Bio-Minerals Combined with Bacillus cereus for Enhancing the Nitrogen Removal Efficiency under Aerobic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source of Microorganisms for Nitrogen Removal
2.2. Bio-FeS and Bio-Pd-FeS for Nitrogen Removal
2.3. Batch Reduction Experiments
2.4. Analytical Methods
3. Results
3.1. The Aerobic Nitrogen Removal Efficiency in a Single Nitrate (NO3−–N) vs. a Mixed Nitrogen (NO3−–N + NO2−–N + NH4+–N) Reactor
3.1.1. BC (Bacillus cereus Strain 146)
3.1.2. Bio-minerals: BF (Bio-FeS) and BPF (Bio-Pd-FeS)
3.1.3. BC + BF (Bacillus cereus Strain 146 + Bio-FeS) and BC + BPF (Bacillus cereus Strain 146 + Bio-Pd-FeS)
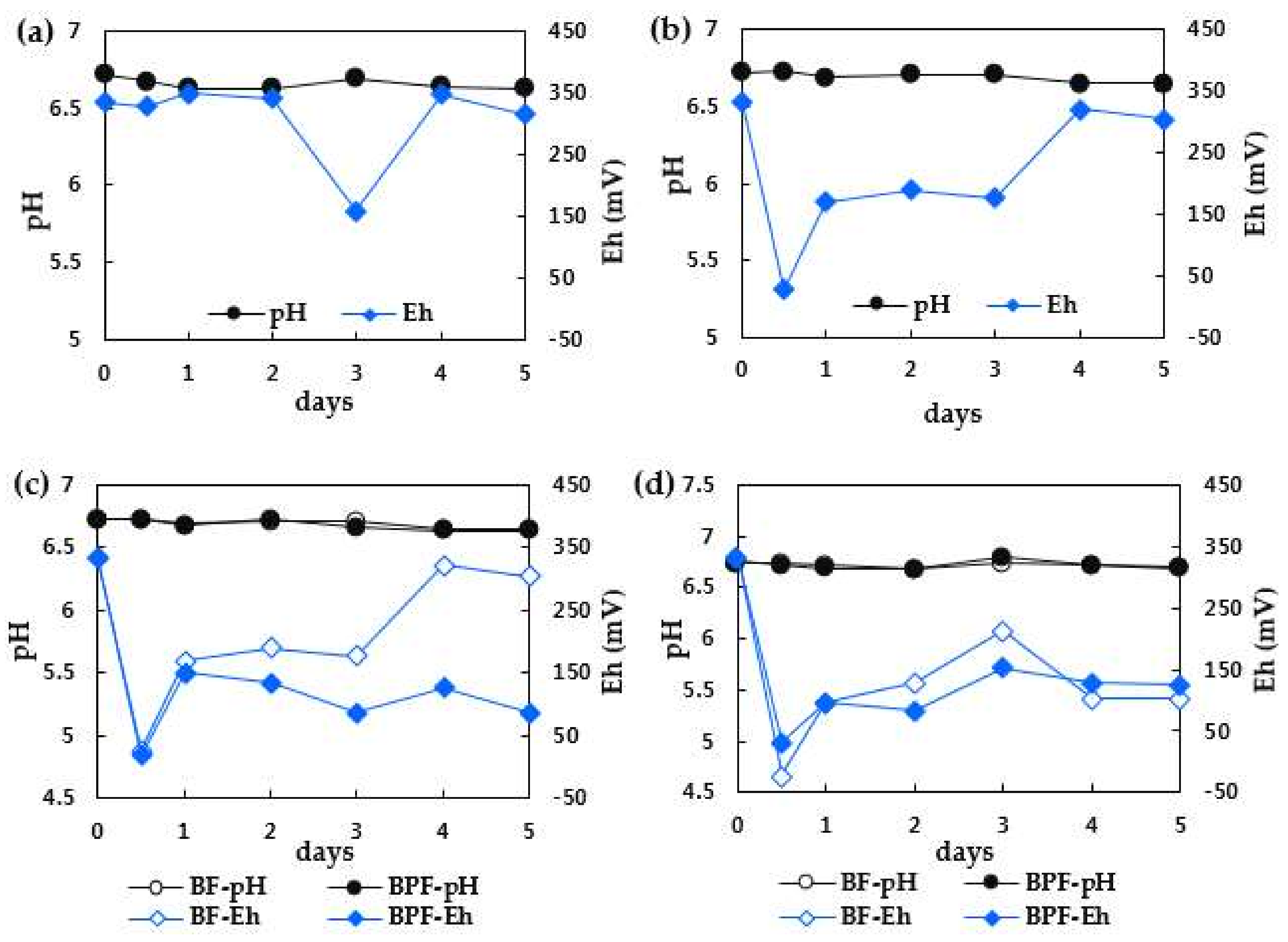
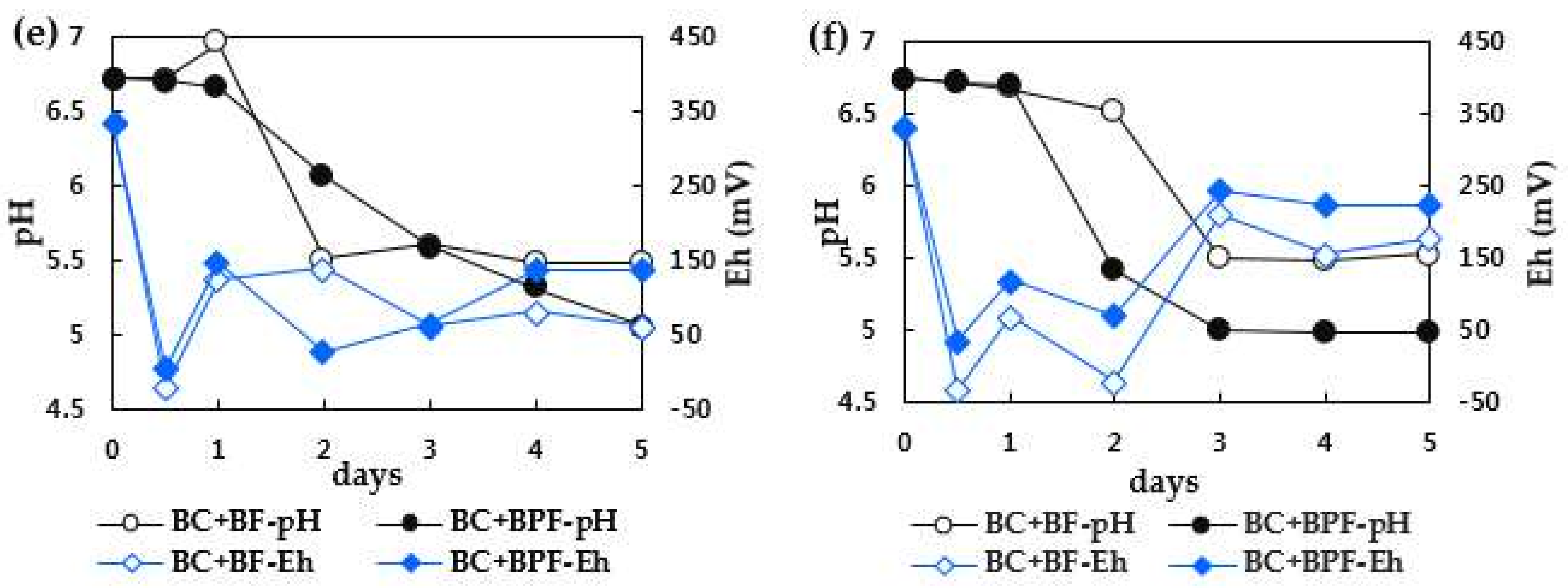
3.2. Redox Potential and pH Changes
3.3. The Amount of Produced SO42− in the Nitrogen Removal Reaction
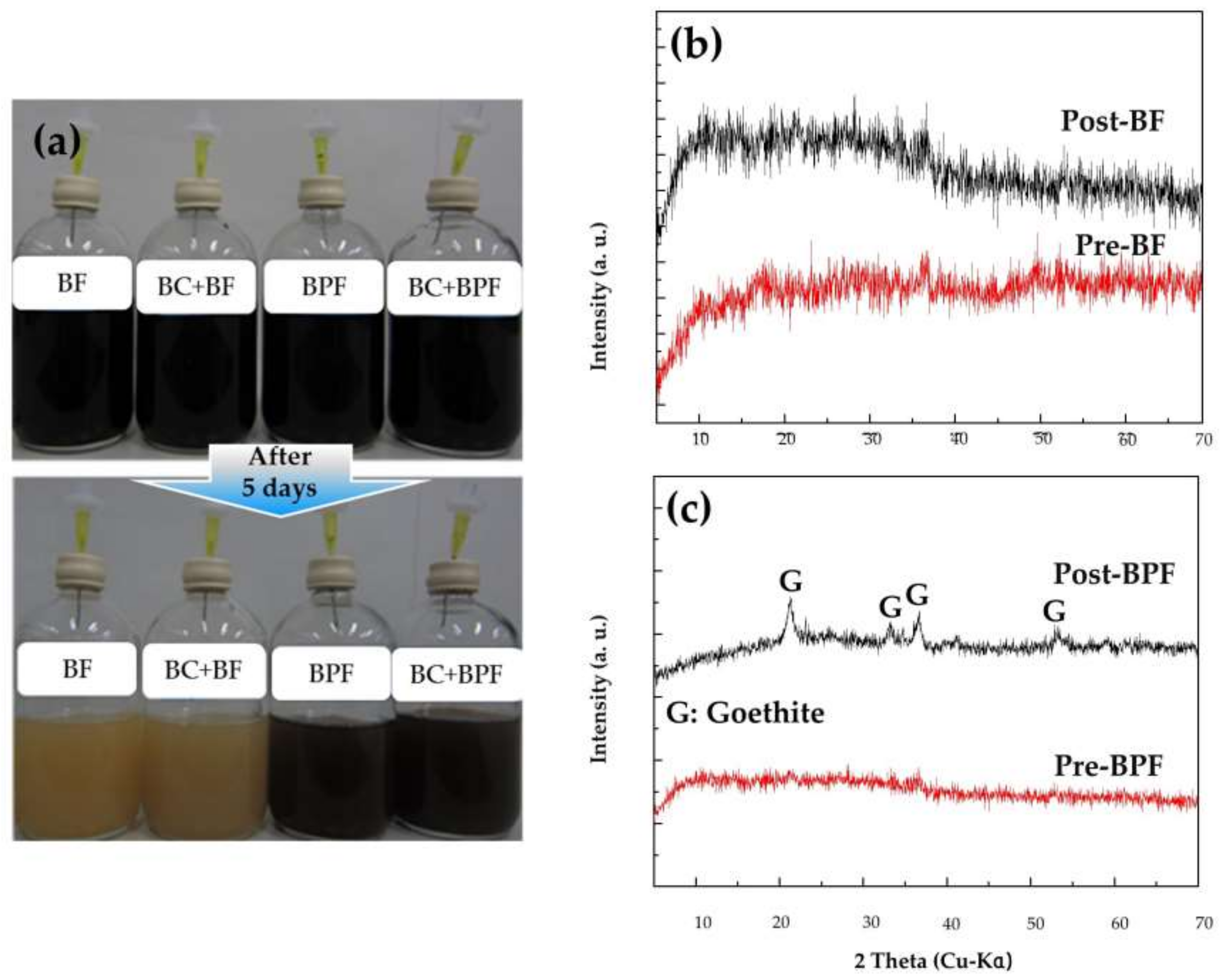
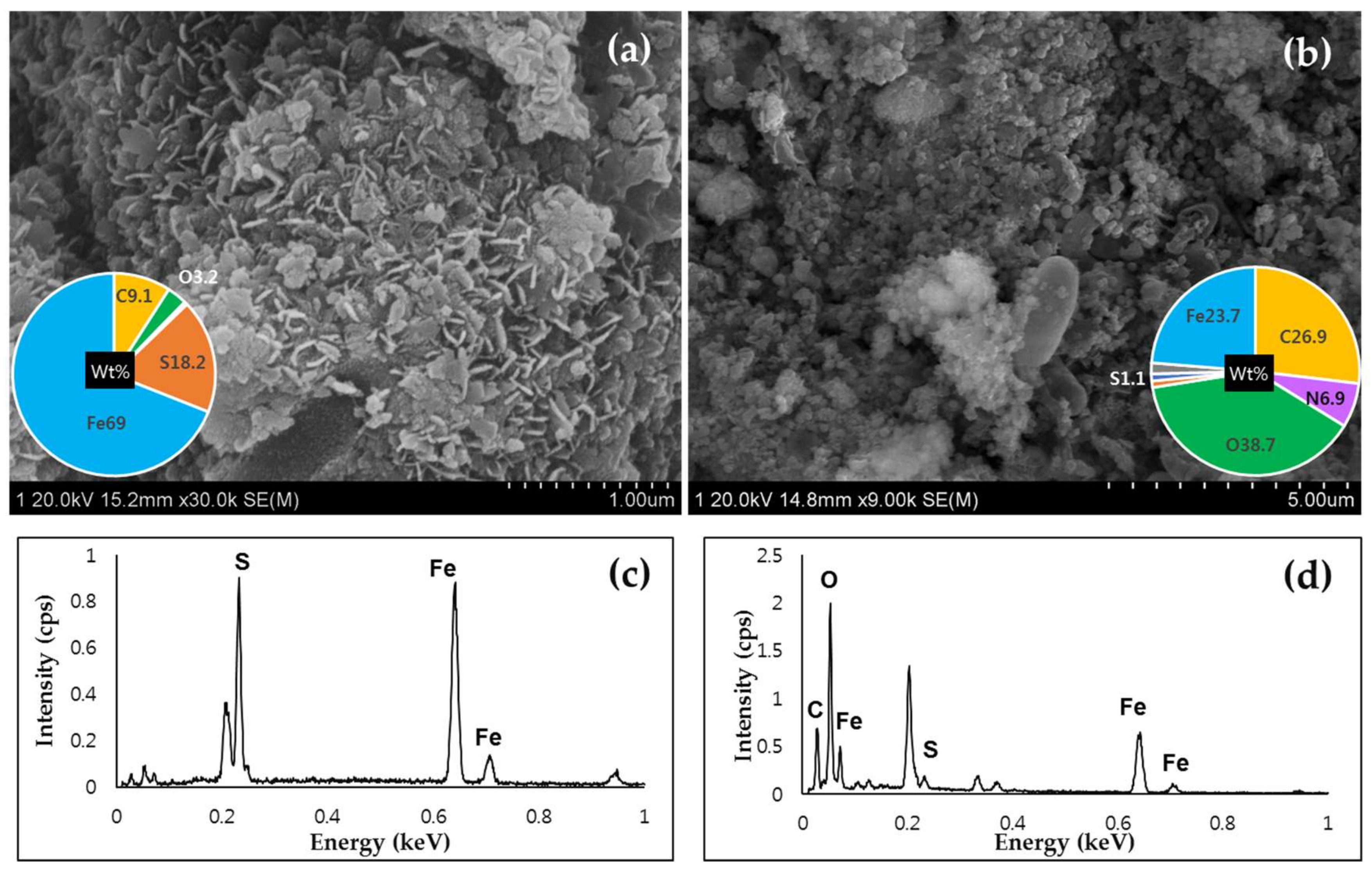
3.4. The Transformation of Bio-Minerals (BF and BPF) Pre- and Post- Reactions
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011; pp. 398–403. [Google Scholar]
- International Groundwater Resources Assessment Centre. Available online: https://un-igrac.org/resource/global-assessment-nitrate-contamination-groundwater (accessed on 17 January 2018).
