1. Introduction
Scheelite particles are brittle and easily overground in the grind process [
1]. As a result, there are always a large number of micro-granular slimes in the scheelite pulp, which weakens the flotation environment and leads to the loss of the valuable minerals [
2]. Decreasing the size of flotation bubbles is regarded as an efficient way to recover the fine fractions [
3]. In the past two to three decades, a lot of research work has been carried out on the development of air bubbles’ sparger design to generate bubbles that are small enough to be used for fine minerals flotation. Luckily, it has been reported that nanoscale bubbles can be produced using a properly designed cavitation Venturi tube in the principle of hydrodynamic cavitation (HC) [
4]. More importantly, nanobubbles (NBs), which refer to tiny bubbles that are mostly smaller than a few hundred nanometers, are confirmed to be advantageous to mineral flotation based on theoretical research and industrial practice [
5,
6].
Due to the high energy efficiency and low maintenance cost, HC is the mostly commonly used cavitation method, and Venturi tube is the most widely used HC device in flotation field [
4]. According to Bernoulli’s equation, once the liquid static pressure reduces to the water vapor pressure, cavitation occurs and NBs generates. On the other hand, cavitation inception and the properties of NBs are influenced by many factors, such as liquid velocity, cavitation time, surfactant concentration, particles in the bulk, etc. [
7,
8]. There is a liquid threshold for cavitation inception in a Venturi tube. When the liquid velocity exceeds the threshold, the higher the speed, the more pronounced the cavitation phenomenon will be [
9]. Xiong [
10] has discovered that it takes a certain time to generate sufficient NBs in the aqueous solution during the cavitation process. The presence of surfactant does not reduce the cavitation threshold, but contributes to the production of more stable and smaller NBs in the solution [
11]. Furthermore, it has been announced that the effect of particles on HC depends on particle surface properties. Specifically, hydrophilic particles have little impact on cavitation inception, while hydrophobic (hydrophobized) particles can act as nuclei, making cavitation more prone to occur [
12].
In mineral flotation, HC can be induced either by HC pretreatments of reagent solution (HCPS) [
13] or by HC pretreatments of pulp (HCPP) [
14]. In particular, HCPS refers to the cavitation triggered by chemical reagent solution passing through the Venturi tube at a high speed, while HCPP means the cavitation incepted by flotation pulp passing through the Venturi tube at a high speed. Studies have shown that NBs generated in the process of HCPS and HCPP can selectively adsorb on the hydrophobic particle surfaces, promote the aggregation of dispersed particles, and meanwhile serve as the nuclei for flotation bubbles attachment, thus enhancing the flotation of ultrafine minerals flotation [
15]. However, the cavitation performance and bubble–particle interaction are largely different in the absence and presence of particles during cavitation. In the flotation with HCPS, NBs are usually produced firstly in the bulk (i.e., bulk nanobubbles, BNBs) and adsorb onto the hydrophobic mineral surfaces as a second step. In the flotation with HCPP, hydrophilic particles have little effect on the initial cavitation; NBs produced mainly BNBs in this case. As the content of hydrophobic particles increases, or the hydrophobicity of mineral surfaces increases, NBs tend to nucleate at the mineral surfaces directly, forming surface NBs (i.e., SNBs) due to the smaller adhesion work between solid particles and water compared with water cohesion work. Therefore, all of the factors that affect the hydrophobicity of the mineral surface can affect the formation of SNBs. Compared with flotation assisted by HCPS, ultrafine particles can attach to NBs without the need of particle–bubble collision in the case of HCPP, which has been proved to be the rate-determining step in froth flotation for ultrafine particles [
16]. These will bring about significant differences in the NBs’ adhesion onto the mineral surface and particle aggregation, which therefore lead to differing flotation environment and final flotation responses [
17,
18].
Much progress has been reported in the last decade for the research, development, and application of HCPS or HCPP and associated NBs in mineral flotation. Specifically, a lot of studies about the application of HCPS and HCPP on mineral flotation have been carried out in the flotation of coal, phosphate, oil sands, etc. [
10]. However, few research studies have been done to compare the flotation performances under these two cavitation modes. To reveal the different flotation responses, the flotation of ultrafine scheelite particles with HCPS and HCPP was compared in this study. The reasons for the differences in flotation behaviors was further analyzed from the perspective of NBs and their roles in particle aggregation.
