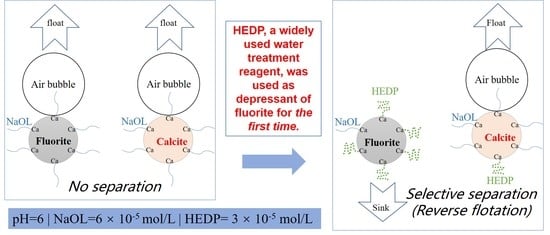
Reverse Flotation Separation of Fluorite from Calcite: A Novel Reagent Scheme
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Zeta Potential Measurements
3. Results and Discussions
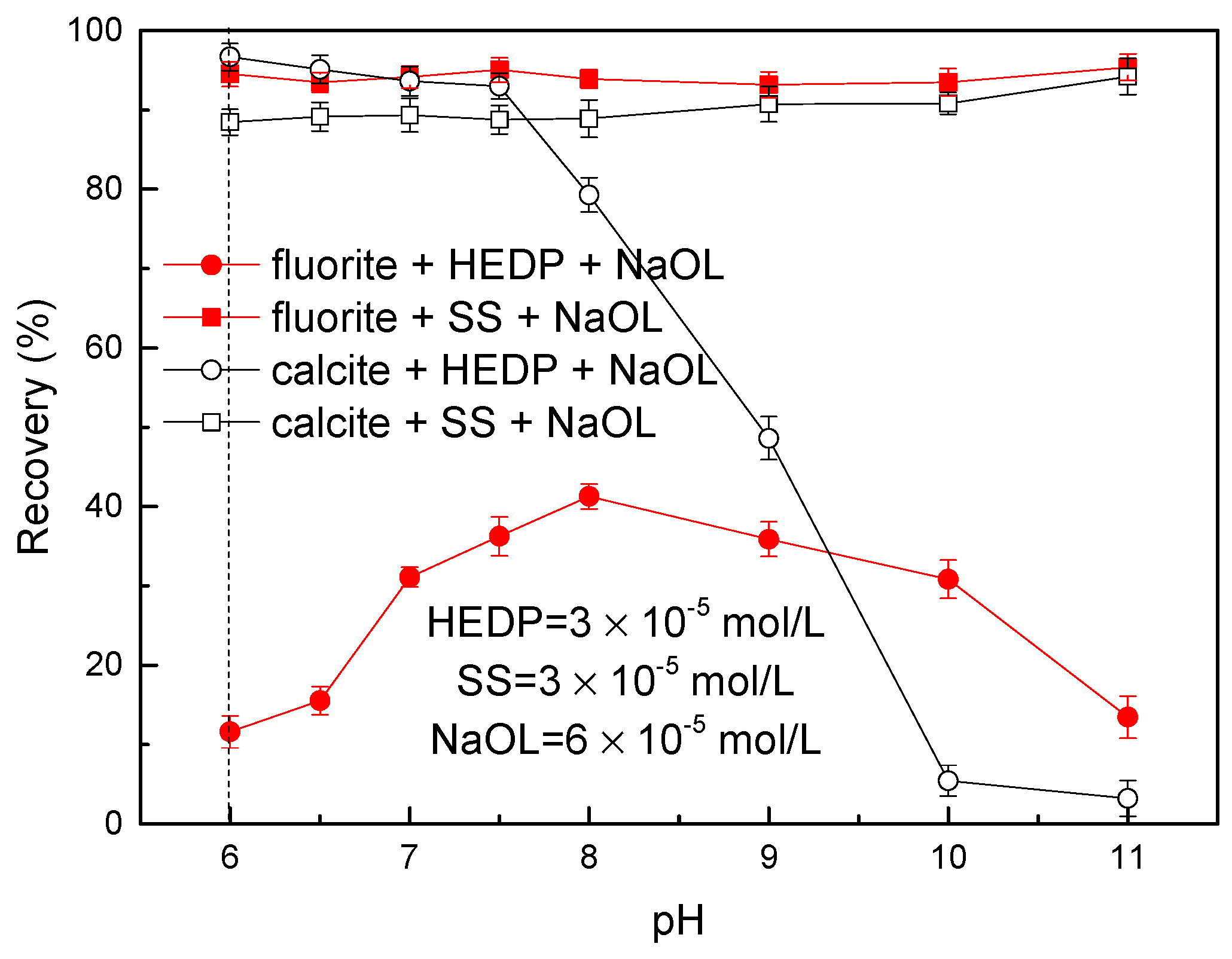
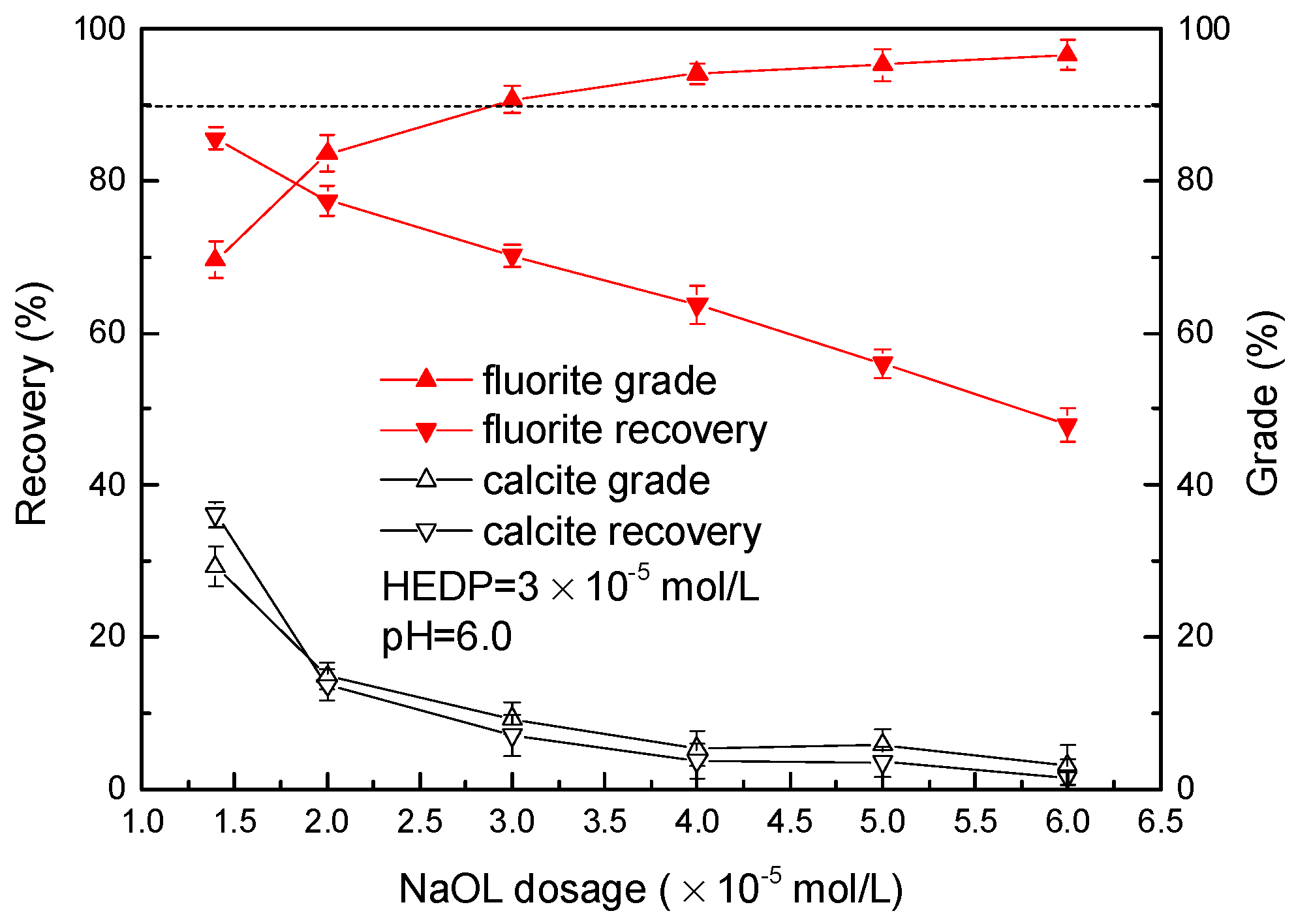
3.1. Flotation Experiment Results
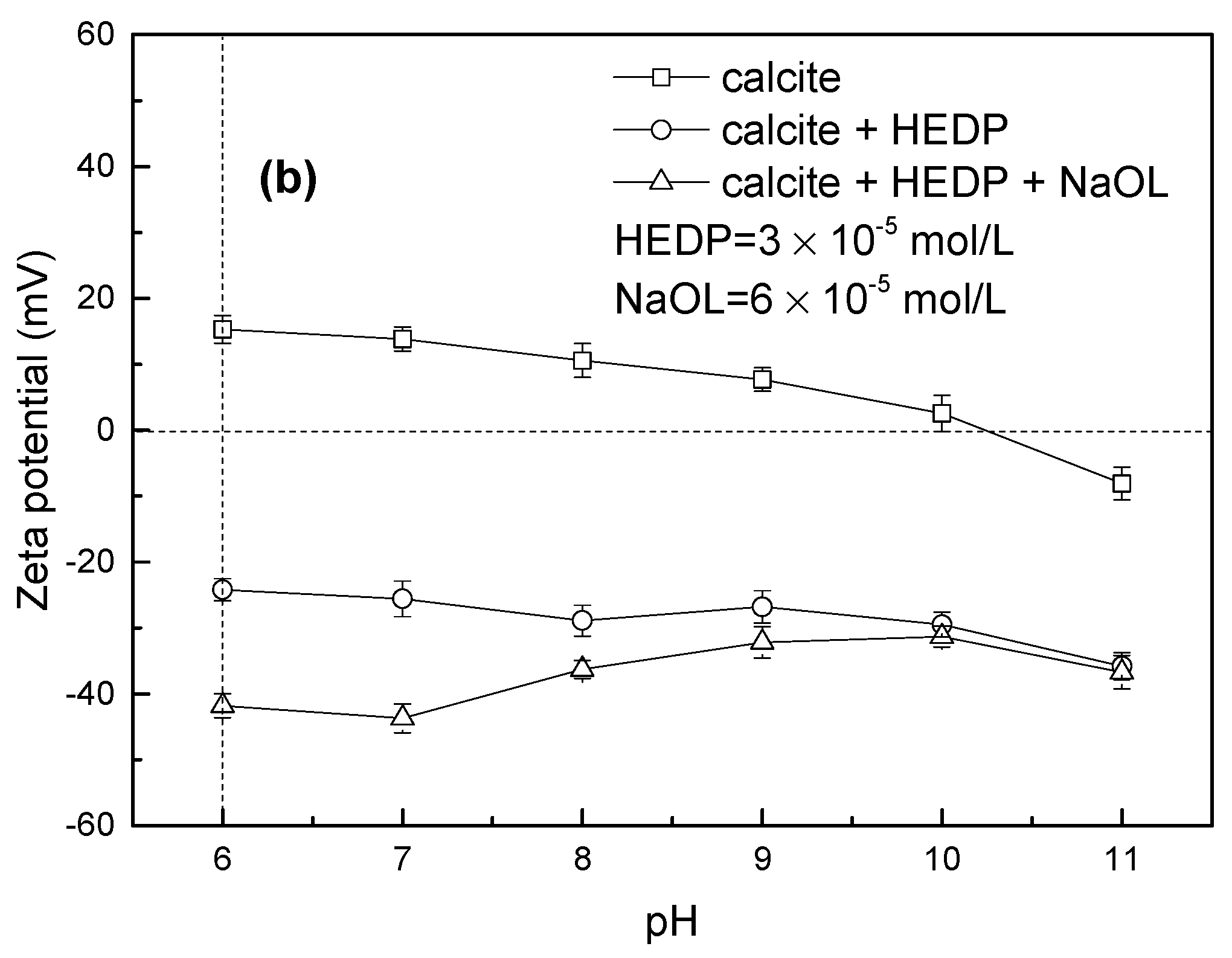
3.2. Zeta Potential Measurement Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Gao, Z.; Xie, L.; Cui, X.; Hu, Y.; Sun, W.; Zeng, H. Probing anisotropic surface properties and surface forces of fluorite crystals. Langmuir 2018, 34, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Gao, Z.; Khoso, S.A.; Gao, J.; Sun, W.; Pu, W.; Hu, Y. Selective adsorption of benzhydroxamic acid on fluorite rendering selective separation of fluorite/calcite. Appl. Surf. Sci. 2018, 435, 752–758. [Google Scholar] [CrossRef]
- Asadi, M.; Mohammadi, M.R.T.; Moosakazemi, F.; Esmaeili, M.J.; Zakeri, M. Development of an environmentally friendly flowsheet to produce acid grade fluorite concentrate. J. Clean. Prod. 2018, 186, 782–798. [Google Scholar] [CrossRef]
- Fa, K.; Nguyen, A.V.; Miller, J.D. Interaction of calcium dioleate collector colloids with calcite and fluorite surfaces as revealed by AFM force measurements and molecular dynamics simulation. Int. J. Miner. Process. 2015, 81, 166–177. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Pradip; Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Li, C.; Gao, Z. Effect of grinding media on the surface property and flotation behavior of scheelite particles. Powder Technol. 2017, 322, 386–392. [Google Scholar] [CrossRef]
