Preliminary Data on the Nanoscale Chemical Characterization of the Inter-Crystalline Organic Matrix of a Calcium Carbonate Biomineral
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Methods
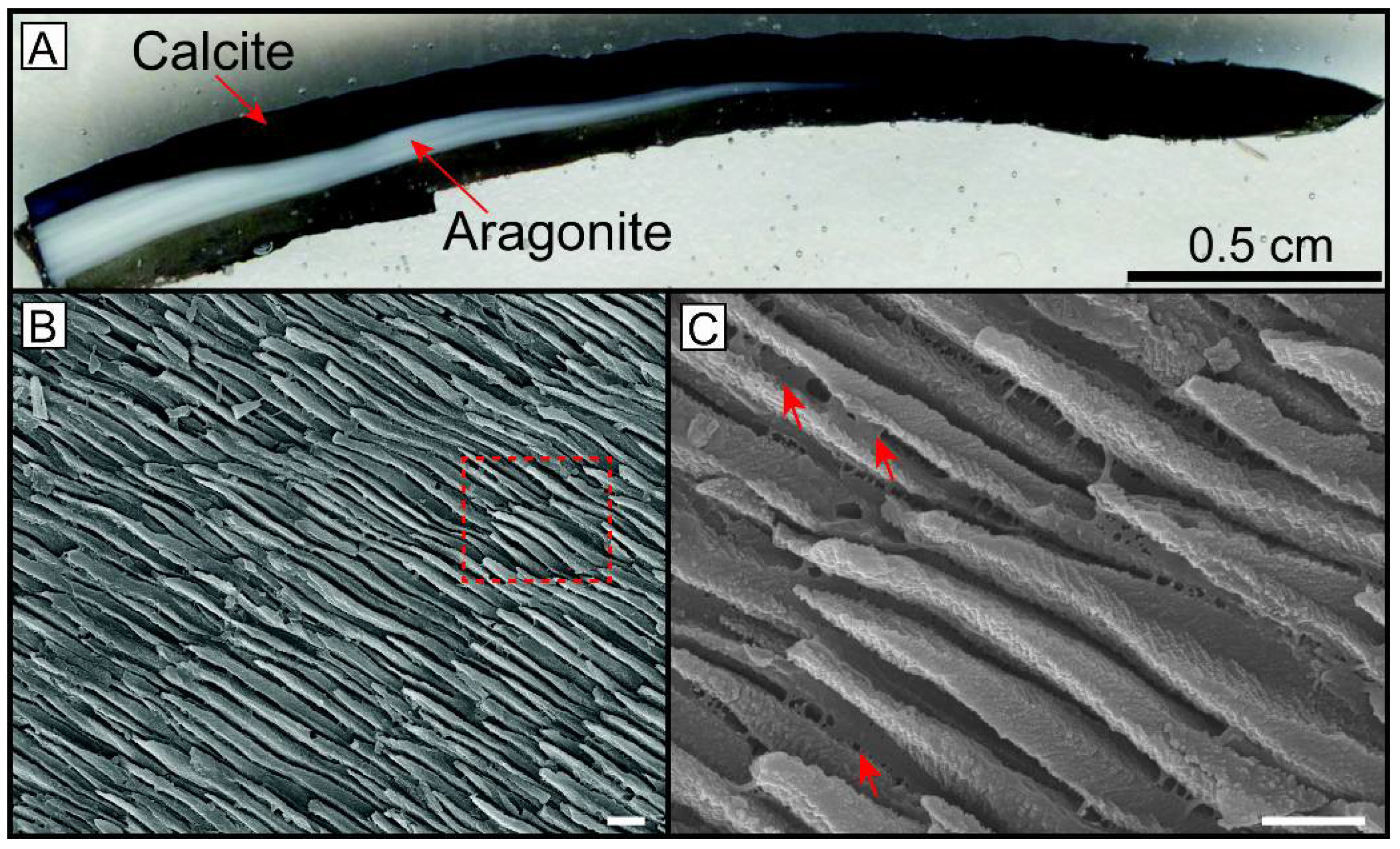
2.2.1. Selection of Regions of Interest
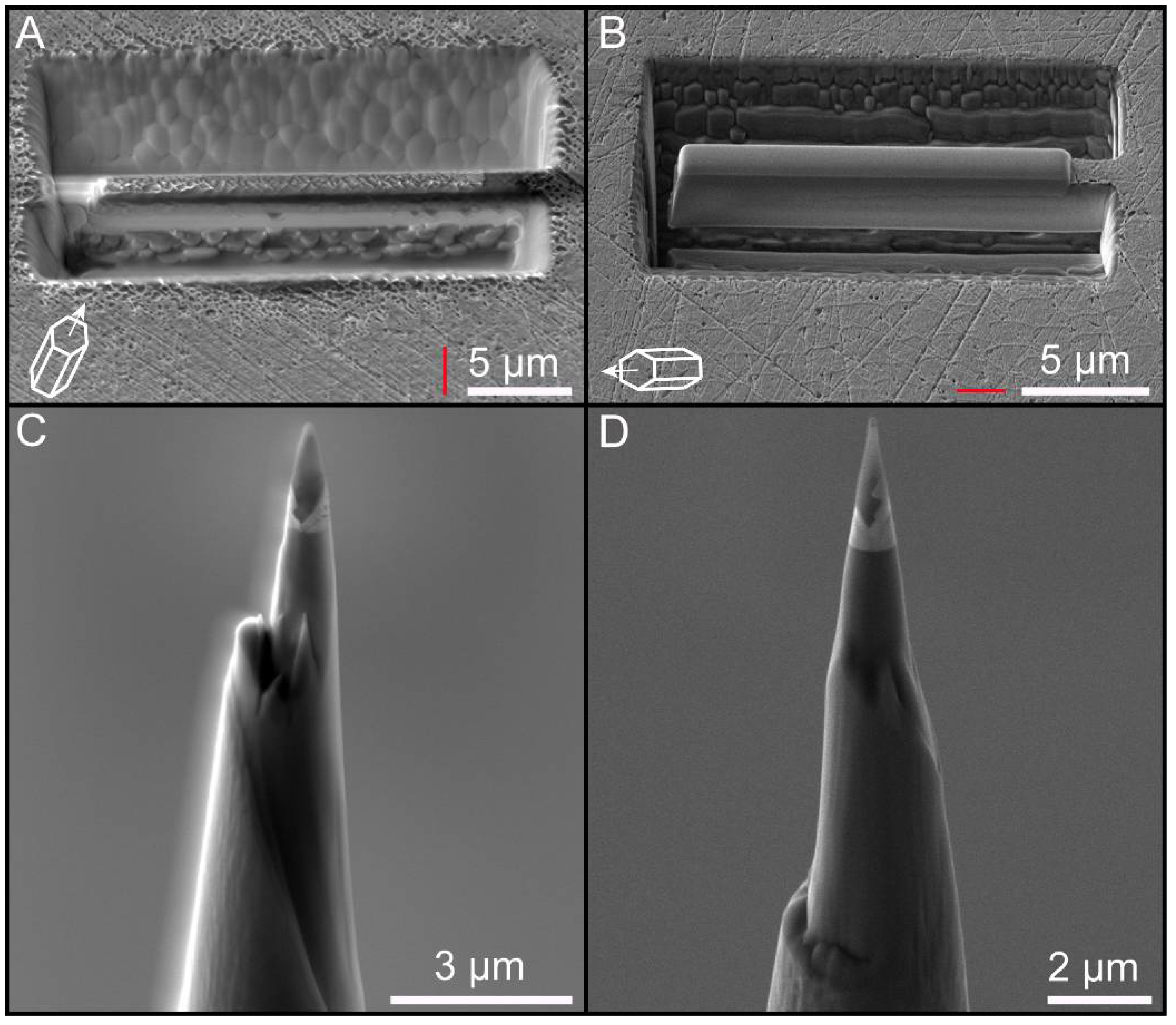
2.2.2. Focused Ion Beam (FIB) Work
2.2.3. Scanning Electron Microscopy (SEM) Imaging
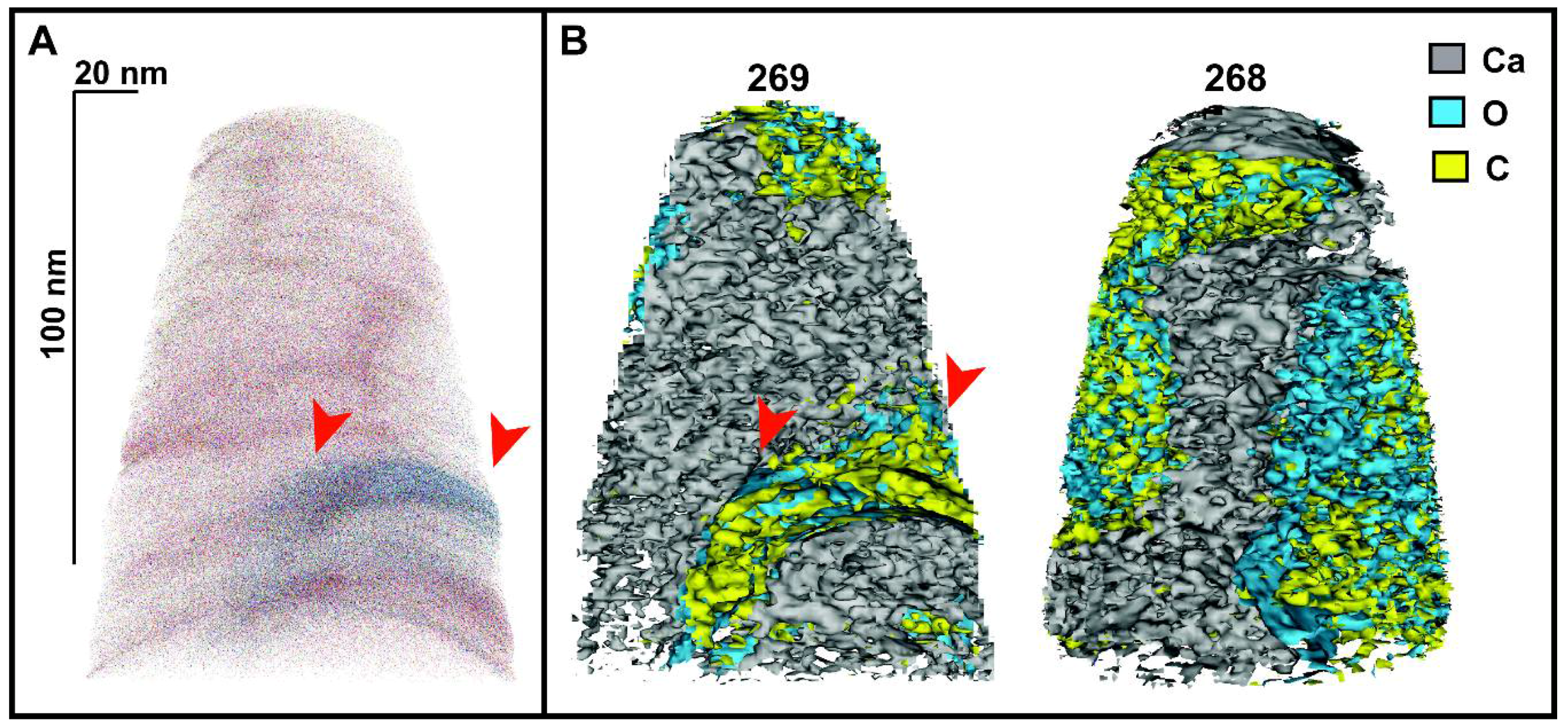
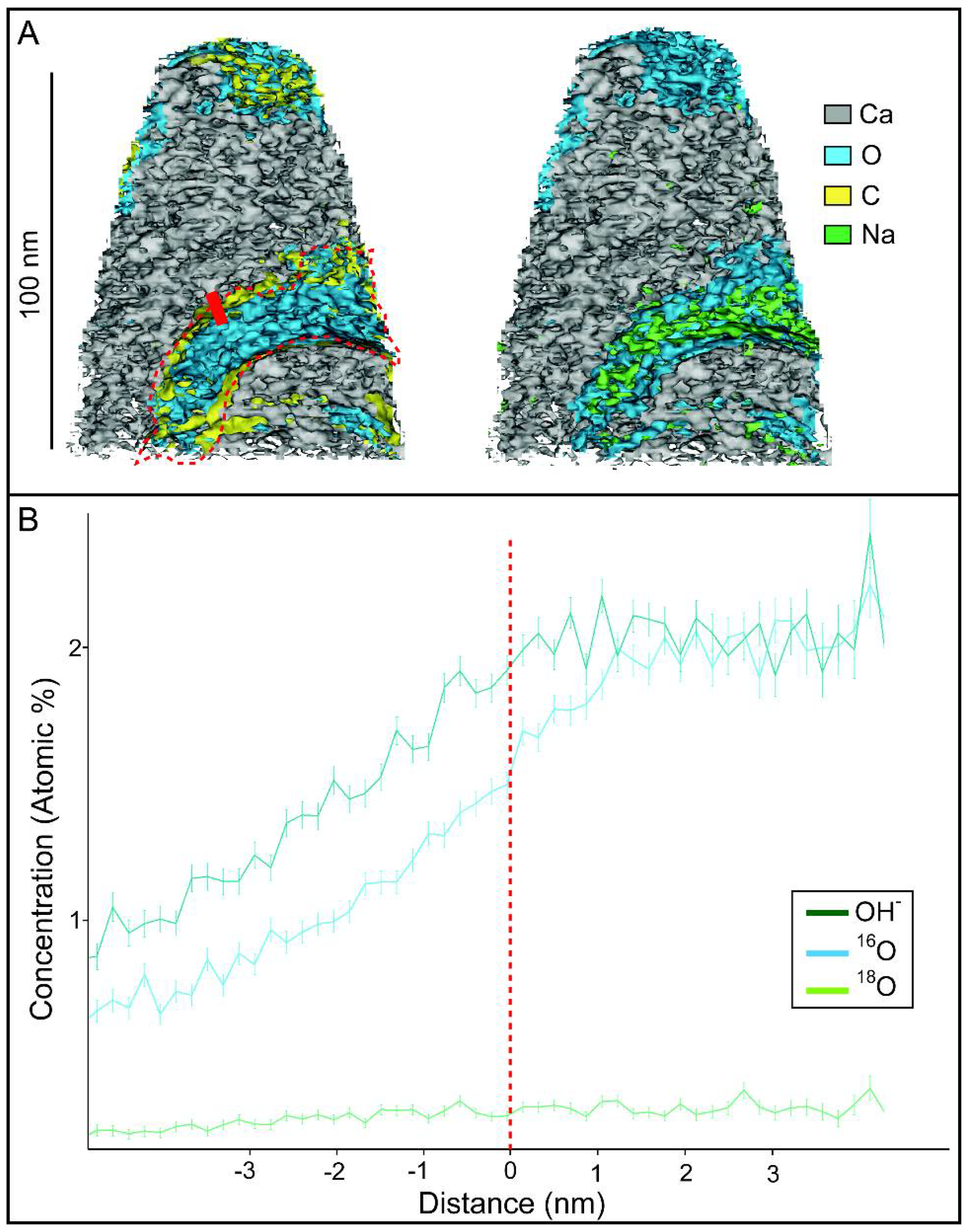
2.2.4. Local Electrode Atom Probe (LEAP) Work and Data Analyses
3. Results
3.1. Quality of Atom Probe Tomography Specimens
3.2. Atom Probe Tomography Chemical Data
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Cuif, J.-P.; Dauphin, Y.; Sorauf, J.E. Biominerals and Fossils through Time; Cambridge University Press: Cambridge, UK, 2011; p. ix + 490. [Google Scholar]
- Pérez-Huerta, A.; Coronado, I.; Hegna, T. Understanding biomineralization in the fossil record. Earth Sci. Rev. 2018, 179, 95–122. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989; 324p. [Google Scholar]
- Weiner, S.; Dove, P.M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 2003, 54, 1–29. [Google Scholar] [CrossRef]
- Dalbeck, P.; England, J.; Cusack, M.; Lee, M.R.; Fallick, A.E. Crystallography and chemistry of the calcium carbonate polymorph switch in M. edulis shell. Eur. J. Mineral. 2006, 18, 601–609. [Google Scholar] [CrossRef]