2. Materials and Methods
2.1. Materials and Reagents
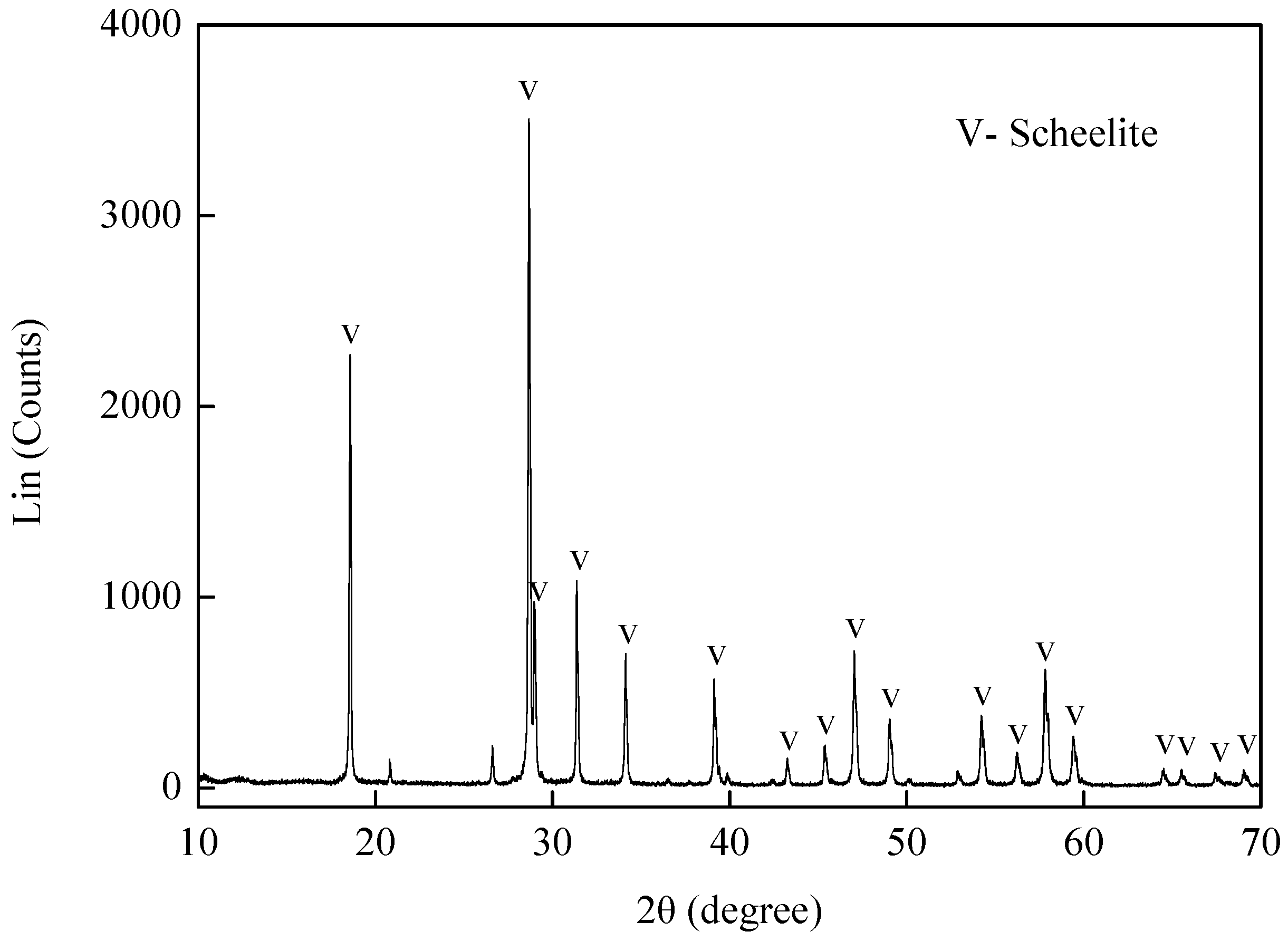
The scheelite sample was obtained from Yuanling, Hunan Province, China. After handpicking, the sample was crushed and dry ground in a ceramic ball mill, then elutriated to collect the –10 μm size fraction for flotation tests, and –2 μm size fraction for zeta potential measurements. The particle size distribution has already shown in our published paper [
19]. The samples were analyzed via a chemical method, showing that the content of WO
3 was 80.24%. The XRD of the sample was shown in
Figure 1, which revealed that the sample was of high purity (99.64%). Sodium oleate (NaOl, AR) was used as the collector in this study. HCl and NaOH acted as pH modifiers. Deionized and double distilled water was used for all of the experiments.
2.2. NBs Generation and Characterization
The NBs generation/cavitation–flotation system used in this study was the same as that shown in a previous study, and all the devices are of the same specifications as the previous ones as well [
19]. First, 100 mL of solution with a pH value of 10 and a certain concentration of NaOl was pumped through a centrifugal pump into a Venturi tube. In order to produce enough NBs for subsequent experiments, we kept the cavitation time as 10 min. When the air-saturated solution reached the throat of the Venturi tube, the solution was depressurized, and cavitation was triggered. As a result, the NBs were formed in the bulk solution. After 10 min of cavitation processing, solution containing NBs were transferred to measure the size distribution of NBs, or a flotation cell of XFG laboratory flotation machine (Jilin exploration machinery factory, Changchun, China) for flotation tests.
The size distribution of NBs generated in the bulk solution was measured through a ZetaSizer Nano ZS 90 instrument (Malvern instruments Ltd., Worcestershire, UK), based on the DLS technique. Average diameter, Db (0.5), was used as instrument output to characterize the NBs’ size. Size measurement was conducted at 20 °C three times, and the average was reported as the final value.
2.3. Flotation Tests
Before we launched the flotation tests in the 100-mL flotation cell, the pulp was preconditioned in the “Reactor” under two different approaches:
- (1)
HCPS. In the absence of mineral particles, 100 mL of NaOl solution was firstly conditioned in the Reactor for 10 min. Then, 4 g of scheelite and 96 mL of pretreated NaOl solution were added into the flotation cell together to make the flotation pulp.
