Effect of Ethylene Diamine Phosphate on the Sulfidization Flotation of Chrysocolla
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Micro-Flotation Experiments
2.3. TEM and BET Measurements
2.4. Adsorption Experiments
2.5. Zeta Potential Measurements
3. Results and Discussion
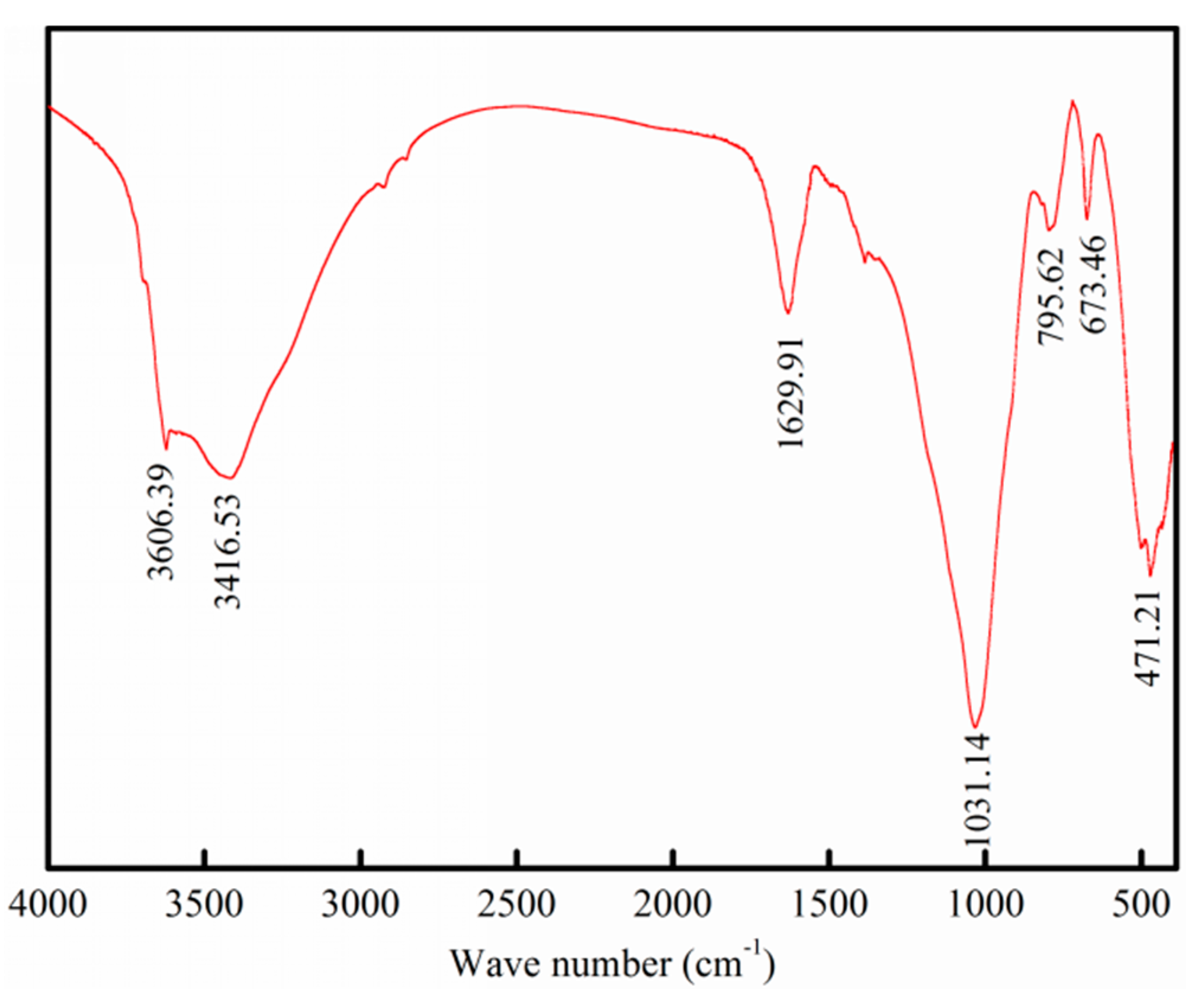
3.1. Surface Structure and Properties of Chrysocolla
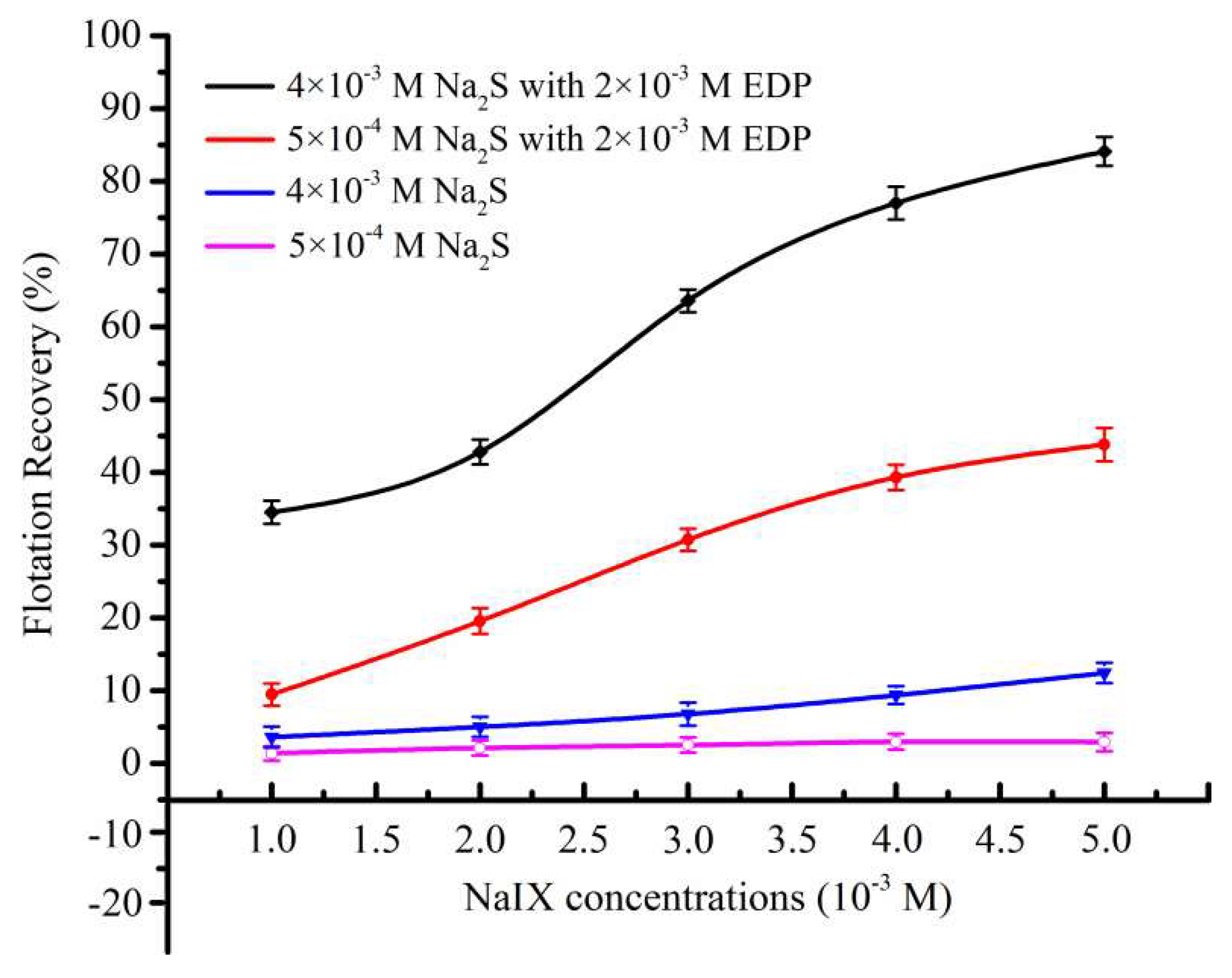
3.2. Effect of EDP on Sulfidization Flotation of Chrysocolla