- Arivalagan, J.; Yarra, T.; Marie, B.; Sleight, V.A.; Duvernois-Berthet, E.; Clark, M.S.; Marie, A.; Berland, S. Insights from the shell proteome: Biomineralization to adaptation. Mol. Biol. Evol. 2017, 34, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Hüning, A.K.; Lange, S.M.; Ramesh, K.; Jacob, D.E.; Jackson, D.J.; Panknin, U.; Gutowska, M.A.; Philipp, E.E.R.; Rosentiel, P.; Lucasson, M.; et al. A shell regeneration assay to identify biomineralization candidate genes in mytilid mussels. Mar. Genom. 2016, 27, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Vander Putten, E.; Dehairs, F.; Keppens, E.; Baeyens, W. High resolution distribution of trace elements in the calcite shell layer of modern Mytilus edulis: Environmental and biological controls. Geochim. Cosmochim. Acta 2000, 64, 997–1011. [Google Scholar] [CrossRef]
- Gillikin, D.P.; Dehairs, F.; Lorrain, A.; Steenmans, D.; Baeyens, W.; André, L. Barium uptake into the shells of the common blue mussel (Mytilus edulis) and the potential for estuarine paleo-chemistry reconstruction. Geochim. Cosmochim. Acta 2006, 70, 395–407. [Google Scholar] [CrossRef]
- Wanamaker, A.D., Jr.; Kreutz, K.J.; Borns, H.W., Jr.; Introne, D.S.; Feindel, S.; Funder, S.; Rawson, P.D.; Barber, B.J. Experimental determination of salinity, temperature, growth, and metabolic effects on shell isotope chemistry of Mytilus edulis collected from Maine and Greenland. Paleoceanography 2007, 22, PA2217. [Google Scholar] [CrossRef]