- Tian, M.; Gao, Z.; Sun, W.; Han, H.; Sun, L.; Hu, Y. Activation role of lead ions in benzohydroxamic acid flotation of oxide minerals: New perspective and new practice. J. Colloid Interface Sci. 2018, 529, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Lu, S. Acidized sodium silicate—An effective modifier in fluorite flotation. Miner. Eng. 1992, 5, 435–444. [Google Scholar] [CrossRef]
- Zhou, W.; Moreno, J.; Torres, R.; Valle, H.; Song, S. Flotation of fluorite from ores by using acidized water glass as depressant. Miner. Eng. 2013, 45, 142–145. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, F.; Gao, H.; Chen, Z.; Peng, Y.; Liu, L. Selective separation of fluorite, barite and calcite with valonea extract and sodium fluosilicate as depressants. Minerals 2017, 7, 24. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Chapter 29—Beneficiation of florite ores. In Handbook of Flotation Reagents Chemistry Theory & Practice; Elsevier: Amsterdam, The Netherlands, 2015; pp. 57–76. [Google Scholar]
- Raju, G.B. Beneficiation of fluorspar by column flotation. Miner. Metall. Process. 2000, 17, 167–172. [Google Scholar]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Gao, Y.; Zhu, Y.; Hu, Y.; Sun, W. Selective flotation of calcite from fluorite: A novel reagent schedule. Minerals 2016, 6, 114. [Google Scholar] [CrossRef]
