An Insight into Flotation Chemistry of Pyrite with Isomeric Xanthates: A Combined Experimental and Computational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Adsorption Procedure
2.2.2. Surface Tension Testing
2.3. Theory Calculations
3. Results and Discussion
3.1. Flotation Solution Chemistry between Pyrite and Isomeric Xanthates
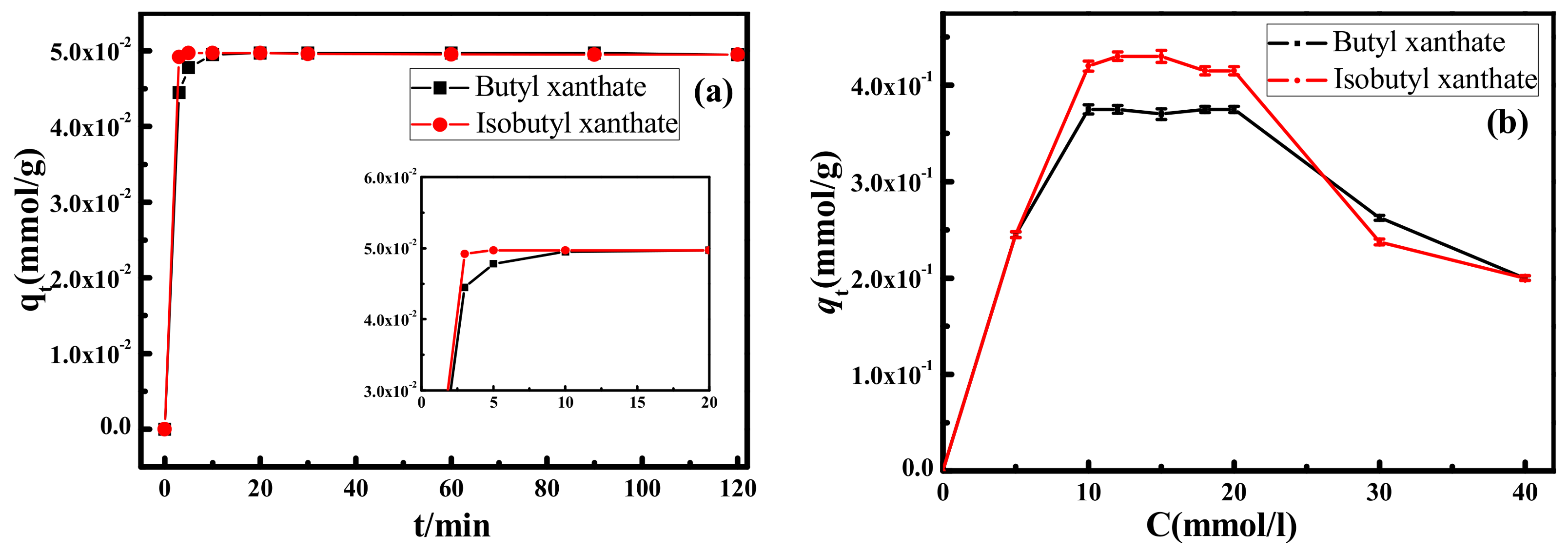
3.1.1. Adsorption of Isomeric Xanthates onto Pyrite Surface
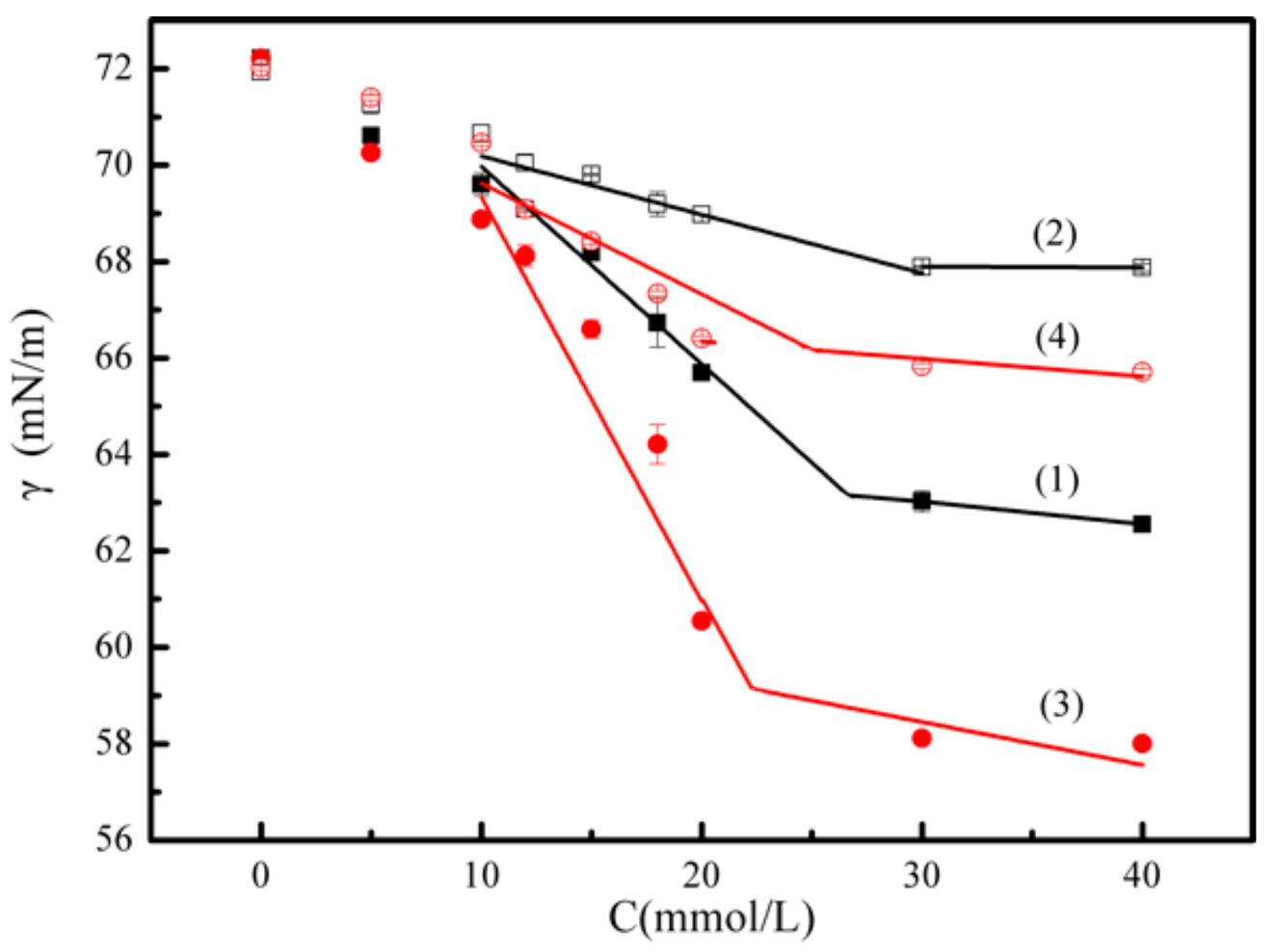
3.1.2. Surface Activity of Isomeric Xanthates in Flotation Chemistry
3.2. MD Simulations between Pyrite and Isomeric Xanthates
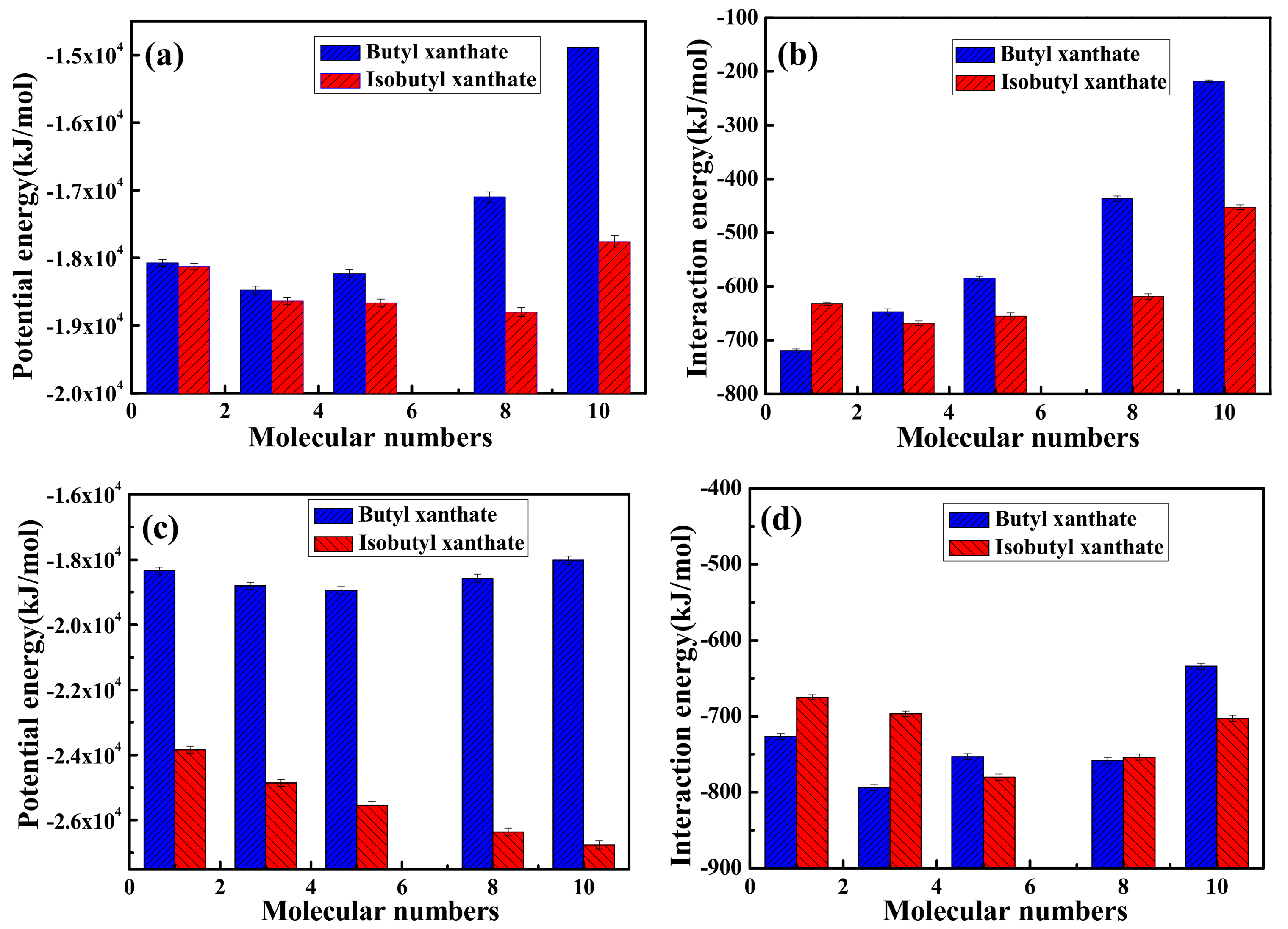
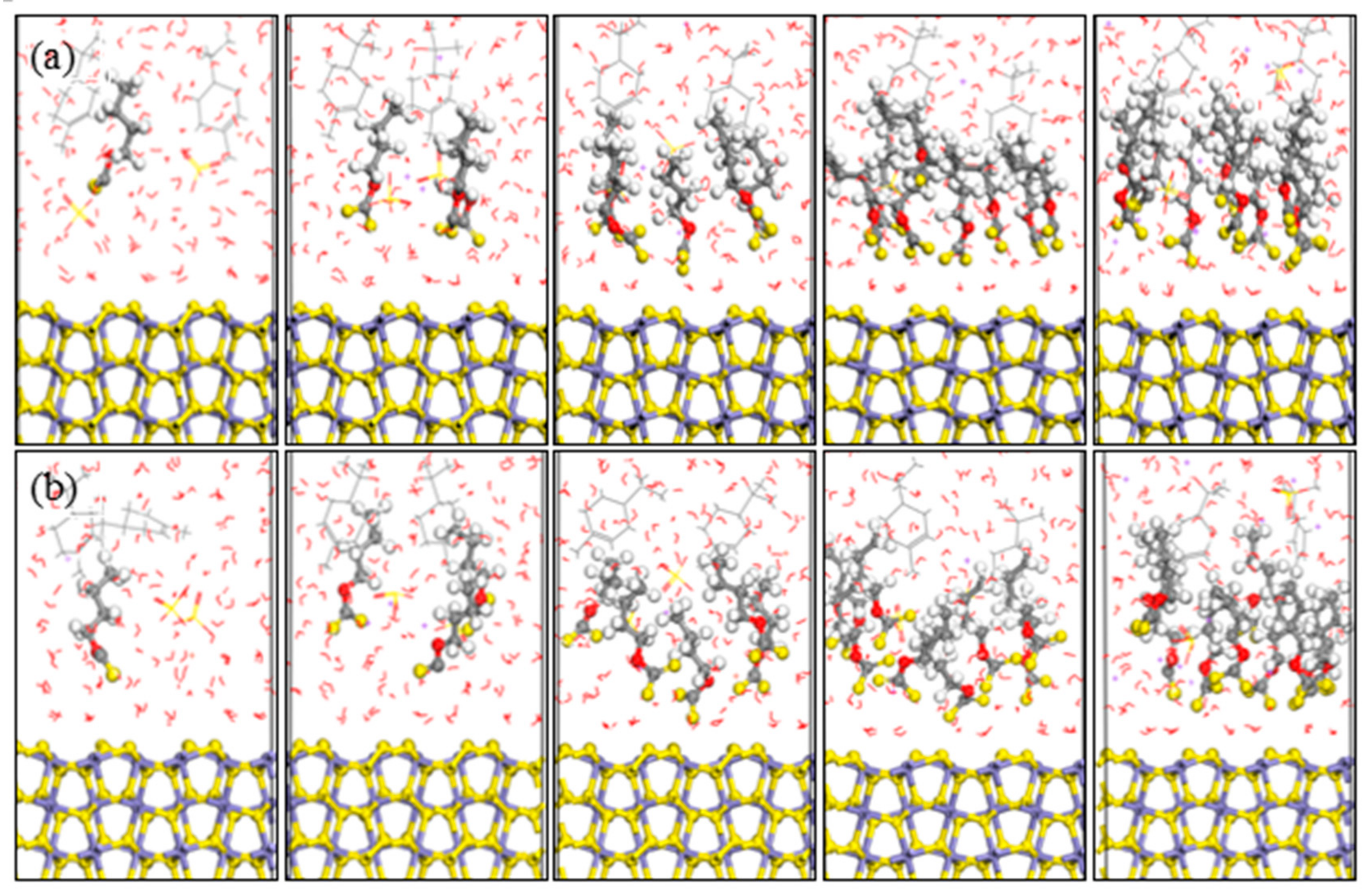
3.2.1. Interactions between Pyrite and Isomeric Xanthates without Frother and Activator
3.2.2. Interactions between Pyrite and Isomeric Xanthates in the Presence of Frother and Activator
3.3. Quantum Chemical Calculations between Pyrite and Isomeric Xanthates
4. Conclusions
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- He, B.Q.; Luo, L. Discussion on the new de-sulfuration method of Chinese high sulfur bauxite. Light Metal 1996, 12, 3–5. [Google Scholar]
- Hu, X.L.; Chen, W.M.; Xie, Q.L. Sulfur phase and sulfur removal in high sulfur-containing bauxite. Trans. Nonferr. Met. Soc. 2011, 21, 1641–1647. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Ma, W.; Yin, Z.; Wu, G. Conversion of sulfur by wet oxidation in the bayer process. Metall. Mater. Trans. B 2015, 46, 1702–1708. [Google Scholar] [CrossRef]