- Freitas, P.S.; Clarke, L.J.; Kennedy, H.; Richardson, C.A. Ion microprobe assessment of the heterogeneity of Mg/Ca, Sr/Ca, and Mn/Ca ratios in Pecten maximus and Mytilus edulis (bivalvia) shell calcite precipitated at constant temperature. Biogeosciences 2009, 6, 1209–1227. [Google Scholar] [CrossRef]
- Gordon, L.M.; Joester, D. Nanoscale chemical tomography of buried organic-inorganic interfaces in the chiton tooth. Nature 2011, 469, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Tran, L.; Joester, D. Atom probe tomography of apatites and bone-type mineralized tissues. ACS Nano 2012, 6, 10667–10675. [Google Scholar] [CrossRef] [PubMed]
- Gordon, L.M.; Cohen, M.J.; MacRenaris, K.W.; Pasteris, J.D.; Seda, T.; Joester, D. Amorphous intergranular phases control the properties of rodent tooth enamel. Science 2015, 347, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Branson, O.; Bonnin, E.A.; Perea, D.E.; Spero, H.J.; Zhu, Z.; Winters, M.; Hönisch, B.; Russell, A.D.; Fehrenbacher, J.S.; Gagnon, A.C. Nanometer-scale chemistry of calcite biomineralization template: Implications for skeletal composition and nucleation. Proc. Natl. Acad. Sci. USA 2016, 113, 12934–12939. [Google Scholar] [CrossRef] [PubMed]
- Thompson, K.; Lawrence, D.; Larson, D.J.; Olson, J.D.; Kelly, T.F.; Gorman, B. In situ site-specific specimen preparation for atom probe tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Huerta, A.; Laiginhas, F.; Reinhard, D.A.; Prosa, T.J.; Martens, R.L. Atom