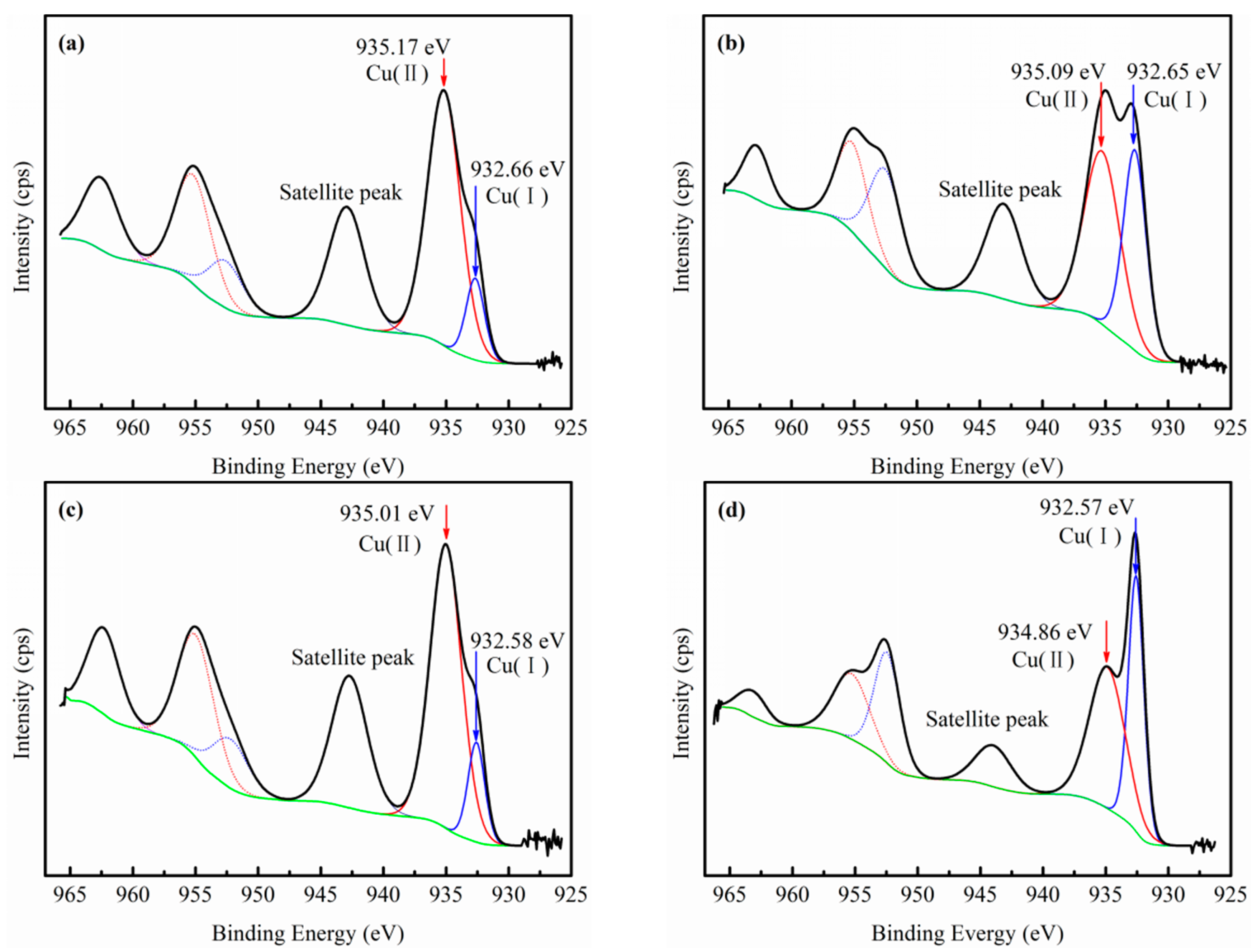
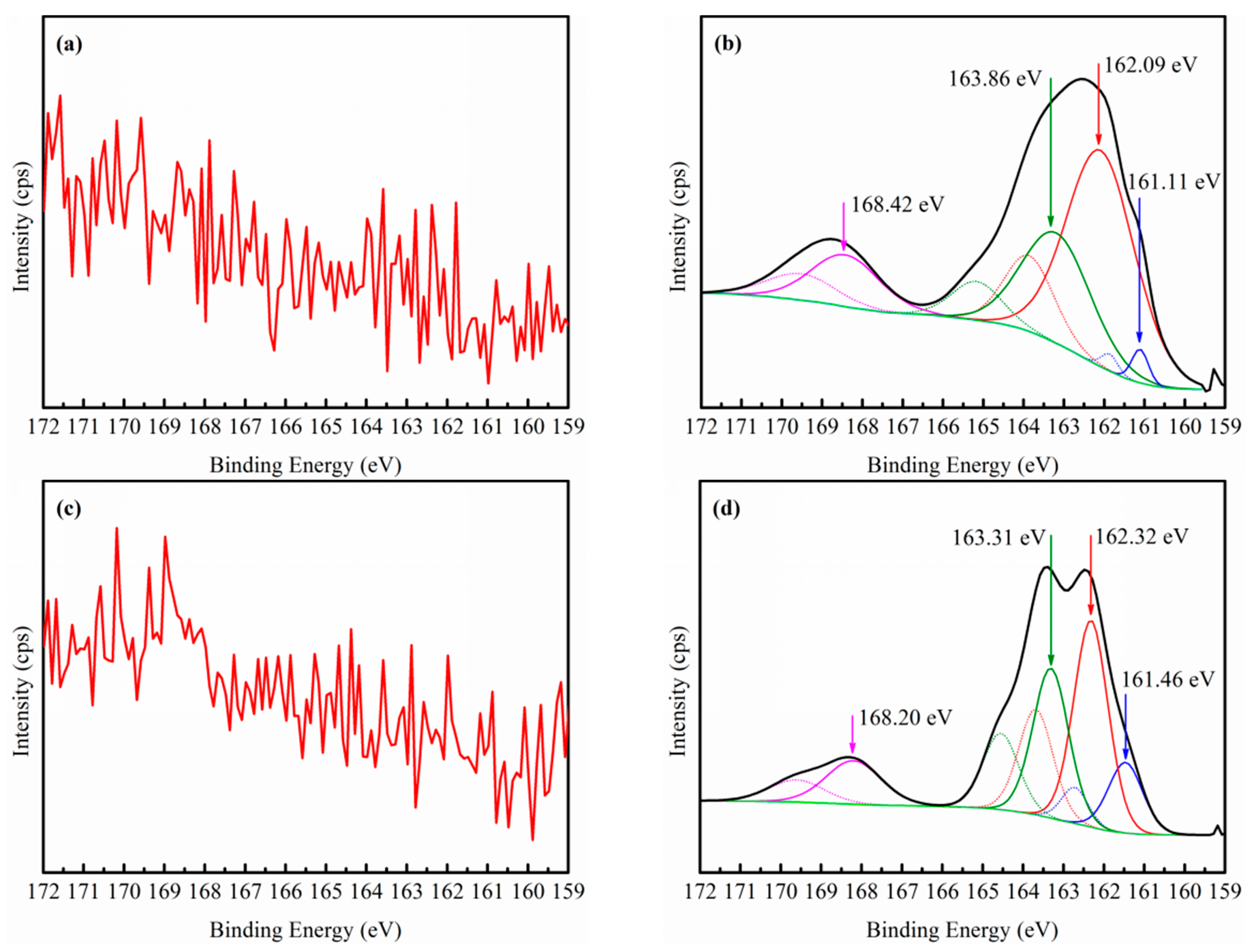
3.3. XPS Analysis
3.4. ICP-AES Analysis
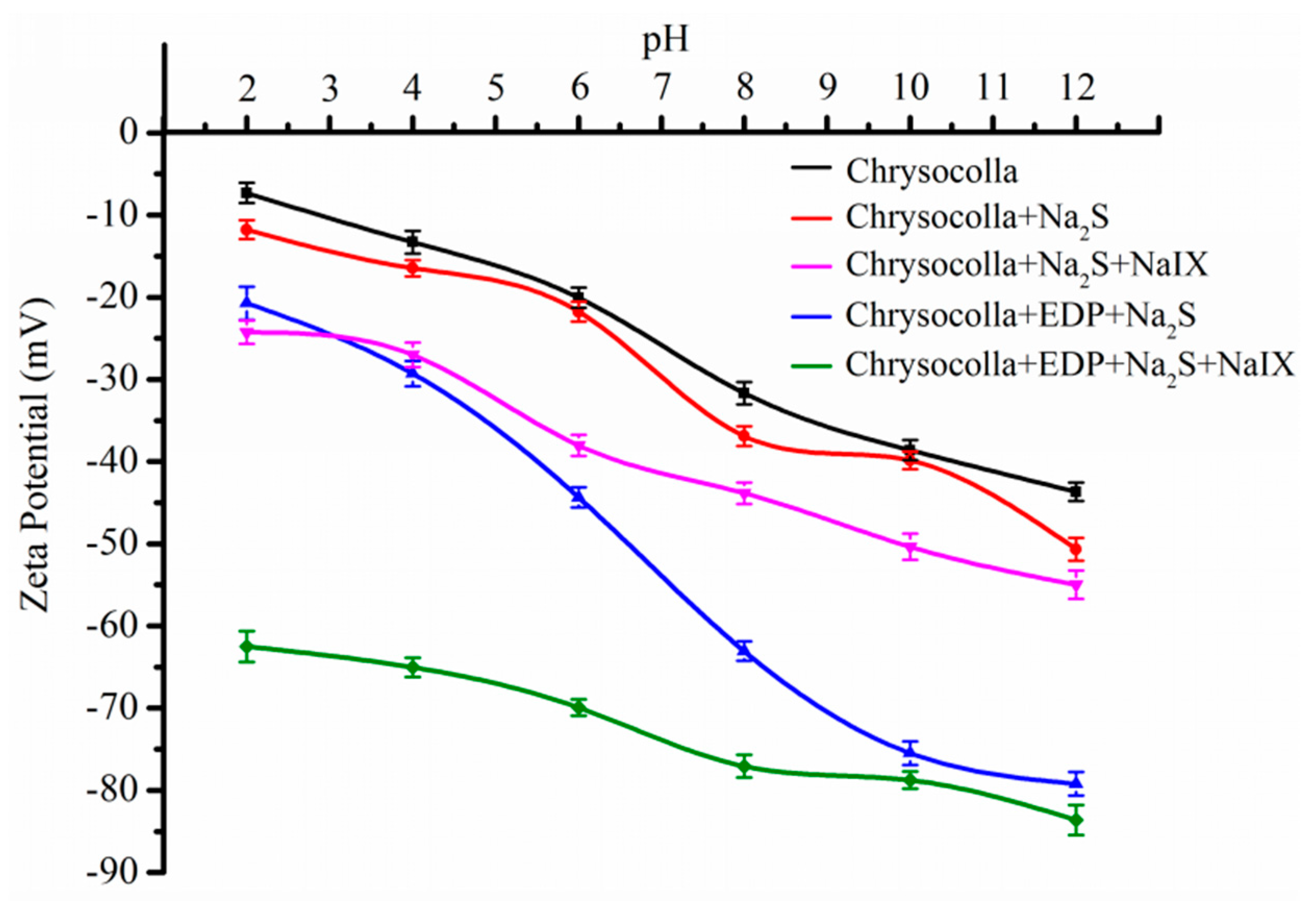
3.5. Zeta Potential Measurements
4. Conclusions
- (1)
- TEM and BET analyses indicate that the chrysocolla’s surface is porous and has a large specific surface area, which may be an important reason large amounts of reagents are consumed when better flotation recovery is obtained for chrysocolla.
- (2)
- EDP has a positive effect on the sulfidization flotation of chrysocolla. Chrysocolla was not floated with Na2S and xanthate in the absence of EDP, while excellent flotation recovery was achieved when chrysocolla was activated with EDP prior to sulfidization using xanthate as a collector.
