 1202 | 38 | 1106 | 0.24 | 21 | 17 | 1.85 | 38 | 0.41 | 5.60 | 584 | 0.37 | 0.21 |
| Objects | Semenov-3 (This Study) | Bracemac-Mcleod [65] | Lahanos [18] | Saf’yanovka [19] | Yubileynoe [67] |
|---|---|---|---|---|---|
| Host Rocks | Basalts | Rhyolites, Andesites, Basalts | Dacites and Rhyodacites | Rhyolites | Basalts and Rhyolites |
| Nodule-Hosted Rocks | Sulfide Breccias | Key Tuffite Horizon | Illite–Hematite Gossanites | Black Shales | Siliceous Rocks |
| Sulfide nodules | Pyrite nodule composed of a porous center with visible inclusions of quartz and barite and a polycrystalline rim with visible inclusions of sphalerite, chalcopyrite and pyrrhotite | Pyrite nodule composed of a porous center (Py I) with inclusions of sphalerite and chalcopyrite, which is overgrown by crystalline pyrite (Py II and Py V) | Marcasite–pyrite nodule composed of a porous pyrite center with inclusions of illite, barite, apatite, galena, aikinite, bornite, tennantite–tetrahedrite, hematite and rutile, which is overgrown by crystalline pyrite and marcasite | Pyrite nodule composed of a porous center with inclusions of chlorite, micas, sphalerite and chalcopyrite and a rim of crystalline pyrite with inclusions of galena | Pyrite nodule composed of a porous center with inclusions of quartz, chlorite, plagioclase, mica, galena, native gold and Bi-, Ag-, Au-, Hg- and Pb-tellurides |
| Trace elements enriched in the porous central zone | Cu, Ag | Cu and Ni; locally, As, Se, Au, Ag, Pb, Zn, Sb, Cd, Sn | Cu, Pb, Ag, Co, Ni and Hg; locally, As and Sb | Cu, Zn, Co, Ni, Mn, Tl, Sb, Au, Se, Bi, Cd, In, Sn | Cu, Zn, Pb, Sb, Bi, As, Au, Ag, Te, Hg, Co, Ni, As |
| Trace elements enriched in the intermediate crystalline zone | As; locally, Pb and Zn | Co, As and Tl; locally, Se | Pb and Cu; locally, Ag and Ni | Pb, Cu, As, Ag, Mo, Ge | Ni, As |
| Trace elements enriched in the crystalline rim | Co, Bi, Mo, Te, U | Tl; locally, Ni, Se | Ni, In, As, Sb, Cd, In, Mo | As, Ag | Au, Ag, Sb, Bi, Cu, Zn, Hg |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melekestseva, I.; Maslennikov, V.V.; Safina, N.P.; Nimis, P.; Maslennikova, S.; Beltenev, V.; Rozhdestvenskaya, I.; Danyushevsky, L.; Large, R.; Artemyev, D.; et al. Sulfide Breccias from the Semenov-3 Hydrothermal Field, Mid-Atlantic Ridge: Authigenic Mineral Formation and Trace Element Pattern. Minerals 2018, 8, 321. https://doi.org/10.3390/min8080321
Melekestseva I, Maslennikov VV, Safina NP, Nimis P, Maslennikova S, Beltenev V, Rozhdestvenskaya I, Danyushevsky L, Large R, Artemyev D, et al. Sulfide Breccias from the Semenov-3 Hydrothermal Field, Mid-Atlantic Ridge: Authigenic Mineral Formation and Trace Element Pattern. Minerals. 2018; 8(8):321. https://doi.org/10.3390/min8080321
Chicago/Turabian StyleMelekestseva, Irina, Valery V. Maslennikov, Nataliya P. Safina, Paolo Nimis, Svetlana Maslennikova, Victor Beltenev, Irina Rozhdestvenskaya, Leonid Danyushevsky, Ross Large, Dmitry Artemyev, and et al. 2018. "Sulfide Breccias from the Semenov-3 Hydrothermal Field, Mid-Atlantic Ridge: Authigenic Mineral Formation and Trace Element Pattern" Minerals 8, no. 8: 321. https://doi.org/10.3390/min8080321