- Yin, J.; Xia, W.; Han, M. Resource utilization of high-sulfur bauxite of low-median grade in Chongqing China. Light Metal 2011, 49, 19–22. [Google Scholar]
- Chen, W.M.; Xie, Q.L.; Hu, X.L. Experimental study on reverse flotation technique for desulfurizing of high-sulfur bauxite. Min. Metall. Eng. 2008, 28, 34–37. [Google Scholar]
- Padilla, R.; Vega, D.; Ruiz, M.C. Pressure leaching of sulfidized chalcopyrite in sulfuric acid-oxygen media. Hydrometallurgy 2007, 86, 80–88. [Google Scholar] [CrossRef]
- Long, X.; Chen, J.; Chen, Y. Adsorption of ethyl xanthate on ZnS(110) surface in the presence of water molecules: A DFT study. Appl. Surf. Sci. 2016, 370, 11–18. [Google Scholar] [CrossRef]
- Padmanabhan, N.P.H.; Sreenivas, T.; Rao, N.K. Processing of ores of titanium, zirconium, hafnium, niobium, tantalum, molybdenum, rhenium, and tungsten: International trends and the Indian scene. High Temp. Mat. Process. 1990, 9, 217–248. [Google Scholar] [CrossRef]
- Wang, X.H.; Forssberg, K.S.E. Mechanisms of pyrite flotation with xanthates. Int. J. Miner. Process. 1991, 33, 275–290. [Google Scholar] [CrossRef]
- Buckley, A. A survey of the application of X-ray photoelectron spectroscopy to flotation research. Colloid Surf. A 1994, 93, 159–172. [Google Scholar] [CrossRef]
- Nagaraj, D.R.; Brinen, J.S. Sims study of adsorption of collectors on pyrite. Int. J. Miner. Process. 2001, 63, 45–57. [Google Scholar] [CrossRef]
- Cao, F.; Sun, C.Y.; Wang, H.J.; Chen, F.W. Effect of alkyl structure on the flotation performance of xanthate collectors. J. Univ. Sci. Technol. B 2014, 36, 1589–1594. [Google Scholar]