- (3)
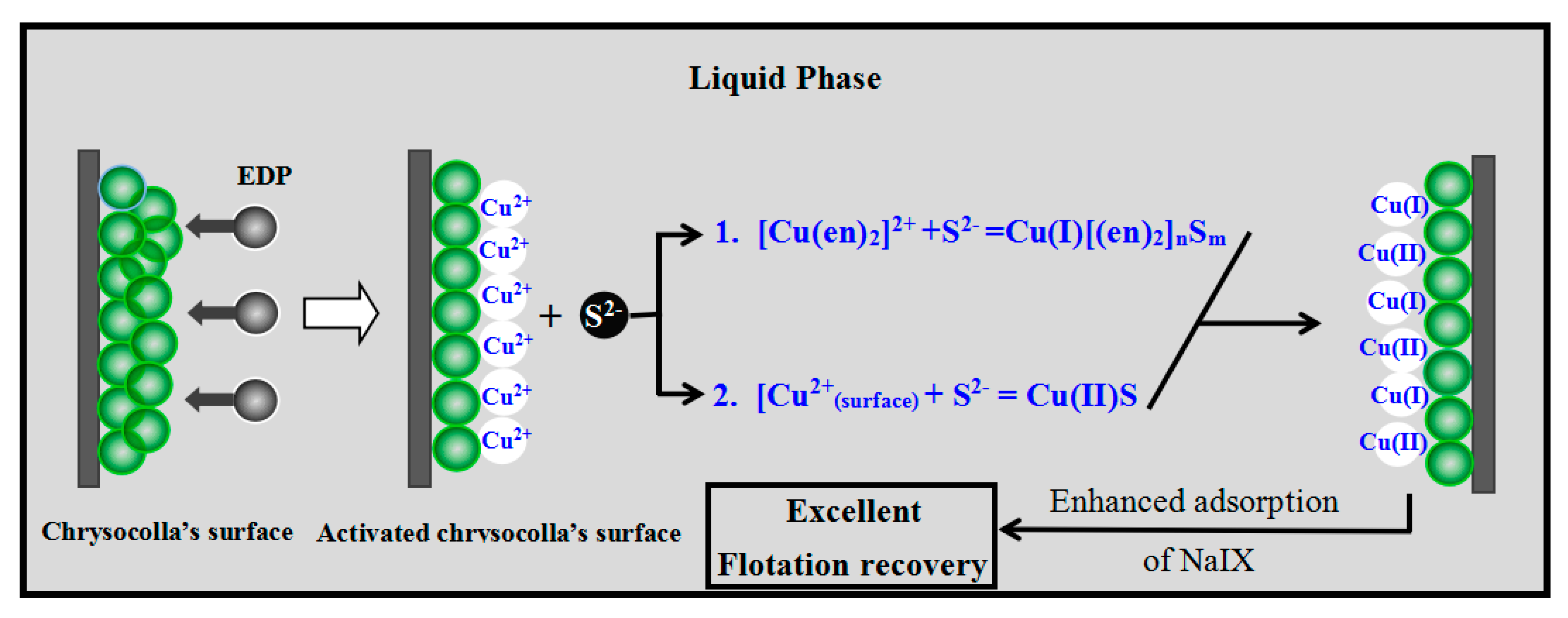
- EDP enhanced the sulfidization-flotation of chrysocolla for two reasons. First, the low-activity Cu sites on the chrysocolla’s surface were dissolved into the pulp solution as copper-ammonia complex ions after the amount of EDP was increased, resulting in more high-activity Cu sites being exposed on the chrysocolla’s surface, which improved the sulfidization reaction on its surface. Second, a redox reaction occurred between the S2− and [Cu(en)2]2+ ions, causing the copper ions in the solution to counter-adsorb onto the chrysocolla’s surface as a new complex, and during this process, the Cu(II) was reduced to Cu(I) and the main sulfidization products were S22−, Sn2−, and SO42−. In conclusion, our results indicate that a denser hydrophobic film were formed on the chrysocolla’s surface, improving the flotation behavior of chrysocolla.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Raghavan, S.; Fuerstenau, D.W. Characterization and pore structure analysis of a copper ore containing chrysocolla. Int. J. Miner. Process. 1997, 4, 381–394. [Google Scholar] [CrossRef]
- Frost, R.L.; Xi, Y.F. Is chrysocolla (Cu, Al)2H2Si2O5(OH)4·nH2O related to spertiniite Cu(OH)2?—Avibrational spectroscopic study. Vib. Spectrosc. 2013, 64, 33–38. [Google Scholar] [CrossRef] [Green Version]
- Bideaux, A.; Nichols, B. Handbook of Mineralogy, Part 1; Mineral Data Publishing: Tucson, AZ, USA, 1995. [Google Scholar]
- Frost, R.L.; Xi, Y.F.; Wood, B.J. Thermogravimetric analysis, PXRD, EDX and XPS study of chrysocolla (Cu, Al)2H2Si2O5(OH)4·nH2O-structural implications. Thermochim. Acta 2012, 545, 157–162. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.C.; Wen, S.M.; Zhao, W.J.; Lu, C.; Bai, X. Leaching of copper from malachite with methane-sulfonic acid. Solvent. Extr. Res. Dev. 2015, 22, 159–168. [Google Scholar] [CrossRef]
- Habashi, F.; Dugdale, R. Leaching studies on chrysocolla. Trans. AIME 1973, 254, 98–102. [Google Scholar]
- Tanda, B.C.; Eksteen, J.J.; Oraby, E.A. An investigation into the leaching behaviour of copper oxide minerals in aqueous alkaline glycine solutions. Hydrometallurgy 2017, 167, 153–162. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, D. Biohydrometallurgy for nonsulfidic minerals—A review. Geomicrobiol. J. 2004, 21, 135–144. [Google Scholar] [CrossRef]
- Lambert, F.; Gaydardzhiev, S.; Léonard, G.; Lewis, G.; Bareel, P.F.; Bastin, D. Copper leaching from waste electric cables by biohydrometallurgy. Miner. Eng. 2015, 76, 38–46. [Google Scholar] [CrossRef]
- Hu, K.J.; Wu, A.X.; Wang, H.J.; Wang, S.Y. A new heterotrophic strain for bioleaching of low grade complex copper ore. Minerals 2016, 6, 12. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Gonzalez, G.; Loskowski, J. The effect of ageing on the flotation of chrysocolla. Miner. Process. Extr. M. 1975, 84, C154. [Google Scholar]