- (2)
HCPP. With a given concentration of NaOl, 4 g of scheelite and 96 mL of solution were mixed well in the Conditioning tank, firstly. Then, the mineral suspension was injected into the Reactor for cavitation treatment for 10 min. After that, the suspension was transferred into the flotation cell to make the flotation pulp.
Although the residence time of solids in the case of HCPP is 10 min longer than that of HCPS in the solution containing NBs, we explored and found that its influence on final flotation recovery was just minor. Firstly, the well-conditioned flotation pulp was conditioned for 3 min in the flotation cell. The final flotation was performed for 5 min. The floated and unfloated particles were collected, filtered, and dried subsequently. The flotation recovery was calculated based on solid mass distributions between the two products. Each microflotation test was implemented three times, and the average was regarded as the final values.
2.4. Zeta-Potential Measurements
The zeta potential of scheelite as a function of pH was measured under different conditions by the same ZetaSizer Nano ZS instrument (Malvern instruments Ltd., Worcestershire, UK) mentioned in
Section 2.2. KCl (0.01 mol/L) was used as the background electrolyte. Before the final measurement, the mineral suspension was stirred for 3 min using a magnetic stirrer and subject to 10 min of standing, so that few free bubbles existed in the suspension (except for NBs). Each data point for zeta potential was an average of three measurements and carried out at 20 °C. Suspension with a high zeta potential is electrically stabilized, while suspension with a low zeta potential tends to aggregate.
2.5. Microscope Tests
Microscope tests were performed to unveil the particle dispersion/aggregation state in the cases of HCPS and HCPP. The procedure of preparing the target mineral suspensions was almost the same as that shown in
Section 2.2, except changing the weight of scheelite added from 4 g to 0.5 g at the beginning of mineral suspension preparation. After cavitation treatment, the mineral suspension was well mixed for 3 min in a beaker gently so that no extra large bubbles formed in this period. The mineral suspension was transferred into a colorimeter tube and settled for 20 min, after which a drop of supernatant liquor of fixed height (2 cm) was extracted out for image analysis. As NBs are of incredible stability in water media, and with a relatively slow setting rate, these operations help to reduce the interference caused by the excessive solids for the latter observation. The observation was finally fulfilled using an optical microscope (Olympus-Cx31rts, Olympus corporation, Tokyo, Japan).
2.6. Shear Yield Stress Measurements
In flotation, pulp rheology is a sensitive indicator of the state of aggregation/dispersion of mineral particles [
20]. Shear yield stress is a measure of the resistance of the floc to the permanent deformation in shear, or the minimum stress needed for the occurrence of flow [
21]. The yield stress of the flotation pulp has been proven to be useful in characterizing the particle aggregation degree in the pulp [
22]. In this study, the yield stress measurement was conducted with a rheometer (Anton Paar MCR102, Anton-Paar Ltd., Graz, Austria) and a vane impeller probe. First, 40 mL of mineral pulp was pretreated as the conditioning operations stated in