- Wang, X.M.; Zhang, T.A.; Lv, G.Z.; Dou, Z.H.; Li, B.; Jiang, X.L. Application comparison of xanthate flotation collectors in high-sulfur bauxite flotation desulfurization. Chin. J. Process. Eng. 2010, 10, 7–12. [Google Scholar]
- Chen, S.H.; Gong, W.Q.; Mei, G.J.; Chen, X.D.; Yan, H.Z. Evaluation of biodegradability of alkyl xanthates flotation collecters. J. Cent. South Univ. 2011, 42, 546–554. [Google Scholar]
- Xu, Y.; Liu, Y.L.; Gao, S.; Jiang, Z.W.; Su, D.; Liu, G.S. Monolayer adsorption of dodecylamine surfactants at the mica/water interface. Chem. Eng. Sci. 2014, 114, 58–69. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Aschauer, U.; Bowen, P. Interaction of biologically relevant ions and organic molecules with titanium oxide (rutile) surfaces: A review on molecular dynamics studies. Colloid Surf. B 2017, 161, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Erastova, V.; Lu, S.; Greenwell, H.C.; Underwood, T.; Xue, H.; Zeng, F.; Chen, G.; Wu, C.; Zhao, R. Understanding model crude oil component interactions on kaolinite silicate and aluminol surfaces: Towards improved understanding of shale oil recovery. Energy Fuels 2017, 32, 1155–1165. [Google Scholar] [CrossRef]
- Nikjoo, H.; O’Neill, P.; Wilson, W.E.; Goodhead, D.T. Computational approach for determining the spectrum of DNA damage induced by ionizing radiation. Radiat. Res. 2001, 156, 577–583. [Google Scholar] [CrossRef]