- Aplan, F.F.; Fuerstenau, D.W. The flotation of chrysocolla by mercaptan. Int. J. Miner. Process. 1984, 13, 105–115. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrera-Urbina, R.; McGlashan, D.W. Studies on the applicability of chelating agents as universal collectors for copper minerals. Int. J. Miner. Process. 2000, 58, 15–33. [Google Scholar] [CrossRef]
- Hope, G.A.; Numprasanthai, A.; Buckley, A.N.; Parker, G.K.; Sheldon, G. Bench-scale flotation of chrysocolla with n-octanohydroxamate. Miner. Eng. 2012, 36–38, 12–20. [Google Scholar] [CrossRef]
- Hope, G.A.; Buckley, A.N.; Parker, G.K.; Numprasanthai, A.; Woods, R.; McLean, J. The interaction of n-octanohydroxamate with chrysocolla and oxide copper surfaces. Miner. Eng. 2012, 36–38, 2–11. [Google Scholar] [CrossRef]
- Castro, S.; Soto, H.; Goldfarb, J.; Laskowski, J. Sulfidizing reactions in the flotation of oxidized copper minerals, II. Role of the adsorption and oxidation of sodium sulphide in the flotation of chrysocolla and malachite. Int. J. Miner. Process. 1974, 1, 151–161. [Google Scholar] [CrossRef]
- Castro, S.; Gaytan, H.; Goldfarb, J. The stabilizing effect of Na2S on the collector coating of chrysocolla. Int. J. Miner. Process. 1976, 3, 71–82. [Google Scholar] [CrossRef]
- Parks, G.A.; Kovacs, C. Thermal activation of chrysocolla for xanthate flotation. Trans. Soc. Min. Eng. 1966, 235, 349–354. [Google Scholar]
- Gonzalez, G.; Soto, H. The effect of thermal treatment on the flotation of chrysocolla. Int. J. Miner. Process. 1978, 5, 153–162. [Google Scholar] [CrossRef]
- Peng, W.S.; Liu, G.K. Infrared Spectrum of Minerals; Science Press: Beijing, China, 1982. [Google Scholar]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M.; Cao, Q.B. Copper sulfide species formed on malachite surfaces in relation to flotation. Ind. Eng. Chem. 2017, 48, 125–132. [Google Scholar] [CrossRef]
- Feng, Q.C.; Zhao, W.J.; Wen, S.M. Surface modification of malachite with ethanediamine and its effect on sulfidization flotation. Appl. Surf. Sci. 2018, 436, 823–831. [Google Scholar] [CrossRef]
- Xu, X.J. The Theory of Oxidized Mineral Flotation Using Organic Chelating Agents as Activators; Yunnan Science & Technology Press: Kunming, China, 2000. [Google Scholar]
- Chen, X.M.; Peng, Y.J.; Bradshaw, D. The separation of chalcopyrite and chalcocite from pyrite in cleaner flotation after regrinding. Int. J. Miner. Process. 2014, 58, 64–72. [Google Scholar] [CrossRef]
- Li, F.X.; Zhong, H.; Xu, H.F.; Jia, H.; Liu, G.Y. Flotation behavior and adsorption mechanism of a-hydroxyoctyl phosphinic acid to malachite. Miner. Eng. 2015, 71, 188–193. [Google Scholar] [CrossRef]
- Kartio, I.J.; Basilio, C.I.; Yoon, R.H. An XPS study of sphalerite activation by copper. Langmuir 1998, 14, 5274–5278. [Google Scholar] [CrossRef]
- Kartio, I.; Laajalehto, K.; Suoninen, E.; Karthe, S.; Szargan, R. Technique for XPS measurements of volatile adsorbed layers: Application to studies of sulphide flotation. Surf. Interface Anal. 1992, 18, 807–810. [Google Scholar] [CrossRef]