probe tomography (APT) of carbonate minerals. Micron 2016, 80, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Perea, D.E.; Liu, J.; Bartrand, J.; Dicken, Q.; Thevuthasan, S.T.; Browning, N.D.; Evans, J.E. Atom probe tomography mapping directly reveals the atomic distribution of phosphorous in resin embedded ferritin. Sci. Rep. 2016, 6, 22321. [Google Scholar] [CrossRef] [PubMed]
- Hare, P.E. Amino acids in the proteins from aragonite and calcite in the shells of Mytilus californianus. Science 1963, 139, 216–217. [Google Scholar] [CrossRef] [PubMed]
- Addadi, L.; Joester, D.; Nudelman, F.; Weiner, S. Mollusk shell formation: A source of new concepts for understanding biomineralization processes. Chem. Eur. J. 2006, 12, 980–987. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Liao, Z.; Wang, X.-X.; Bao, L.-F.; Fan, M.-H.; Li, X.-M.; Wu, C.-W.; Xia, S.-W. Layer-by-layer proteomic analysis of Mytilus gallopronvincialis shells. PLoS ONE 2015, 10, e0133913. [Google Scholar] [CrossRef] [PubMed]
- Schöne, B.R.; Zhang, Z.; Jacob, D.E.; Gillikin, D.P.; Tütken, T.; Garbe-Schönberg, D.; McConnaughey, T.; Soldati, A. Effect of organic matrices on the determination of the trace element chemistry (Mg, Sr, Mg/Ca, Sr/Ca) of aragonitic bivalve shells (Artica islandica)—Comparison of ICP-OES and LA-ICP-MS data. Geochem. J. 2010, 44, 23–37. [Google Scholar]