- Wang, L.; Hu, Y.; Sun, W.; Sun, Y. Molecular dynamics simulation study of the interaction of mixed cationic/anionic surfactants with muscovite. Appl. Surf. Sci. 2015, 327, 364–370. [Google Scholar] [CrossRef]
- Montalti, M.; Fornasiero, D.; Ralston, J. Ultraviolet-visible spectroscopic study of the kinetics of adsorption of ethyl xanthate on pyrite. J. Colloid Interface Sci. 1991, 143, 440–450. [Google Scholar] [CrossRef]
- Tariq, M.; Freire, M.G.; Saramago, B.; Coutinho, J.A.; Lopes, J.N.; Rebelo, L.P. Surface tension of ionic liquids and ionic liquid solutions. Chem. Soc. Rev. 2012, 41, 829–868. [Google Scholar] [CrossRef] [PubMed]
- And, G.L.; Watson, P.R. Surface tension measurements of N-alkylimidazolium ionic liquids. Langmuir 2001, 17, 6138–6141. [Google Scholar]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloid Surf. A 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Dong, B.; Li, N.; Zheng, L.; Yu, L.; Inoue, T. Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 2007, 23, 4178–4182. [Google Scholar] [CrossRef] [PubMed]
- Rappe, A.K.; Casewit, C.J.; Colwell, K.S.; Iii, W.A.G.; Skiff, W.M. Uff, a full periodic table force field for molecular mechanics and molecular dynamics simulations. J. Am. Chem. Soc. 1992, 114, 10024–10035. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, J.; Long, X.; Guo, J. Study of H2O adsorption on sulfides surfaces and thermokinetic analysis. J. Ind. Eng. Chem. 2014, 20, 605–609. [Google Scholar] [CrossRef]
- Underwood, T.; Erastova, V.; Greenwell, H.C. Wetting effects and molecular adsorption at hydrated kaolinite clay mineral surfaces. J. Phys. Chem. C 2016, 120, 11433–11449. [Google Scholar] [CrossRef]