- Elyanow, D.; Persechino, J. Advances in Nitrate Removal. Available online: https://www.sueswatertechnologies.com (accessed on 3 February 2018).
- Naik, S.; Setty, Y. Biological denitrification of wastewater—A mini review on carbon source. In Proceedings of the International Conference on Chemical, Environmental Science and Engineering, Pattaya, Thailand, 28–29 July 2012. [Google Scholar]
- Lee, H.W.; Park, Y.K.; Choi, E.; Lee, J.W. Bacterial community and biological nitrate removal: Comparisons of autotrophic and heterophic reactors for denitrification with raw sewage. J. Microbial. Biotechnol. 2008, 18, 1826–1835. [Google Scholar] [CrossRef]
- Zhao, B.; He, Y.L.; Zhang, X.F. Nitrogen removal capability through simultaneous heterotrophic nitrification and aerobic denitrification by Bacillus sp. LY. Environ. Technol. 2010, 31, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Park, K.J.; Cho, K.S.; Nam, S.W.; Park, T.J.; Bajpai, R. Aerobic nitrification-denitrification by heterotrophic Bacillus strains. Bioresource Technol. 2005, 96, 1897–1906. [Google Scholar] [CrossRef] [PubMed]
- Zhu, I.; Getting, T. A review of nitrate reduction using inorganic materials. Environ. Technol. Rev. 2012, 1, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Su, C.; Puls, R. Nitrate reduction by zerovalent iron: Effects of formate, oxalate, citrate, chloride, sulfate, borate, and phosphate. Environ. Sci. Technol. 2004, 38, 2715–2720. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.; Liljestrand, H.M.; Khim, J. Nitrate reduction by zero-valent iron under different pH regimes. Appl. Geochem. 2004, 19, 335–342. [Google Scholar] [CrossRef]
- Guo, X.; Yang, Z.; Lie, H.; Lv, X.; Tu, Q.; Ren, Q.; Xia, X.; Jing, C. Common oxidants active the reactivity of zero-valent iron (ZVI) and hence remarkably enhance nitrate reduction from water. Sep. Purif. Technol. 2015, 146, 227–234. [Google Scholar] [CrossRef]
- Choi, J.; Shin, W.S.; Choi, S.J.; Kim, Y.H. Reductive denitrification using zero-valent iron and bimetallic iron Environ. Technol. 2009, 30, 939–946. [Google Scholar] [CrossRef]
- Sun, W.; Li, Q.; Gao, S.; Shang, J.K. Monometallic Pd/Fe3O4 catalyst for denitrification of water. Appl. Catal. B Environ. 2012, 125, 1–9. [Google Scholar] [CrossRef]
- Jung, J.; Bae, S.; Lee, W. Nitrate reduction by maghemite supported Cu-Pd bimetallic catalyst. Appl. Catal. B Environ. 2012, 127, 148–158. [Google Scholar] [CrossRef]
- Ottley, C.J.; Davison, W.; Edmunds, W.M. Chemical catalysis of nitrate reduction by iron (II). Geochim. Cosmochim. Acta 1997, 61, 1819–1828. [Google Scholar] [CrossRef]
- Brunet, R.C.; Garcia-Gil, L.J. Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol. Ecol. 1996, 21, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Henderson, A.D.; Demond, A.H. Permeability of iron sulfide (FeS)-based materials for groundwater remediation. Water Res. 2013, 47, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Feng, C.; Liu, Y.; Li, R.; Kong, Z.; Chen, N.; Tong, S.; Hao, C.; Liu, Y. Pyrite-based autotrophic denitrification for remediation of nitrate contaminated groundwater. Bioresour. Technol. 2014, 173, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Till, B.A.; Weathers, L.J.; Alvarez, P.J.J. Fe (0)-supported autotrophic denitrification. Environ. Sci. Technol. 1998, 32, 634–639. [Google Scholar] [CrossRef]