- Rollion-Bard, C.; Mangin, D.; Champenois, M. Development and application of oxygen and carbon isotopic measurements of biogenic carbonates by ion probe. Geostand. Geoanal. Res. 2007, 31, 39–50. [Google Scholar] [CrossRef]

| Specimen/Data Set | M26_268 | M27_269 | M30_299 | M24_742 | M14_744 |
|---|---|---|---|---|---|
| Sample Description | Mussel Calcite Prism | Mussel Calcite Prism | Mussel Calcite Prism | Mussel Calcite Prism_90 | Mussel Calcite Prism_90 |
| Instrument Model | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS | LEAP 5000 XS |
| Instrument Settings | |||||
| Laser Wavelength (nm) | 355 | 355 | 355 | 355 | 355 |
| Laser Pulse Rate (pJ) | 50 | 50 | 50 | 500 | 500 |
| Laser Pulse Energy (kHz) | 200 | 200 | 200 | 200 | 200 |
| Evaporation Control | Detection Rate | Detection Rate | Detection Rate | Detection Rate | Detection Rate |
| Target Detection Rate (%) | 0.2 | 0.3 | 0.2 | 0.5 | 0.5 |
| Nominal Flight Path (mm) | 100 | 100 | 100 | 100 | 100 |
| Temperature (K) | 40 | 40 | 40 | 50 | 50 |
| Pressure (torr) | 5.4 × 10−11 | 6.2 × 10−11 | 6.3 × 10−11 | 5.0 × 10−11 | 5.1 × 10−11 |
| ToF offset, to (ns) | 279.94 | 279.94 | 279.94 | 279.94 | 279.94 |
| Data Analysis | |||||
| Software | IVAS 3.6.12 | IVAS 3.6.12 | IVAS 3.6.12 | IVAS 3.6.12 | IVAS 3.6.12 |
| Total Ions: | 17,353,786 | 19,344,916 | 14,354,477 | 5,949,513 | 2,638,187 |
| Single | 12,422,920 | 13,540,671 | 10,277,531 | 4,582,591 | 1,977,676 |