- Nosé, S. A unified formulation of the constant temperature molecular dynamics methods. J. Chem. Phys. 1984, 81, 511–519. [Google Scholar] [CrossRef]
- Karasawa, N.; Iii, W.A.G. Acceleration of convergence for lattice sums. J. Chem. Phys. 1989, 93, 7320–7327. [Google Scholar] [CrossRef]
- Williams, D.E. Accelerated convergence of crystal-lattice potential sums. Acta Crystollogr. E 1971, 27, 452–455. [Google Scholar] [CrossRef]
- Pradip; Rai, B.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Dong, F.; Gao, Z.; Wu, H.; Wang, Z. Anisotropic adsorption of oleate on diaspore and kaolinite crystals: Implications for their flotation separation. Appl. Surf. Sci. 2014, 321, 331–338. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Yin, L. The effect of 5-nitroindazole as an inhibitor for the corrosion of copper in a 3.0% NaCl solution. Rsc Adv. 2015, 5, 63866–63873. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533. [Google Scholar] [CrossRef] [PubMed]
- Somasundaran, P.; Wang, D. Chapter 5 application of flotation agents and their structure-property relationships. Dev. Miner. Process. 2006, 17, 143–201. [Google Scholar]
- Liu, G.; Yang, X.; Zhong, H. Molecular design of flotation collectors: A recent progress. Adv. Colloid Interface Sci. 2017, 246, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Rueda, J.A.; Ruffini, R.; Wu, Y.B.; Xue, S.S. Surface tension of the core-crust interface of neutron stars with global charge neutrality. Phys. Rev. C 2014, 89, 196–204. [Google Scholar] [CrossRef]