- Seo, H.; Roh, Y. Mixed contaminants removal efficiency using bio-FeS nanoparticles. J. Nanosci. Nanotechnol. 2017, 17, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kalkowski, I.; Conrad, R. Metabolism of nitric oxide in denitrifying Pseudomonas aeruginosa and nitrate-respiring Bacillus cereus. FEMS Microbiol. Lett. 1991, 82, 107–112. [Google Scholar] [CrossRef]
- Rout, P.R.; Bhunia, P.; Dash, R.R. Simultaneous removal of nitrogen and phosphate from domestic wastewater using Bacillus cereus GS-5 strain exhibiting heterotrophic nitrification, aerobic denitrification and denitrifying phosphorous removal. Bioresource Technol. 2017, 244, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Cha, D.K. Microbial reduction of nitrate in the presence of nanoscale zero-valent iron. Chemosphere 2008, 72, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Gordon, A.D.; Smirnov, A.; Shumlas, S.L.; Singireddy, S.; DeCesare, M.; Schoonen, M.A.A.; Strongin, D.R. Reduction of nitrite and nitrate on nano-dimensioned FeS. Oringins Life Evol. Biosph. 2013, 43, 305–322. [Google Scholar] [CrossRef] [PubMed]
- Guy, K.A.; Xu, H.; Yang, J.C.; Werth, C.J.; Shapley, J.R. Catalytic nitrate and nitrite reduction with Pd-Cu/PVP colloids in water: Composition, structure, and reactivity correlations. J. Phys. Chem. C 2009, 113, 8177–8185. [Google Scholar] [CrossRef]
- Chirita, P.; Descostes, M.; Schlegel, M.L. Oxidation of FeS by oxygen-bearing acidic solutions. J. Colloid Interf. Sci. 2008, 321, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Sheela, B.; Khasim, B.S.; Yellaji, R.O. Simultaneous nitrification and denitrification of ammonical wastewaters using Bacillus species SBI isolated from domestic sewage. IJLBPR 2015, 4, 17–21. [Google Scholar] [CrossRef]
- Luther, G.W., III; Findlay, A.J.; MacDonald, D.J.; Owings, S.M.; Hanson, T.E.; Beinart, R.A.; Girguis, P.R. Thermodynamics and kinetics of sulfide oxidation by oxygen: A look at inorganically controlled reactions and biologically mediated processes in the environment. Front. Microbiol. 2011, 2, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eby, G.N. Principles of Environmental Geochemistry; Waveland Press, Inc.: Long Grove, IL, USA, 2016; pp. 108–109. [Google Scholar]



| Ingredients | (g/L) |
|---|---|
| NaHCO3 | 0.3 |
| CaCl2∙2H2O | 0.025 |
| KH2PO4 | 0.3 |
| MgCl2∙6H2O | 0.1 |
| HEPES (C8H18N2O4S) | 7.2 |
| Trace minerals | 1 mL |
| Vitamins | 1 mL |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, H.; Roh, Y. Bio-Minerals Combined with Bacillus cereus for Enhancing the Nitrogen Removal Efficiency under Aerobic Conditions. Minerals 2018, 8, 253. https://doi.org/10.3390/min8060253
Seo H, Roh Y. Bio-Minerals Combined with Bacillus cereus for Enhancing the Nitrogen Removal Efficiency under Aerobic Conditions. Minerals. 2018; 8(6):253. https://doi.org/10.3390/min8060253
Chicago/Turabian StyleSeo, Hyunhee, and Yul Roh. 2018. "Bio-Minerals Combined with Bacillus cereus for Enhancing the Nitrogen Removal Efficiency under Aerobic Conditions" Minerals 8, no. 6: 253. https://doi.org/10.3390/min8060253




