Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite
Abstract
:1. Introduction
2. Experiments
2.1. Materials
2.2. Micro-Flotation Experiments
2.3. Zeta Potential Measurements
2.4. Contact Angle Measurements
2.5. XPS Analysis
3. Results and Discussion
3.1. Micro-flotation Experiments
3.2. Zeta Potential Measurements
3.3. Contact Angle Measurements
3.4. XPS Analysis
3.5. Depression Mechanism of NaPP
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cao, Q.; Cheng, J.; Wen, S.; Li, C.; Bai, S.; Liu, D. A mixed collector system for phosphate flotation. Miner. Eng. 2015, 78, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Helbig, C.; Baldauf, H.; Mahnke, J.; Stöckelhuber, K.W.; Schulze, H.J. Investigation of Langmuir monofilms and flotation experiments with anionic/cationic collector mixtures. Int. J. Miner. Process. 1998, 53, 135–144. [Google Scholar] [CrossRef]
- Gharabaghi, M.; Irannajad, M.; Noaparast, M. A review of the beneficiation of calcareous phosphate ores using organic acid leaching. Hydrometallurgy 2010, 103, 96–107. [Google Scholar] [CrossRef]
- Li, G.; Cao, Y.; Liu, J.; Wang, D. Cyclonic flotation column of siliceous phosphate ore. Int. J. Miner. Process. 2012, 110–111, 6–11. [Google Scholar] [CrossRef]
- Dos Santos, M.A.; Santana, R.C.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A.S. Effect of ionic species on the performance of apatite flotation. Sep. Purif. Technol. 2010, 76, 15–20. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Luo, H.; Cheng, R.; Liu, F. Selective reverse flotation of apatite from dolomite in collophanite ore using saponified gutter oil fatty acid as a collector. Int. J. Miner. Process. 2017, 165, 20–27. [Google Scholar] [CrossRef]