- Monte, M.B.M.; Lins, F.F.; Oliveira, J.F. Selective flotation of gold from pyrite under oxidizing conditions. Int. J. Miner. Process. 1997, 51, 255–267. [Google Scholar] [CrossRef]
- Aristilde, L.; Sposito, G. Molecular modeling of metal complexation by a fluoroquinolone antibiotic. Environ. Toxicol. Chem. 2008, 27, 2304–2310. [Google Scholar] [CrossRef] [PubMed]
- Rosso, K.M.; Becker, U.; Hochella, M.F. Atomically resolved electronic structure of pyrite {100} surfaces; an experimental and theoretical investigation with implications for reactivity. Am. Mineral. 1999, 84, 1535–1548. [Google Scholar] [CrossRef]
- Dewar, M.J.S. A critique of frontier orbital theory. J. Mol. Struc. Theochem. 1989, 200, 301–323. [Google Scholar] [CrossRef]
- Li, Y.Q.; Chen, J.H.; Chen, Y.; Guo, J. Density functional theory study of influence of impurity on electronic properties and reactivity of pyrite. Trans. Nonferr. Met. Soc. China 2011, 21, 1887–1895. [Google Scholar] [CrossRef]
- Guo, L.; Zhu, S.; Zhang, S.; He, Q.; Li, W. Theoretical studies of three triazole derivatives as corrosion inhibitors for mild steel in acidic medium. Corros. Sci. 2014, 87, 366–375. [Google Scholar] [CrossRef]
- Khaled, K.F.; Hamed, M.N.H.; Abdel-Azim, K.M.; Abdelshafi, N.S. Inhibition of copper corrosion in 3.5% nacl solutions by a new pyrimidine derivative: Electrochemical and computer simulation techniques. J. Solid State Electrochem. 2011, 15, 663–673. [Google Scholar] [CrossRef]