Section 2.3, after which the well-conditioned pulp was transferred into the sample holder for the measurement. The shear stress sweep ranged from 0.01 Pa to 10 Pa within six min to fulfill the stress range. Yield stress was acquired from the shear stress versus shear strain curves where shear strain suddenly increased as shear stress increased. It was calculated by the point where the tangent lines of the strain curves intersected. More details of the measurement procedures can also be seen in Zhang [
23].
3. Results and Discussion
3.1. Ultrafine Scheelite Particles Flotation with HCPS and HCPP
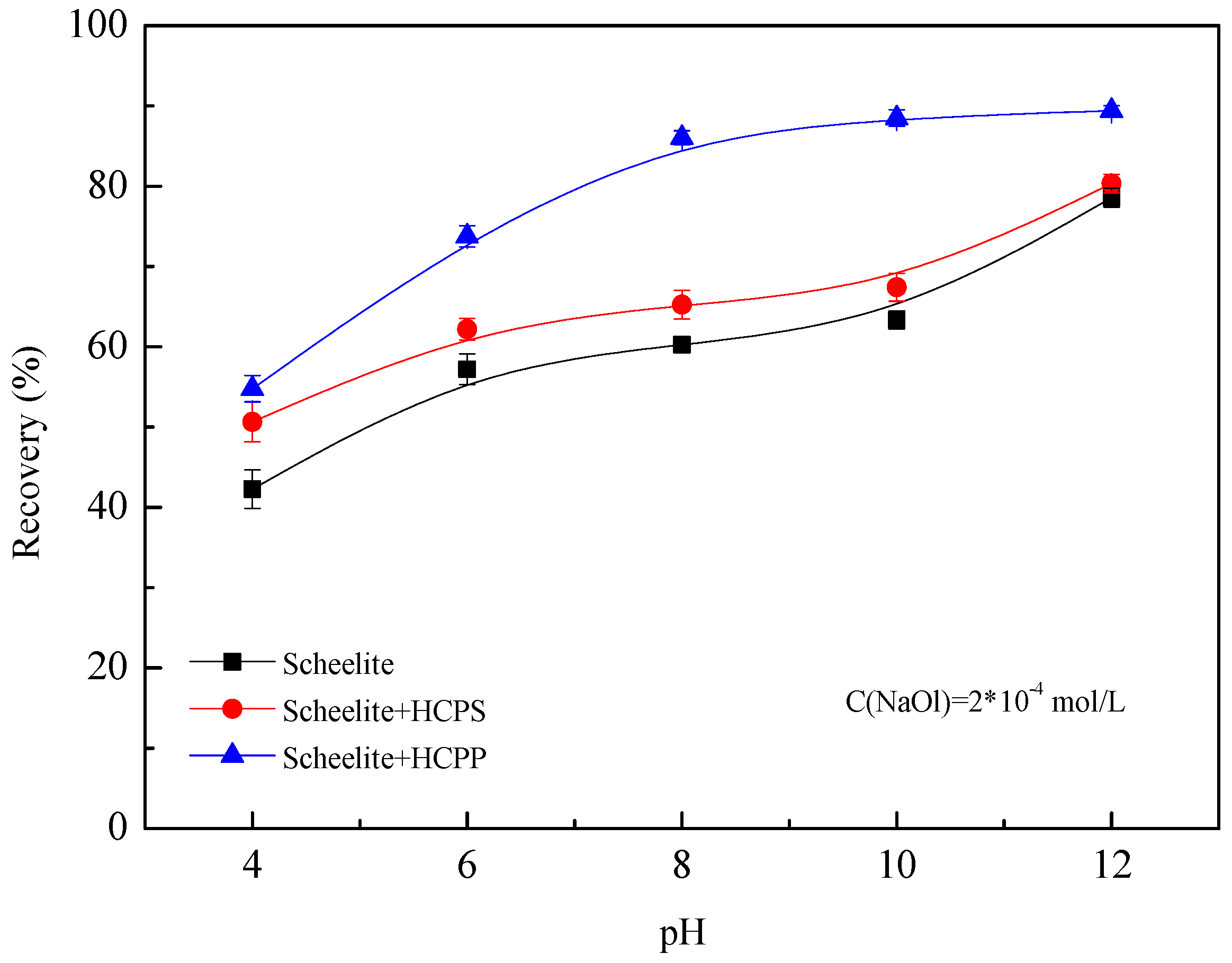
Figure 2 demonstrates the results of the flotation recovery of ultrafine scheelite particles as a function of solution pH under different conditions. Noticeably, both HCPS and HCPP lead to higher flotation recoveries at the same pH conditions, confirming that HC enhances the flotation of ultrafine particles. Compared with HCPS, HCPP promotes the flotation recovery more significantly, especially under intermediately and weakly alkaline conditions (pH 8–10). This may be related to the surface hydrophobicity of scheelite in NaOl solution. Under acidic conditions, the adsorption of NaOl on scheelite is so weak that the surface of scheelite is not hydrophobic enough. The relatively hydrophilic particles have little effect on cavitation, resulting in similar cavitation and flotation performances in the cases of HCPS and HCPP. As pH increases (e.g., pH 8–10), the chemisorption of NaOl on scheelite significantly improves the surface hydrophobicity of scheelite [
24], which therefore promotes the cavitation and NBs formation in the process of HCPP. In addition, when the pH of pulp is high (e.g., above 10), the effect of HCPS on enhancing ultra-fine scheelite particles flotation seems to be weaken, which may be mainly attributed to the increased foaming ability of NaOl and associated entrainment [
25].
Figure 3 shows the comparative results of the flotation recovery of ultrafine scheelite particles as a function of NaOl concentration. It is clear that preconditioning minerals with HC leads to higher flotation recoveries compared with conventional flotation, which reconfirms that HC can promote the flotation of ultrafine scheelite particles. Under the same NaOl concentration condition, HCPP promotes the scheelite flotation more noticeably than HCPS. Moreover, the effect of HCPS on improving final flotation recovery gradually decreases as the NaOl concentration increases, while the enhancement on final recovery is much less affected by NaOl concentration in the case of HCPP. Based on the statement in
Section 1, two different kinds of NBs exist in the solution pretreated by HC. In the case of HCPS, BNBs dominant, while BNBs or SNBs may exist in the case of HCPP, depending on the degree of hydrophobicity of scheelite. The above findings indicate that BNBs and SNBs may play different roles in enhancing the flotation of ultrafine particles.
Decreasing bubble size and increasing apparent particle size are the two basic approaches to improving fine and ultrafine mineral flotation [
3]. Previous research studies have proven that NBs generated in the process of HC play roles in both of these two approaches, which are also convinced to be the main contributors for HC-enhanced flotation [
26,
27]. However, how do NBs affect the bubble average size and particle apparent size in the case of HCPS and HCPP? Considering the different flotation performances revealed above, we will try to clarify the flotation response under different cavitation conditions mainly from these two aspects.