- Abdel-Khalek, N.A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonates gangues. Miner. Eng. 2000, 13, 789–793. [Google Scholar] [CrossRef]
- Mohammadkhani, M.; Noaparast, M.; Shafaei, S.Z.; Amini, A.; Amini, E.; Abdollahi, H. Double reverse flotation of a very low grade sedimentary phosphate rock, rich in carbonate and silicate. Int. J. Miner. Process. 2011, 100, 157–165. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, R.W. Dolomite depressants in the flotation of apatite and collophane from dolomite. Miner. Eng. 1997, 10, 537–545. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Y.; Guo, X.; Guo, W. The depression effect and mechanism of NSFC on dolomite in the flotation of phosphate ore. Sep. Purif. Technol. 2016, 161, 88–95. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Hou, B.; Ye, J.; Mao, S.; Li, X. Flotation separation of quartz from collophane using an amine collector and its adsorption mechanisms. Powder Technol. 2017, 318, 224–229. [Google Scholar] [CrossRef]
- Amankonah, J.O.; Somasundaran, P. Effects of dissolved mineral species on the electrokinetic behavior of calcite and apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abouzeid, A.Z.M. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Liu, X.; Ruan, Y.; Li, C.; Cheng, R. Effect and mechanism of phosphoric acid in the apatite/dolomite flotation system. Int. J. Miner. Process. 2017, 167, 95–102. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Hernáinz, F.; Calero, M.; Blázquez, G. Flotation of low-grade phosphate ore. Adv. Powder Technol. 2004, 15, 421–433. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.D. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Luo, H.; Cheng, R.; Li, C.; Zhang, J. Effect of citric acid and flotation performance of combined depressant on collophanite ore. Miner. Eng. 2017, 109, 162–168. [Google Scholar] [CrossRef]
- Rashchi, F.; Finch, J.A. Polyphosphates: A review their chemistry and application with particular reference to mineral processing. Miner. Eng. 2000, 13, 1019–1035. [Google Scholar] [CrossRef]
- Edwards, C.R.; Kipkie, W.B.; Agar, G.E. The effect of slime coatings of the serpentine minerals, chrysotile and lizardite, on pentlandite flotation. Int. J. Miner. Process. 1980, 7, 33–42. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C.; Xu, F. The flotation separation of scheelite from calcite and fluorite using dextran sulfate sodium as depressant. Int. J. Miner. Process. 2017, 169, 53–59. [Google Scholar] [CrossRef]
- Yongxin, L.; Changgen, L. Selective flotation of scheelite from calcium minerals with sodium oleate as a collector and phosphates as modifiers. I. Selective flotation of scheelite. Int. J. Miner. Process. 1983, 10, 205–218. [Google Scholar] [CrossRef]
- Merma, A.G.; Torem, M.L.; Morán, J.J.V.; Monte, M.B.M. On the fundamental aspects of apatite and quartz flotation using a Gram positive strain as a bioreagent. Miner. Eng. 2013, 48, 61–67. [Google Scholar] [CrossRef]