| Xanthate | CMC (mmol/L) | γcmc (mN/m) | Πcmc (mN/m) | Γmax (μmol/m2) | Amin (Å2) | |
|---|---|---|---|---|---|---|
| After adsorption | Butyl xanthate | 29.7 ± 0.2 | 67.9 ± 0.1 | 4.15 ± 0.1 | 48.9 ± 0.57 | 3.40 ± 0.29 |
| Isobutyl xanthate | 25.3 ± 0.2 | 66.1 ± 0.1 | 5.95 ± 0.1 | 92.8 ± 2.6 | 1.79 ± 0.064 | |
| Before adsorption | Butyl xanthate | 26.5 ± 0.2 | 63.1 ± 0.1 | 8.86 ± 0.1 | 165.2 ± 2.3 | 1.01 ± 0.072 |
| Isobutyl xanthate | 22.2 ± 0.2 | 59.2 ± 0.1 | 10.9 ± 0.1 | 338.2 ± 6.6 | 0.491 ± 0.025 | |
| Adsorbate | Butyl Xanthate Ion | Isobutyl Xanthate Ion | H2O |
|---|---|---|---|
| Adsorption energy | −100.79 ± 2.6 | −104.76 ± 2.5 | −26.45 ± 1.2 |
| Ensemble | Numbers of Xanthates | 1 | 3 | 5 | 8 | 10 |
|---|---|---|---|---|---|---|
| NVT | Butyl xanthate | −2837.43 ± 92.93 | −3605.57 ± 109.18 | −4254.63 ± 118.41 | −5065.44 ± 140.39 | −5364.72 ± 151.69 |
| Isobutyl xanthate | −2736.72 ± 88.93 | −3606.63 ± 104.61 | −4238.93 ± 105.77 | −5335.12 ± 117.29 | −5720.40 ± 154.66 | |
| NPT | Butyl xanthate | −2694.52 ± 93.42 | −3352.89 ± 97.58 | −4051.46 ± 100.27 | −4768.22 ± 105.37 | −5224.37 ± 113.88 |
| Isobutyl xanthate | −2651.09 ± 92.01 | −3328.48 ± 96.51 | −4052.44 ± 99.89 | −5126.57 ± 107.28 | −5725.45 ± 111.37 |
| Ensemble | Numbers of Xanthates | 1 | 3 | 5 | 8 | 10 |
|---|---|---|---|---|---|---|
| NVT | Butyl xanthate | −10,393.07 ± 115.01 | −12,286.21 ± 132.44 | −12,730.93 ± 168.57 | −14,779.68 ± 142.91 | −18,323.18 ± 162.99 |
| Isobutyl xanthate | −10,471.61 ± 111.32 | −12,241.89 ± 135.85 | −14,007.95 ± 141.68 | −16,591.48 ± 147.31 | −18,559.74 ± 200.99 | |