- Fischer, K. Distribution and elimination of HEDP in aquatic test systems. Water Res. 1993, 27, 485–493. [Google Scholar] [CrossRef]
- Browning, F.H.; Fogler, H.S. Effect of precipitating conditions on the formation of calcium−HEDP precipitates. Langmuir 1996, 12, 5231–5238. [Google Scholar] [CrossRef]
- Boulahlib-Bendaoud, Y.; Ghizellaoui, S. Use of the hedp for the inhibition of the tartar of ground waters. Energy Procedia 2012, 18, 1501–1510. [Google Scholar] [CrossRef]
- Khormali, A.; Petrakov, D.G.; Moghaddam, R.N. Study of adsorption/desorption properties of a new scale inhibitor package to prevent calcium carbonate formation during water injection in oil reservoirs. J. Petrol. Sci. Eng. 2017, 153, 257–267. [Google Scholar] [CrossRef]
- Pina, C.M.; Putnis, C.V.; Becker, U.; Biswas, S.; Carroll, E.C.; Bosbach, D.; Putnis, A. An atomic force microscopy and molecular simulations study of the inhibition of barite growth by phosphonates. Surf. Sci. 2004, 553, 61–74. [Google Scholar] [CrossRef] [Green Version]
- Spanos, N.; Kanellopoulou, D.G.; Koutsoukos, P.G. The interaction of diphosphonates with calcitic surfaces: Understanding the inhibition activity in marble dissolution. Langmuir 2006, 22, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Devyatov, F.V.; Musin, D.R. Complex formation of 1-hydroxyethylidene-1,1-diphosphonic acid with gadolinium(III) and calcium(II) in the aqueous solutions. Russ. J. Gen. Chem. 2013, 83, 1990–1996. [Google Scholar] [CrossRef]
