Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres
Abstract
:1. Introduction
2. Materials and Methods
3. Nomenclature and Systematization
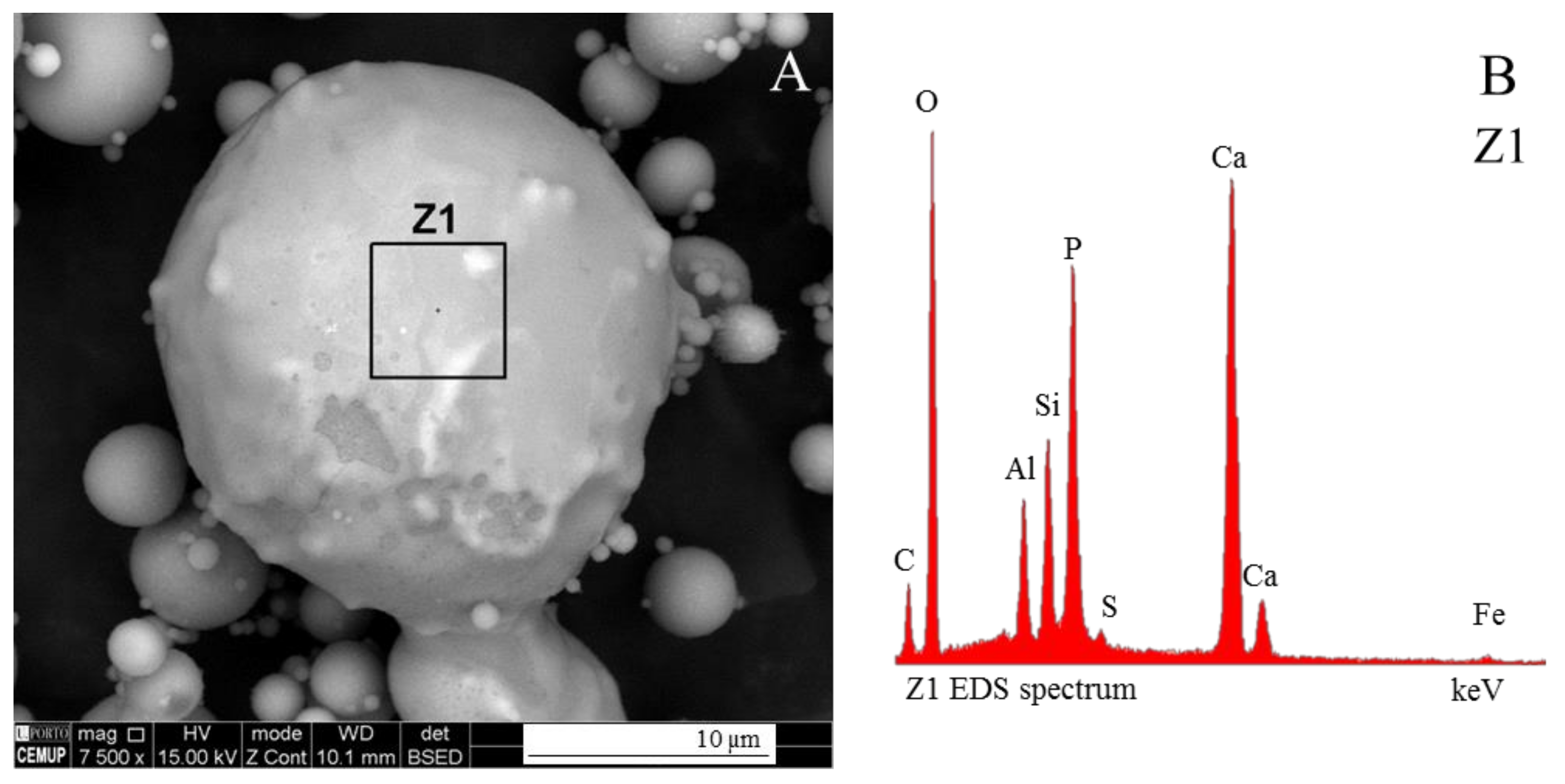
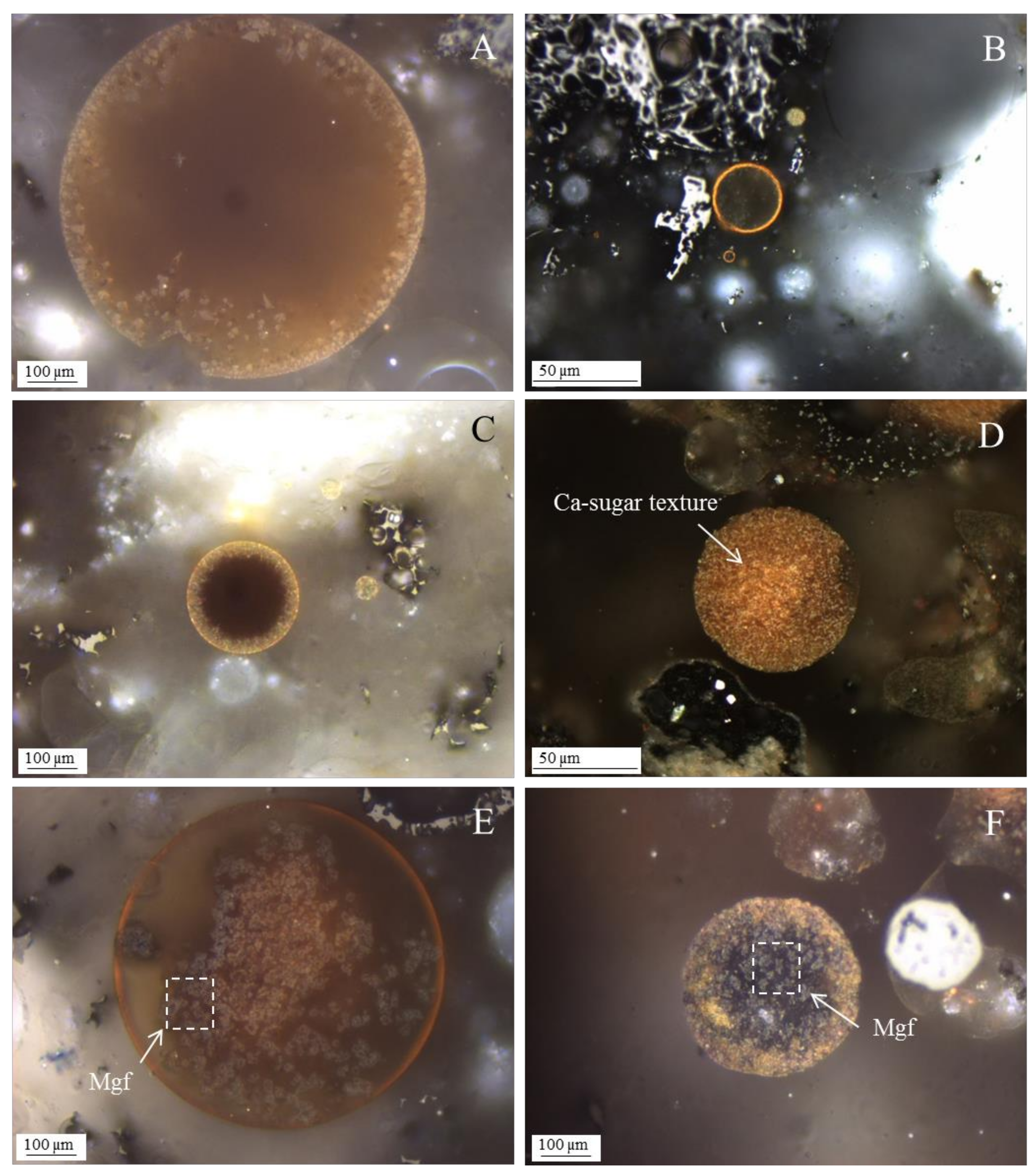