- Lu, Y.; Drelich, J.; Miller, J.D. Oleate Adsorption at an Apatite Surface Studied by Ex-Situ FTIR Internal Reflection Spectroscopy. J. Colloid Interface Sci. 1998, 202, 462–476. [Google Scholar] [CrossRef]
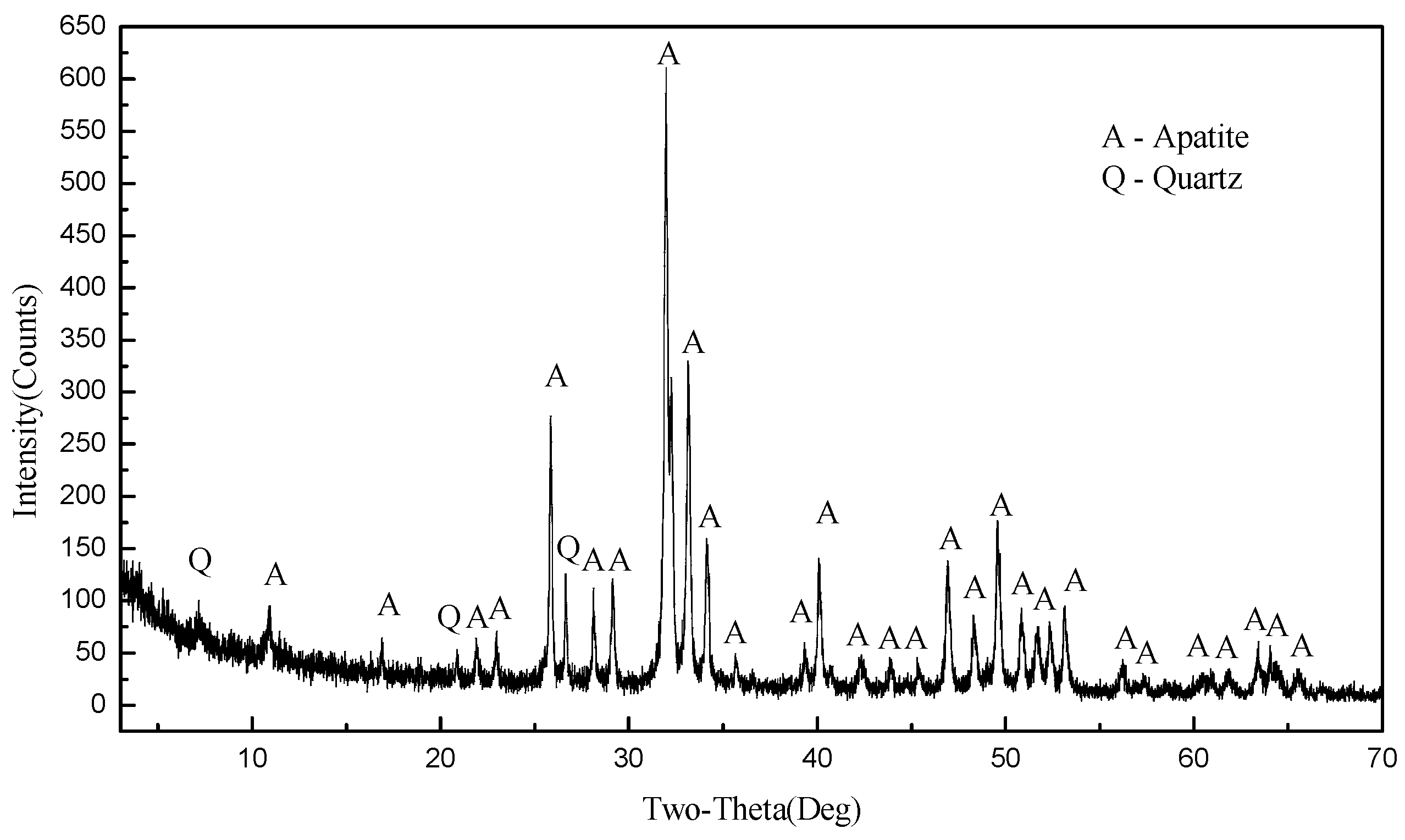



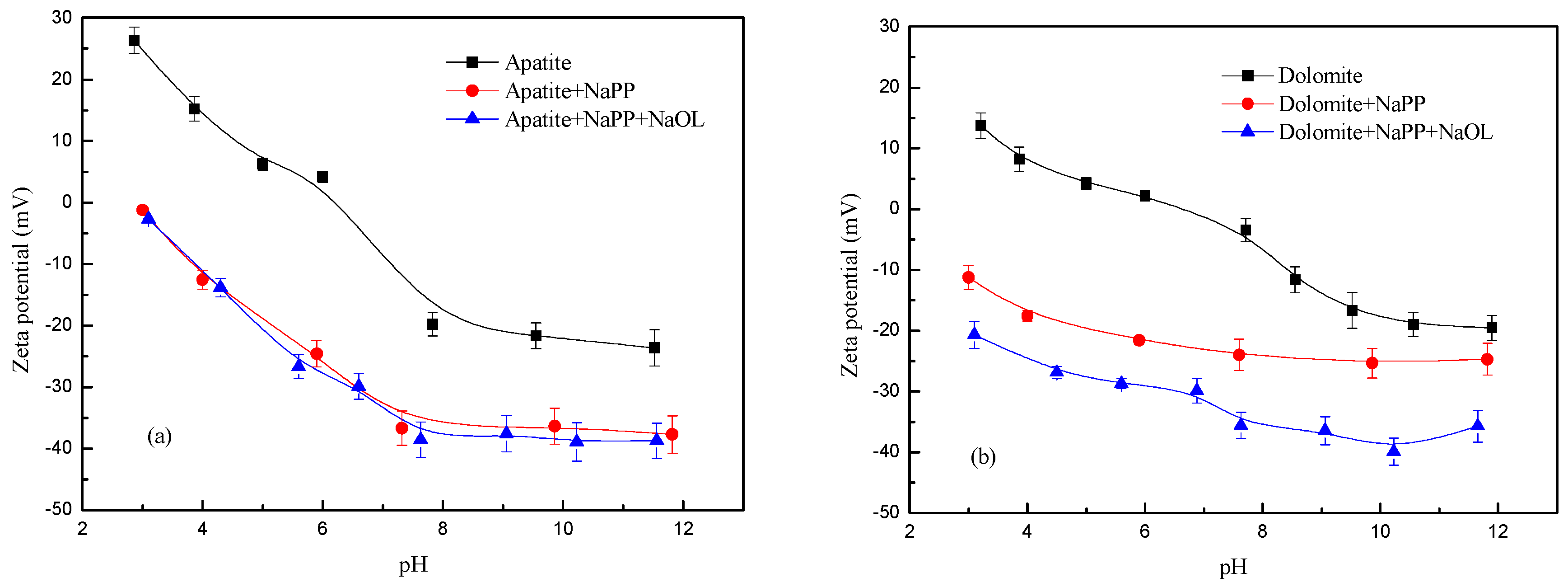



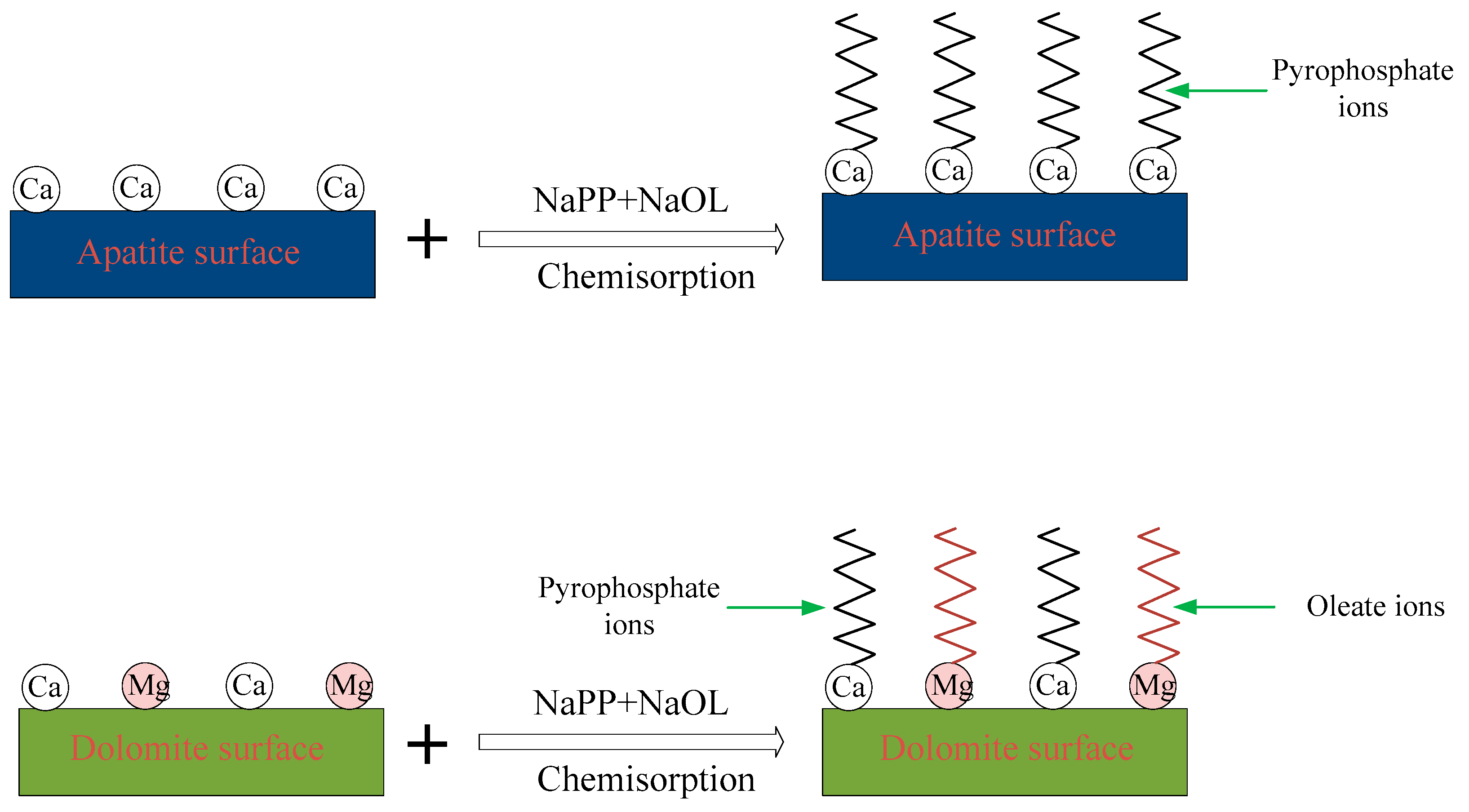
| NaPP (mg/L) | Production | Yield (%) | Grade (%) | Recovery (%) | ||
|---|---|---|---|---|---|---|
| P2O5 | MgO | P2O5 | MgO | |||
| 0 | Concentrate | 29.4 | 26.4 | 3.8 | 39.4 | 11.3 |
| Tailing | 70.6 | 16.9 | 12.4 | 60.6 | 88.7 | |
| Feed | 100.0 | 19.7 | 9.9 | 100.0 | 100.0 | |
| 100 | Concentrate | 55.7 | 34.1 | 2.9 | 96.5 | 16.3 |
| Tailing | 44.3 | 1.5 | 18.6 | 3.5 | 83.7 | |
| Feed | 100.0 | 19.7 | 9.9 | 100.0 | 100.0 | |
| Sample | Binding Energy (eV) | Chemical Shift (eV) | ||
|---|---|---|---|---|
| Ca | Mg | Ca | Mg | |
| Apatite | 347.54 | - | - | - |
| Apatite + NaOL | 347.03 | - | −0.51 | - |
| Apatite + NaPP + NaOL | 347.49 | - | −0.05 | - |
| Dolomite | 347.14 | 1303.79 | - | - |
| Dolomite + NaOL | 346.51 | 1303.50 | −0.63 | −0.29 |
| Dolomite + NaPP + NaOL | 347.07 | 1303.23 | −0.07 | −0.56 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. https://doi.org/10.3390/min8070278
Chen Y, Feng Q, Zhang G, Liu D, Liu R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals. 2018; 8(7):278. https://doi.org/10.3390/min8070278
Chicago/Turabian StyleChen, Yanfei, Qiming Feng, Guofan Zhang, Dezhi Liu, and Runzhe Liu. 2018. "Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite" Minerals 8, no. 7: 278. https://doi.org/10.3390/min8070278