3.2. NBs Properties and their Influence on Flotation Bubble Size Distribution
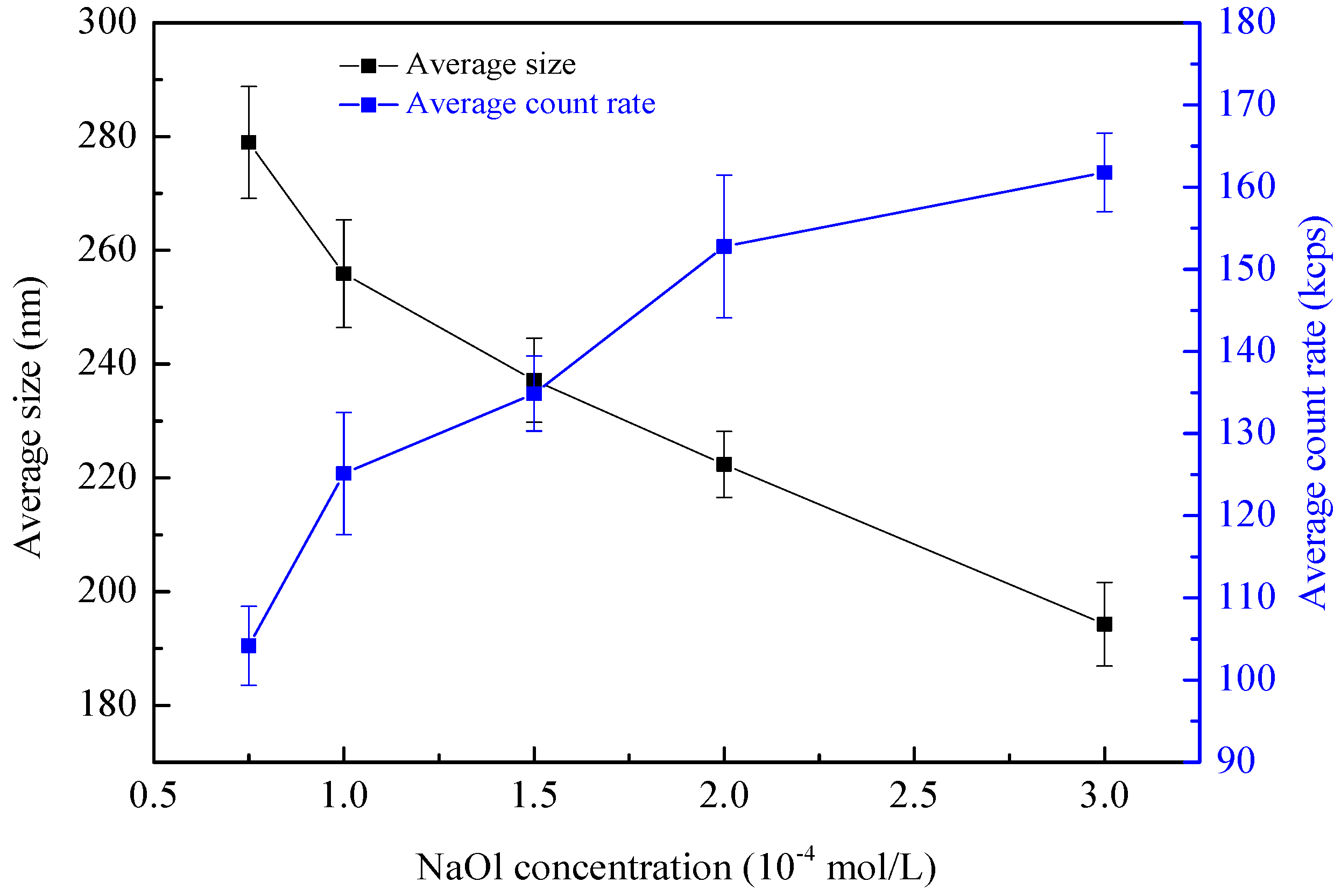
NBs are of incredible stability in aqueous solution, which allows them to remain in water long enough for the non-real-time measurement [
28]. In our study, NBs generated by HC are characterized by the average bubble size and average count rate (shown in
Figure 4). It is clear that NBs with an average size around 200–300 nm have been produced in the HC process. Using the same technique, Calgaroto [
26] discovered that NBs with a size distribution ranging from 100 nm to 800 nm are produced by depressurizing air-saturated water solutions at a high flow velocity. Furthermore, with the increase of NaOl concentration, the average bubble size decreases and the average count rate increases. Research has shown that the number of tiny bubbles in solution are positively correlated with the value of count rate [
29]. It indicates that more and smaller NBs are produced as the concentration of NaOl increases. With the increasing of NaOl concentration, the surface tension of solution decreases, benefiting the maintenance of newly generated bubbles by preventing bubbles bursting and coalescence as well [
29]. In addition, according to the nuclei theory, there are many gas nuclei stored in the cavities of the particles [
30]. It means that the introduction of particles into the aqueous media increases the total gas nuclei of the cavitation system, which therefore promotes the cavitation inception as well as NBs’ generation. So, it is demonstrated that many more NBs may be produced in the case of HCPP than HCPS, although the NBs generated by HCPS can hardly characterized due to the presence of solids.
In flotation, these nanoscale bubbles are smaller than conventional-size flotation bubbles for several orders of magnitude in size. The existence of NBs in the pulp significantly reduce the average size of flotation bubbles. As a result, the particle–bubble collision probability, a determining step for ultrafine mineral flotation, can be increased remarkably.