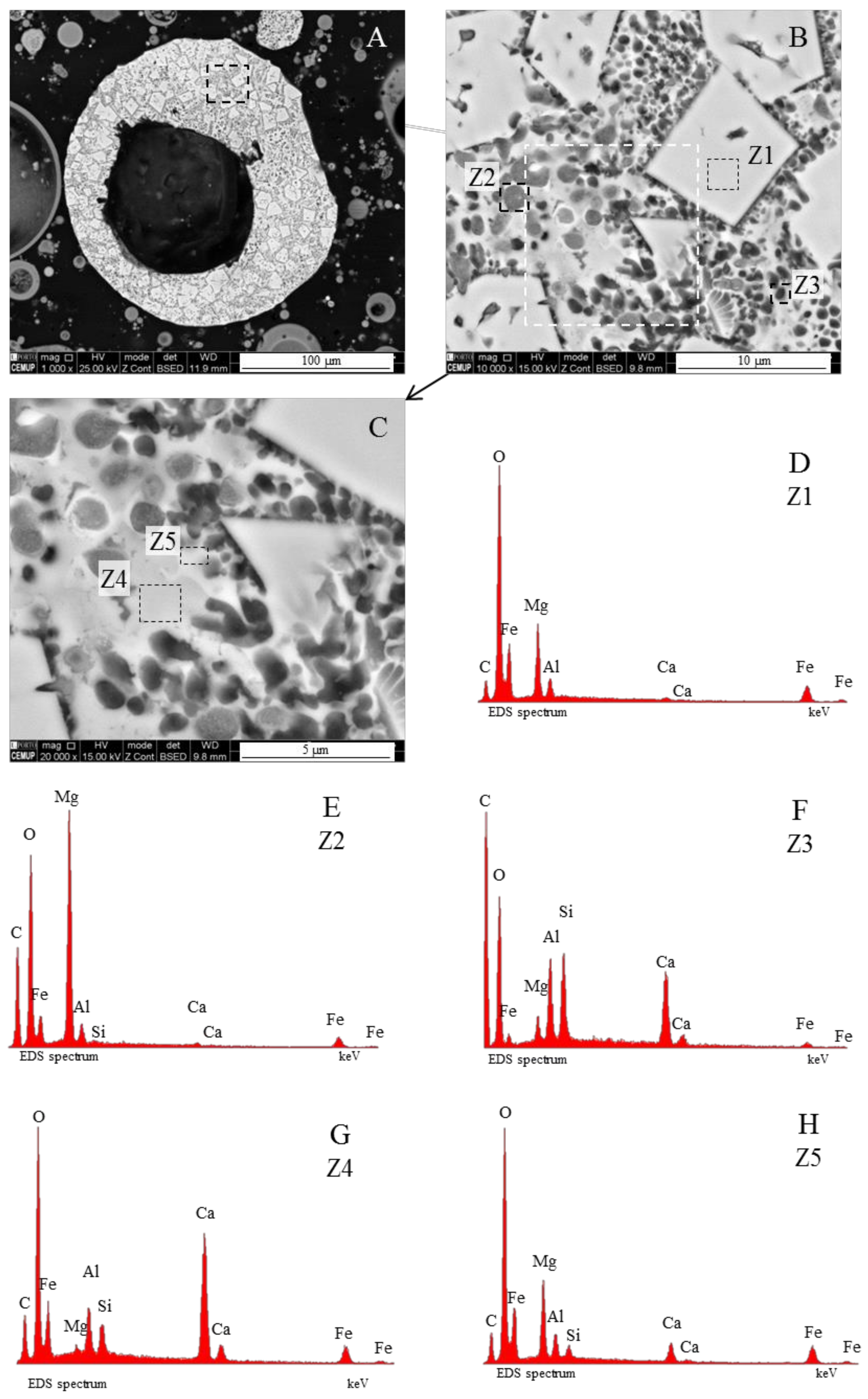
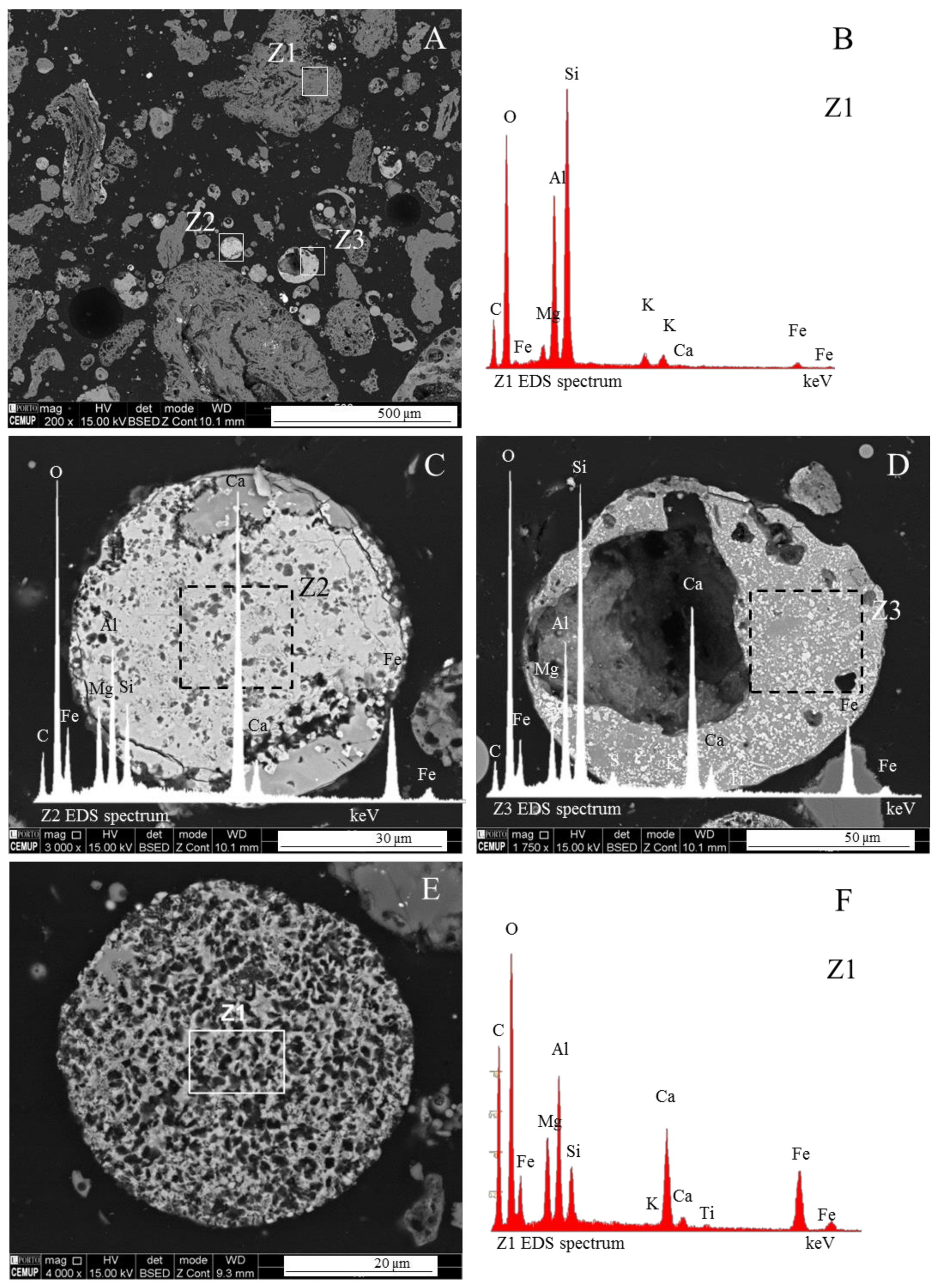