| Multiple | 4,795,460 | 5,640,146 | 3,954,829 | 1,253,233 | 587,989 |
| Partial | 135,406 | 164,099 | 122,117 | 113,689 | 72,522 |
| Reconstructed Ions: | 15,845,723 | 14,964,253 | 12,423,780 | 3,371,329 | 1,447,152 |
| Ranged | 2,613,374 | 3,505,144 | 2,154,459 | 1,223,980 | 355,479 |
| Unranged | 13,232,349 | 11,459,109 | 10,269,321 | 2,147,349 | 1,091,673 |
| Background (ppm/nsec) | 12 | 26 | 9 | 43 | 17 |
| Reconstruction | |||||
| Final tip state | Fractured | Fractured | Fractured | Fractured | Fractured |
| Pre-/Post-analysis Imaging | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. | SEM/n.a. |
| Radius Evolution Model | “shank” | “shank” | “shank” | “shank” | “shank” |
| Vinitial; Vfinal | 2382 V; 5104 V | 2134 V; 6029 V | 1537 V; 4085 V | 2651 V; 5069 V | 3527 V; 3986 V |
| Specimen | 268 | ||
| Element | Atom Count | Atomic % | 1s error |
| Ca | 1,009,230 | 39.59 | 0.0466 |
| O | 1,228,475 | 48.19 | 0.0529 |
| C | 301,983 | 11.85 | 0.0228 |
| Na | 5585 | 0.22 | 0.0029 |
| H | 3277 | 0.13 | 0.0022 |
| N | 711 | 0.03 | 0.0010 |
| Total | 2,549,261 | 100 | 0.13 |
| Specimen | 269 | ||
| Ion Type | Atom Count | Atomic % | 1s error |
| Ca | 1,837,349 | 39.76 | 0.0347 |
| O | 1,915,350 | 41.45 | 0.0356 |
| C | 675,984 | 14.63 | 0.0190 |
| Na | 22,001 | 0.48 | 0.0032 |
| H | 170,212 | 3.68 | 0.0091 |
| N | 575 | 0.01 | 0.0005 |
| Total | 4,621,471 | 100 | 0.10 |
| Specimen | 299 | ||
| Ion Type | Atom Count | Atomic % | 1s error |