3.3. Effect of NBs on Ultrafine Scheelite Particles’ Aggregation
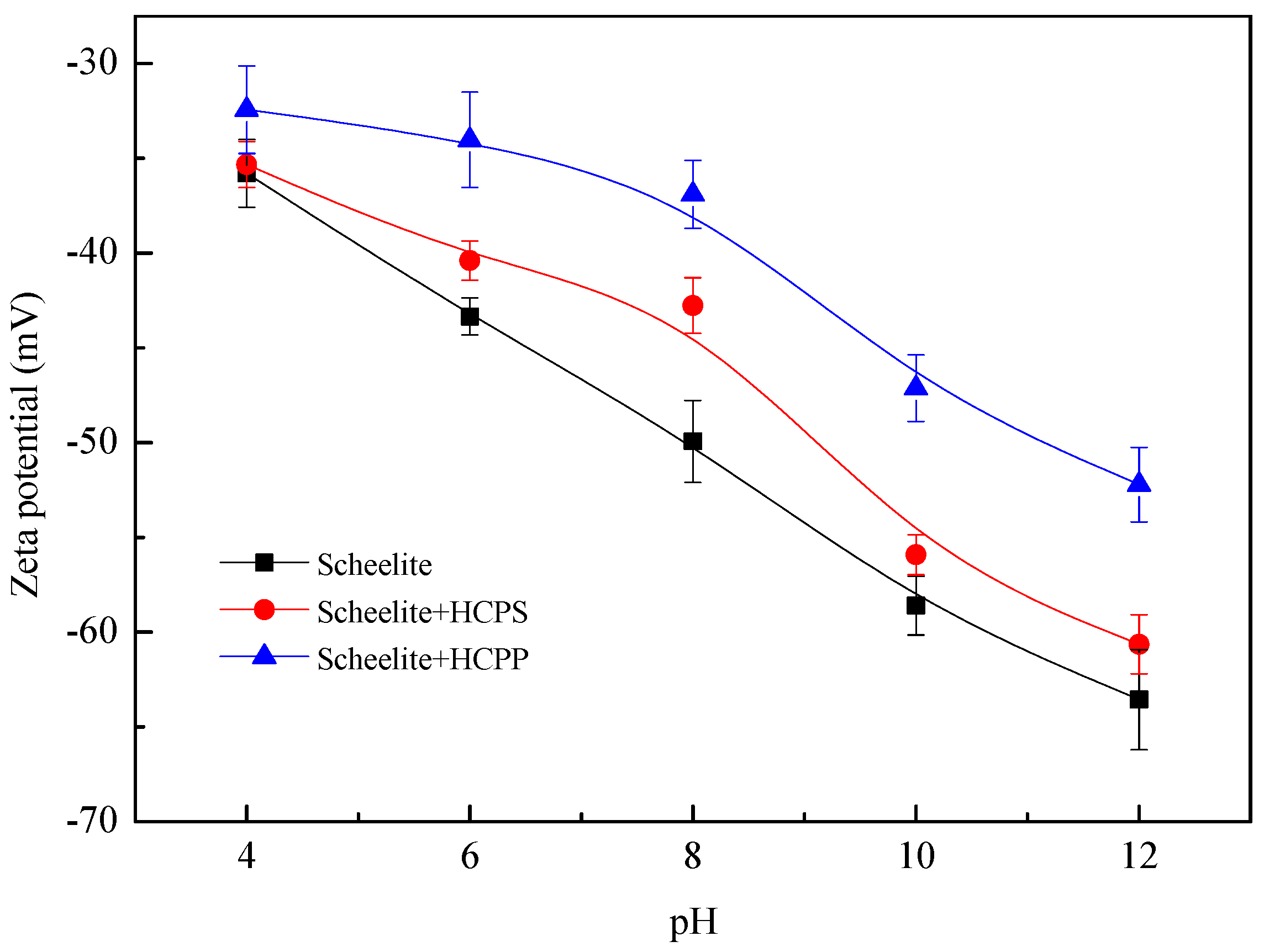
Figure 5 presents the zeta potential of scheelite as a function of pH under different conditions. It shows that the zeta potential values change to be much smaller after scheelite is treated by HC in pH values above 4. Based on our previous study, the adsorption of NBs generated in the process of HC on the mineral surfaces is the main contributing factor [
19]. The mechanism of NBs adhesion leading to the decrease of mineral particles’ surface potential is still unclear, with several possible explanations being proposed based on the previous literature [
31,
32]. Besides, in the case of HCPP, the surface negative potentials of the scheelite particles are significantly reduced, while the zeta potentials of scheelite have smaller changes in the case of HCPS. As we have stated above, SNBs can nucleate on the surface of hydrophobized particles directly in the case of HCPP, while only some BNBs are introduced when HCPS occurs. The zeta potentials results indicate that the attachment of BNBs on hydrophobic surface is much more difficult, compared with the SNBs directly nucleating on the surface with the same hydrophobicity.