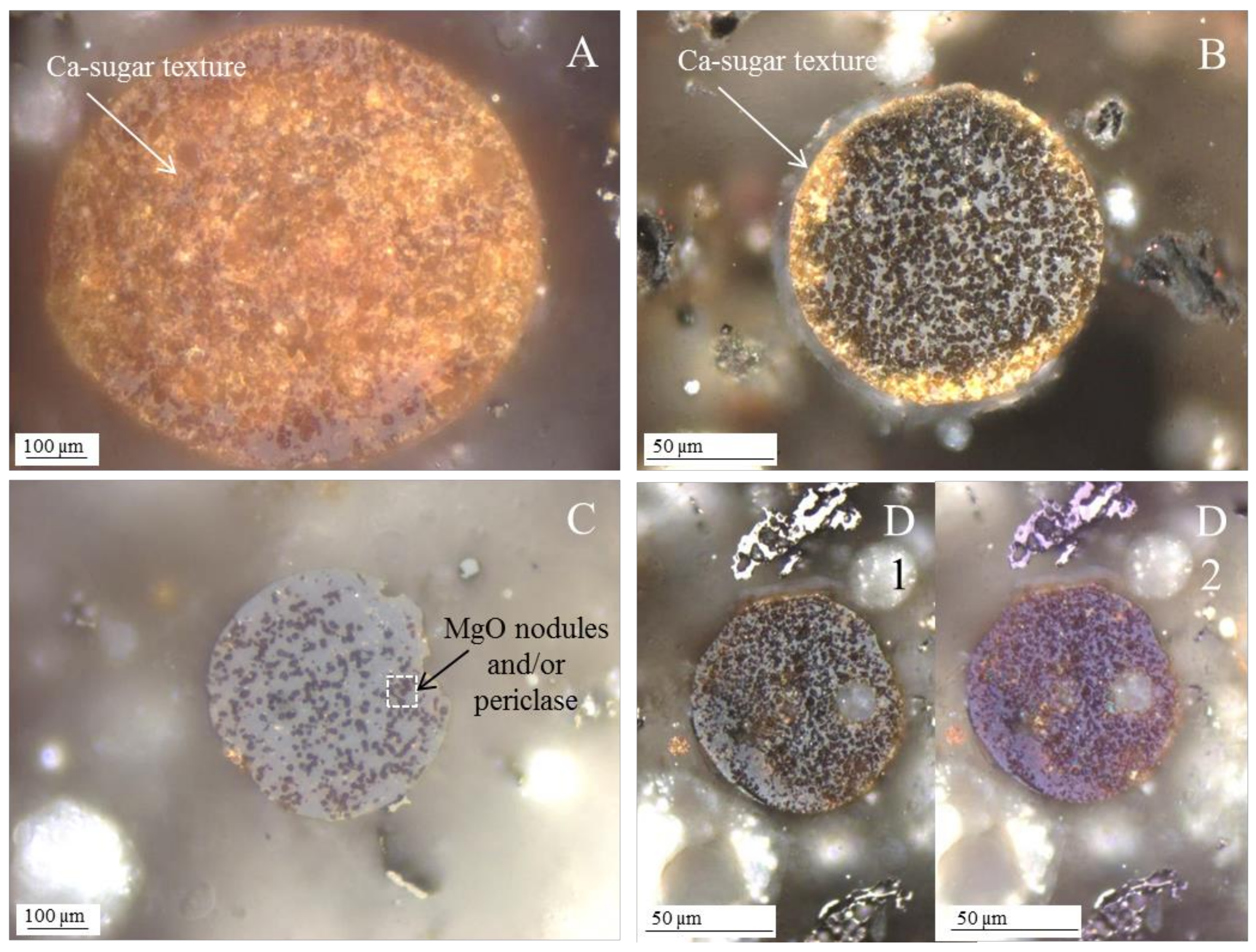
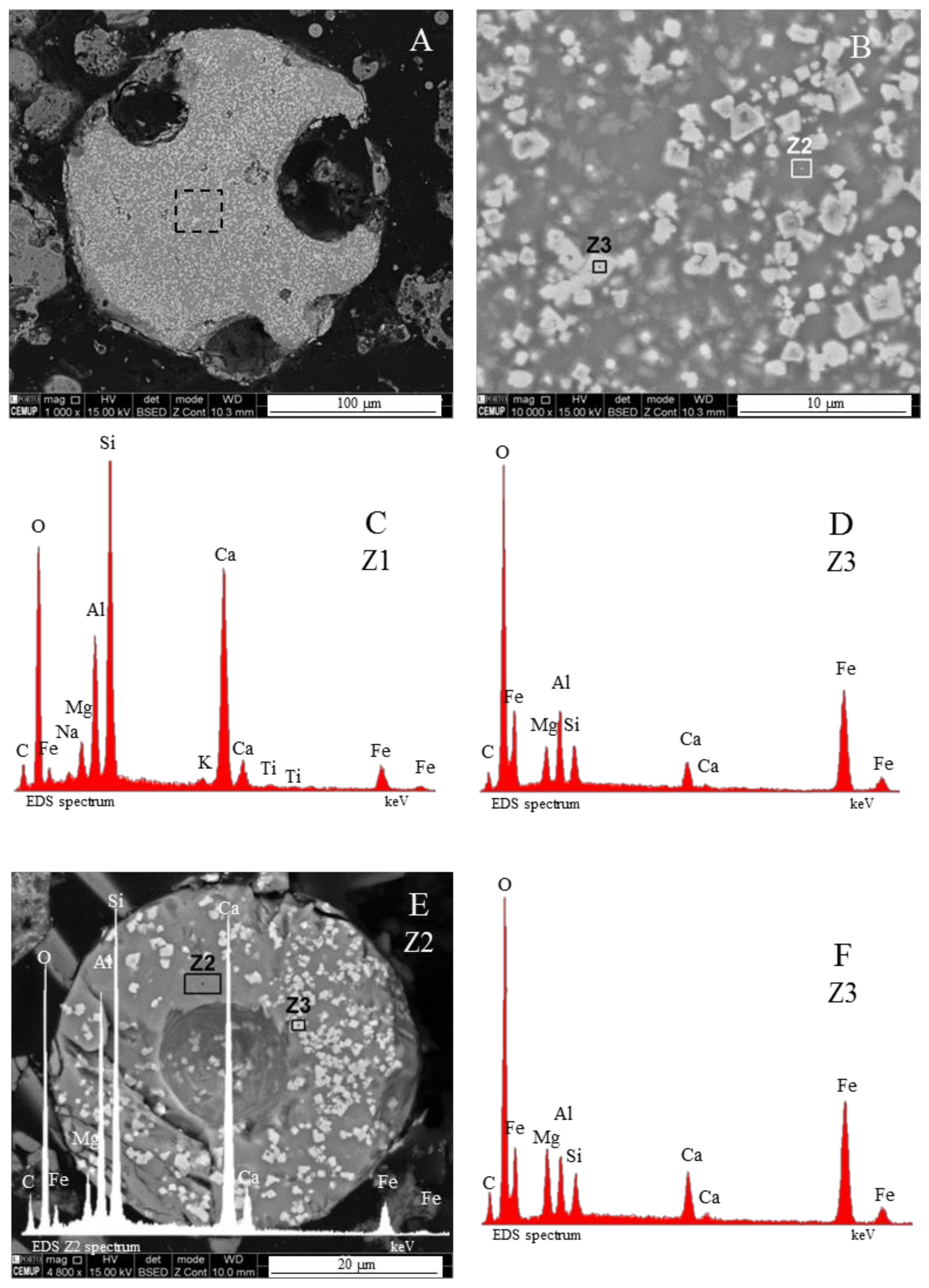