- Skinner, W.M.; Prestidge, C.A.; Smart, R.S.C. Irradiation effects during XPS studies of Cu (II) activation of zinc sulphide. Surf. Interface Anal. 1996, 24, 620–626. [Google Scholar] [CrossRef]
- Smart, R.S.C.; Skinner, W.M.; Gerson, A.R. XPS of sulphide mineral surfaces: Metal-deficient, polysulphides, defects and elemental sulphur. Surf. Interface Anal. 1999, 28, 101–105. [Google Scholar] [CrossRef]
- Buckley, A.; Woods, R. An X-ray photoelectron spectroscopic study of the oxidation of chalcopyrite. Aust. J. Chem. 1984, 37, 2403–2413. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Deng, J.S.; Zhao, W.J. Combined DFT and XPS investigation of enhanced adsorption of sulfide species onto cerussite by surface modification with chloride. Appl. Surf. Sci. 2017, 425, 8–15. [Google Scholar] [CrossRef]
- Xie, G.Y.; Zhang, M.X.; Bian, B.X.; Fan, M.Q. Xuankuangxue; China University of Mining and Technology Press: Xuzhou, China, 2012. [Google Scholar]
- Gonzalez, G.; Laskowski, J. The point of zero charge of oxidized copper minerals: Tenorite, malachite and chrysocolla. Electroanal. Chem. 1974, 53, 452–456. [Google Scholar] [CrossRef]




| Element/Oxide | Cu | Fe | Mn | Al2O3 | MgO | CaO | SiO2 |
|---|---|---|---|---|---|---|---|
| wt (%) | 31.02 | 0.25 | <0.005 | 5.77 | 0.17 | 0.25 | 40.57 |
| Minerals | Pore Size (nm) | Pore Volume (cm3/g) | Specific Surface Area (m2/g) | Particle Size (μm) |
|---|---|---|---|---|
| Chrysocolla | 2.26 | 0.1751 | 242.51 | 45–74 |
| Malachite | – | – | 0.363 | 45–74 |
| Element | Atomic Concentration of Cu, S, and N Species (%) | |||
|---|---|---|---|---|
| a | b | c | d | |
| Cu(II) | 7.05 | 5.62 | 7.52 | 6.16 |
| Cu(I) | 1.26 | 4.15 | 1.43 | 7.28 |
| S | 0.00 | 6.31 | 0.00 | 8.65 |
| N | – | 1.65 | – | 2.46 |
| Conditions | Colloid | Cu Ions Concentrations | S Ions Concentrations |
|---|---|---|---|
| Chrysocolla + aqueous solution | – | 26.43 | – |
| Chrysocolla + 2 × 10−3 M EDP | 0.00 | 75.38 | – |
| Chrysocolla + 4 × 10−3 M Na2S | 93.33 | 0.54 | 25.42 |
| Chrysocolla + 2 × 10−3 M EDP + 4 × 10−3 M Na2S | <0.000 | 0.38 | 9.63 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, P.; Liu, D.; Xu, X.; Jia, X.; Zhang, X.; Liu, D.; Liu, R. Effect of Ethylene Diamine Phosphate on the Sulfidization Flotation of Chrysocolla. Minerals 2018, 8, 216. https://doi.org/10.3390/min8050216
Shen P, Liu D, Xu X, Jia X, Zhang X, Liu D, Liu R. Effect of Ethylene Diamine Phosphate on the Sulfidization Flotation of Chrysocolla. Minerals. 2018; 8(5):216. https://doi.org/10.3390/min8050216
Chicago/Turabian StyleShen, Peilun, Dianwen Liu, Xiaohui Xu, Xiaodong Jia, Xiaolin Zhang, Dan Liu, and Ruizeng Liu. 2018. "Effect of Ethylene Diamine Phosphate on the Sulfidization Flotation of Chrysocolla" Minerals 8, no. 5: 216. https://doi.org/10.3390/min8050216