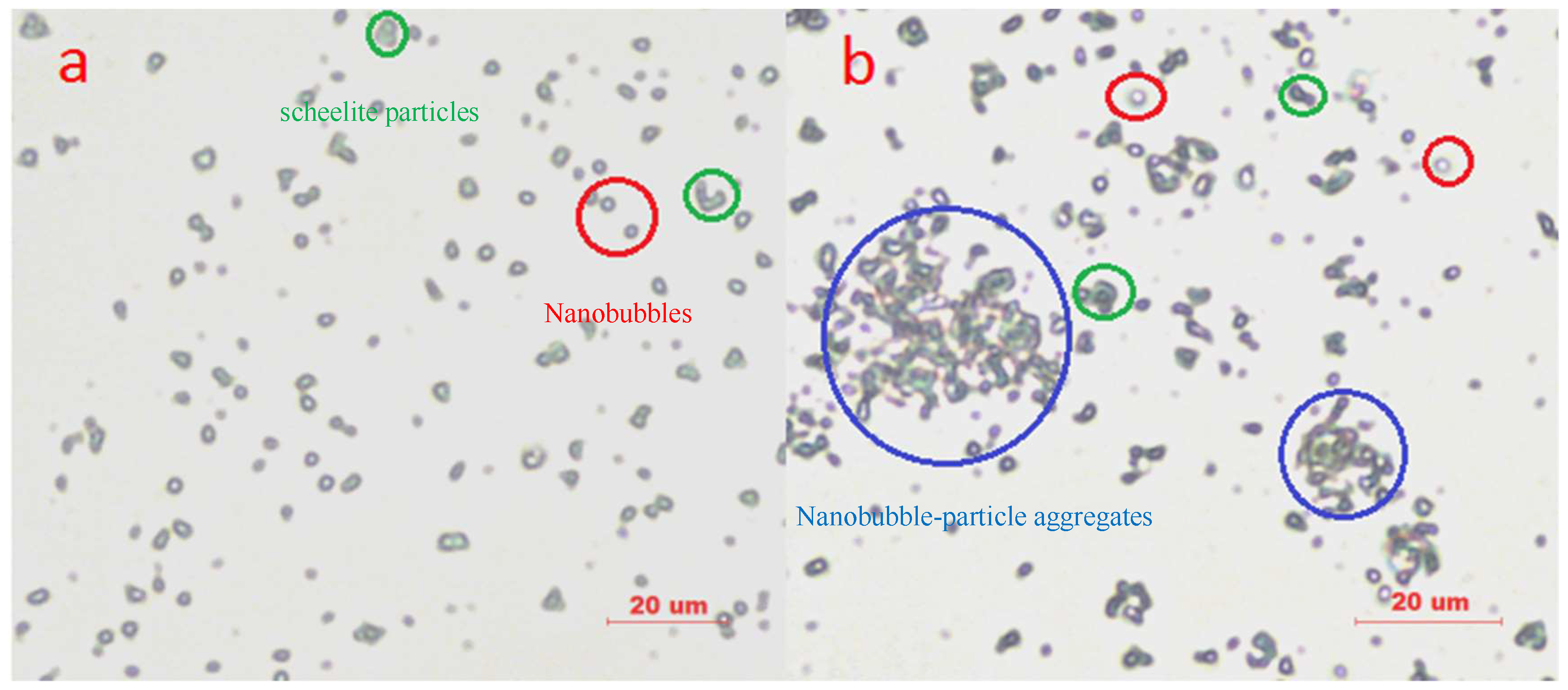
Figure 6 indicates the interaction of particles and NBs through photo-micrographs. Generally, NBs generated by HCPS and HCPP are present in the mineral suspension in three states: free nuclei, adhering onto the surface of mineral particles, and forming bubble–particle aggregates. In the mineral suspension with HCPS, NBs are mainly free in the suspension, and particles are basically disperse. In contrast, in the mineral suspension with HCPP, most of NBs mainly adhere onto the surface of the hydrophobized particles or even form large particle–bubble aggregates. The results confirm that SNBs are more likely to adhere onto the hydrophobized particles’ surface than BNBs, which is consistent with the data of zeta-potential measurements.