| Ca | 1,210,035 | 49.63 | 0.0552 |
| O | 980,140 | 40.20 | 0.0481 |
| C | 224,252 | 9.20 | 0.0203 |
| Na | 8043 | 0.33 | 0.0037 |
| H | 15,268 | 0.63 | 0.0051 |
| N | 568 | 0.02 | 0.0010 |
| Total | 2,438,305 | 100 | 0.13 |
| Specimen | 742 | ||
| Ion Type | Atom Count | Atomic % | 1s error |
| Ca | 1,140,973 | 60.20 | 0.0713 |
| O | 639,550 | 33.75 | 0.0488 |
| C | 107,881 | 5.69 | 0.0178 |
| Na | 6806 | 0.36 | 0.0044 |
| Total | 1,895,210 | 100 | 0.14 |
| Specimen | 744 | ||
| Ion Type | Atom Count | Atomic % | 1s error |
| Ca | 321,589 | 60.66 | 0.1356 |
| O | 177,418 | 33.47 | 0.0918 |
| C | 30,081 | 5.67 | 0.0336 |
| Na | 1059 | 0.20 | 0.0061 |
| Total | 530,147 | 100 | 0.27 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Huerta, A.; Laiginhas, F. Preliminary Data on the Nanoscale Chemical Characterization of the Inter-Crystalline Organic Matrix of a Calcium Carbonate Biomineral. Minerals 2018, 8, 223. https://doi.org/10.3390/min8060223
Pérez-Huerta A, Laiginhas F. Preliminary Data on the Nanoscale Chemical Characterization of the Inter-Crystalline Organic Matrix of a Calcium Carbonate Biomineral. Minerals. 2018; 8(6):223. https://doi.org/10.3390/min8060223
Chicago/Turabian StylePérez-Huerta, Alberto, and Fernando Laiginhas. 2018. "Preliminary Data on the Nanoscale Chemical Characterization of the Inter-Crystalline Organic Matrix of a Calcium Carbonate Biomineral" Minerals 8, no. 6: 223. https://doi.org/10.3390/min8060223