- Wang, J.; Gao, Z.; Gao, Y.; Hu, Y.; Sun, W. Flotation separation of scheelite from calcite using mixed cationic/anionic collectors. Miner. Eng. 2016, 98, 261–263. [Google Scholar] [CrossRef]
- Miller, J.D.; Fa, K.; Calara, J.V.; Paruchuri, V.K. The surface charge of fluorite in the absence of surface carbonation. Colloids Surf. A Physicochem. Eng. Asp. 2004, 238, 91–97. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Miner. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Hu, Y.; Chi, R.; Xu, Z. Solution chemistry study of salt-type mineral flotation systems: Role of inorganic dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar]
- Moulin, P.; Roques, H. Zeta potential measurement of calcium carbonate. J. Colloid Interface Sci. 2003, 261, 115–126. [Google Scholar] [CrossRef]
- Browning, F.H.; Fogler, H.S. Effect of synthesis parameters on the properties of calcium phosphonate precipitates. Langmuir 1995, 11, 4143–4152. [Google Scholar] [CrossRef]
- Sergienko, V.S. Specific structural features of 1-hydroxyethane-1,1-siphosphonic acid (HEDP) and its salts with organic and alkali-metal cations. Crystallogr. Rep. 2000, 45, 64–70. [Google Scholar] [CrossRef]