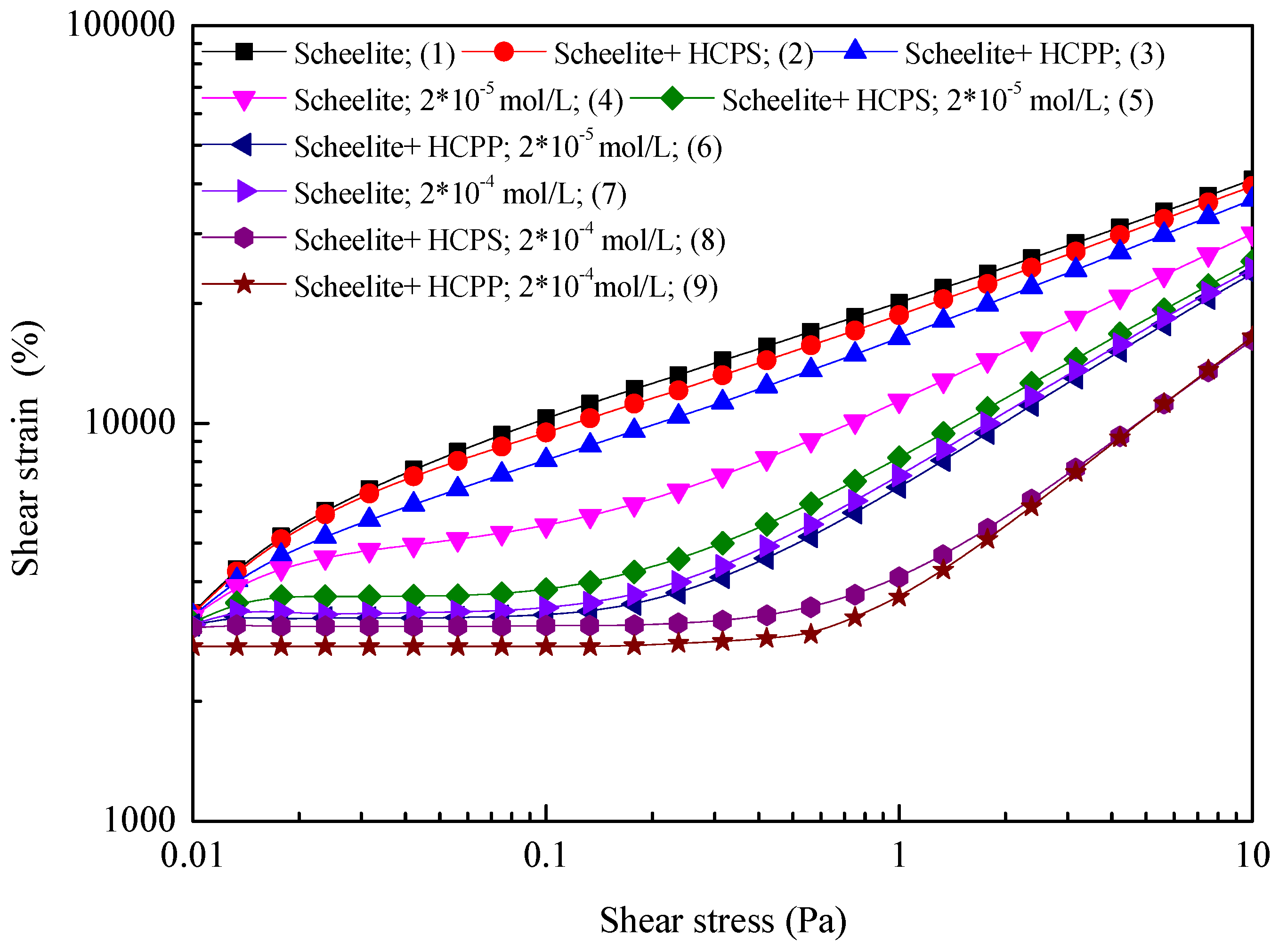
To further clarify the particles’ aggregation induced by NBs, the shear yield stress of pulp pretreated in different ways was investigated (shown in
Figure 7 and
Table 1). Here, τ
0 is the shear yield stress, presenting the force needed for breaking the system. It is clear that τ
0 is not available in the absence of NaOl regardless of cavitation pretreatments. It suggests that it is difficult for scheelite particles to form aggregates in the shearing process without NaOl added. In the presence of NaOl, the shear yield stress of the mineral starts to appear, and increases as the NaOl concentration increases. When the concentration of NaOl increases from 2 × 10
−5 M to 2 × 10
−4 M, the shear yield stress of the pulp increases from 0.015 Pa to 0.092 Pa. It indicates that the addition of NaOl promotes the aggregation of ultrafine scheelite particles, which has been reported in our previous research [
19]. After the scheelite surface is hydrophobized by NaOl, the cavitation pretreatment of pulp contributes to larger shear yield stresses both in the case of HCPS and HCPP. This reconfirms that the NBs generated by HC promote the aggregation of solids. More importantly, compared with the rheological behaviors of scheelite particles under the HCPS and HCPP conditions, it is easy to find that HCPP leads to a greater shear yield stress (0.133 Pa versus 0.102 Pa and 0.422 Pa versus 0.369 Pa), suggesting that HCPP promotes the aggregation of solids more significantly than HCPS. This finding supports the results shown in microscope tests.
In the flotation with HCPS, the generation of NBs decreases the average size distribution of flotation bubbles, therefore increasing the particle–bubble collision probability. Meanwhile, these NBs (i.e., BNBs) also promote the flotation of ultrafine particles to some extent. As a result, the flotation of ultrafine scheelite particles is enhanced. However, as the NaOl concentration increases, the enhancement of HCPS on the flotation of ultrafine minerals tends to be negligible, suggesting that the enhancement of ultrafine mineral flotation induced by HCPS is limited. In contrast, the improvement on final recovery is more significant in the flotation with HCPP. Initially, more NBs are produced, leading to a greater increase in the particle–bubble collision probability. Moreover, the adherence of NBs on hydrophobized particles reduces the surface zeta potentials of particles and promotes the interaction among NBs and ultrafine particles, all of which benefit the particle aggregation. The aggregation of ultrafine scheelite particles in the case of HCPP is more significant, which may be another reason why HCPP results in better flotation performance.
During the experiments, the interaction of NBs and particles is only briefly mentioned, which is a deficiency in our study. Fortunately, some novel techniques have been introduced into this field, such as nanoparticle tracking analysis (NTA) and Atomic Force Microscope (AFM) [
33], which provides us chance for further study in the interaction of NBs and mineral particles.