| NPT | Butyl xanthate | −10,367.21 ± 103.21 | −12,051.86 ± 103.70 | −12,559.27 ± 116.23 | −14,436.87 ± 125.32 | −18,229.68 ± 122.63 |
| Isobutyl xanthate | −10,211.36 ± 104.24 | −12,013.49 ± 103.70 | −13,941.43 ± 113.77 | −16,443.92 ± 117.75 | −18,347.99 ± 125.90 |
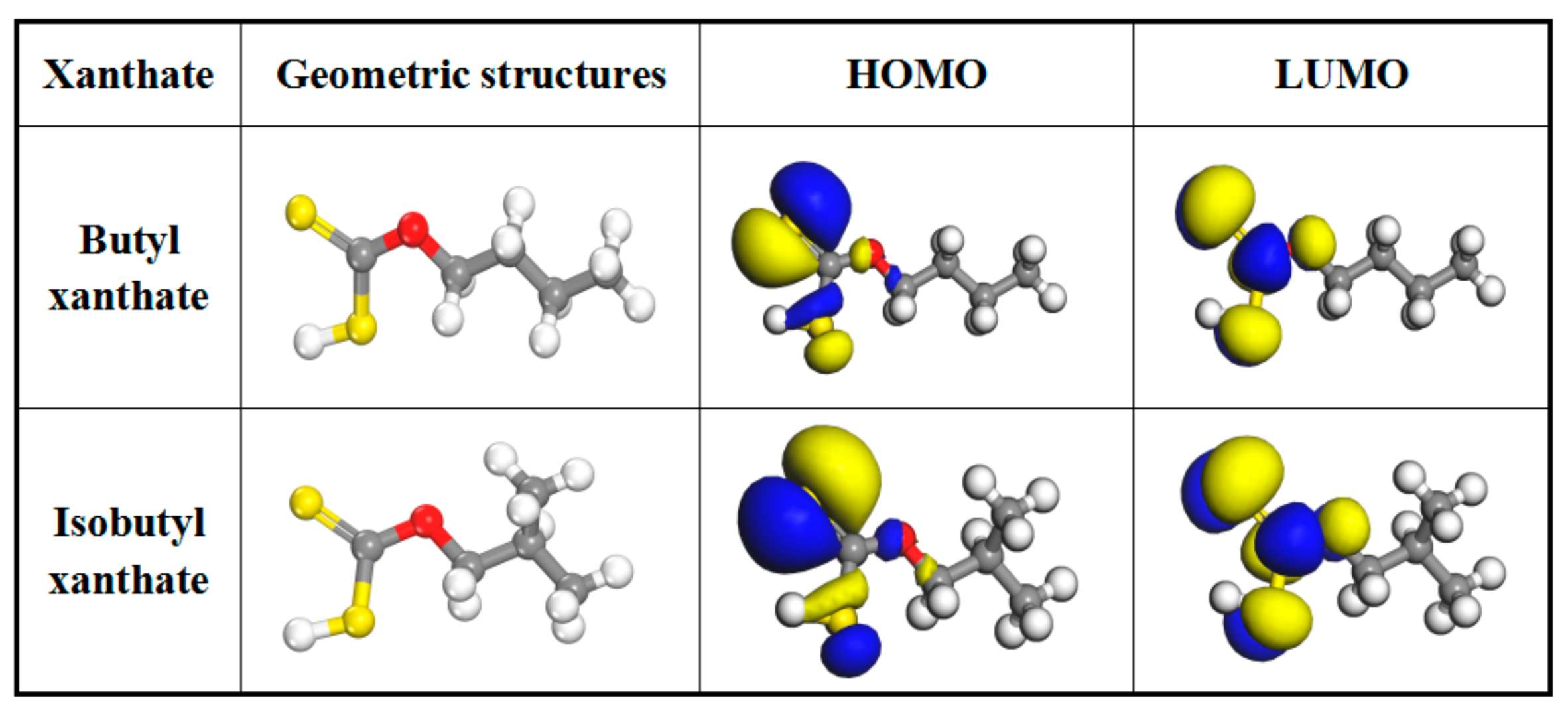
| The Energy of Molecule Orbital/eV | Energy Gap/eV | μ/Debye | |||
|---|---|---|---|---|---|
| HOMO | LUMO | ∣∆E1∣ | ∣∆E2∣ | ||
| Pyrite | −5.878 | −4.438 | - | - | - |
| Butyl xanthate | −5.214 | −2.174 | 0.776 | 3.040 | 3.845 |
| Isobutyl xanthate | −5.058 | −2.083 | 0.620 | 2.975 | 4.012 |
| Butyl xanthate-pyrite | −5.399 | −5.318 | - | 0.261 | - |
| Isobutyl xanthate-pyrite | −5.287 | −5.238 | - | 0.049 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, G.; Su, S.; Huang, Y.; Peng, W.; Cao, Y.; Liu, J. An Insight into Flotation Chemistry of Pyrite with Isomeric Xanthates: A Combined Experimental and Computational Study. Minerals 2018, 8, 166. https://doi.org/10.3390/min8040166
Han G, Su S, Huang Y, Peng W, Cao Y, Liu J. An Insight into Flotation Chemistry of Pyrite with Isomeric Xanthates: A Combined Experimental and Computational Study. Minerals. 2018; 8(4):166. https://doi.org/10.3390/min8040166
Chicago/Turabian StyleHan, Guihong, Shengpeng Su, Yanfang Huang, Weijun Peng, Yijun Cao, and Jiongtian Liu. 2018. "An Insight into Flotation Chemistry of Pyrite with Isomeric Xanthates: A Combined Experimental and Computational Study" Minerals 8, no. 4: 166. https://doi.org/10.3390/min8040166