- Guan, Q.; Hu, Y.; Tang, H.; Sun, W.; Gao, Z. Preparation of α-CaSO4·½H2O with tunable morphology from flue gas desulphurization gypsum using malic acid as modifier: A theoretical and experimental study. J. Colloid Interface Sci. 2018, 530, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.R. Comprehensive Handbook of Chemical Bond Energies; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Gao, Z.; Li, C.; Sun, W.; Hu, Y. Anisotropic surface properties of calcite: A consideration of surface broken bonds. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 53–61. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Sun, W.; Hu, Y.-H.; Liu, X.W. Anisotropic surface broken bond properties and wettability of calcite and fluorite crystals. Trans. Nonferr. Met. Soc. China 2012, 22, 1203–1208. [Google Scholar] [CrossRef]
- Zheng, R.; Ren, Z.; Gao, H.; Qian, Y. Flotation behavior of different colored fluorites using sodium oleate as a collector. Minerals 2017, 7, 159. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhou, Z.; Gao, Y.; Sun, W.; Hu, Y.; Gao, Z. Reverse Flotation Separation of Fluorite from Calcite: A Novel Reagent Scheme. Minerals 2018, 8, 313. https://doi.org/10.3390/min8080313
Wang J, Zhou Z, Gao Y, Sun W, Hu Y, Gao Z. Reverse Flotation Separation of Fluorite from Calcite: A Novel Reagent Scheme. Minerals. 2018; 8(8):313. https://doi.org/10.3390/min8080313
Chicago/Turabian StyleWang, Jianjun, Zihan Zhou, Yuesheng Gao, Wei Sun, Yuehua Hu, and Zhiyong Gao. 2018. "Reverse Flotation Separation of Fluorite from Calcite: A Novel Reagent Scheme" Minerals 8, no. 8: 313. https://doi.org/10.3390/min8080313