3.1. Calcispheres
3.2. Calcimagnesiaspheres
3.3. Magnesiaspheres
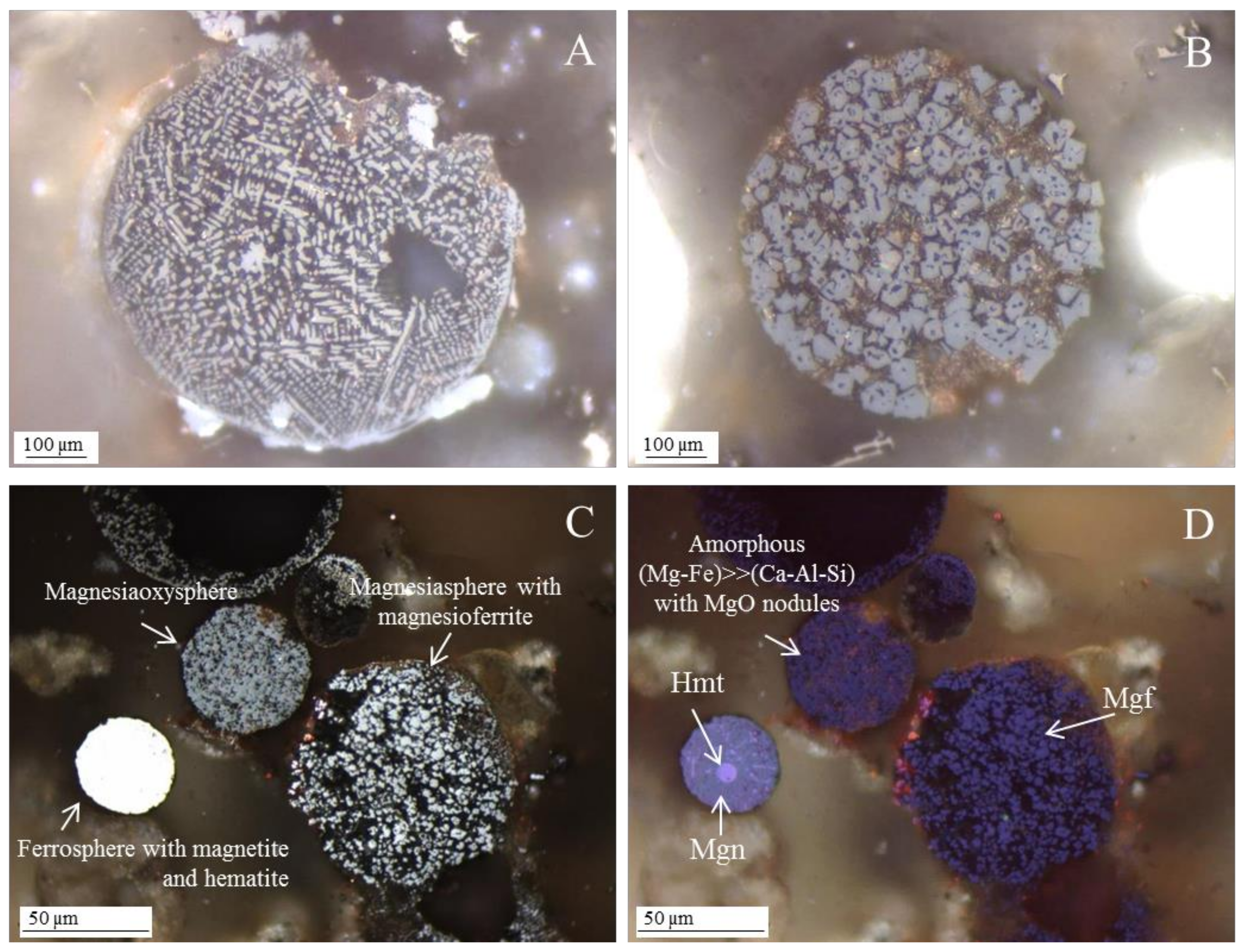
3.3.1. Magnesiapheres: Magnesiaferrospheres Type
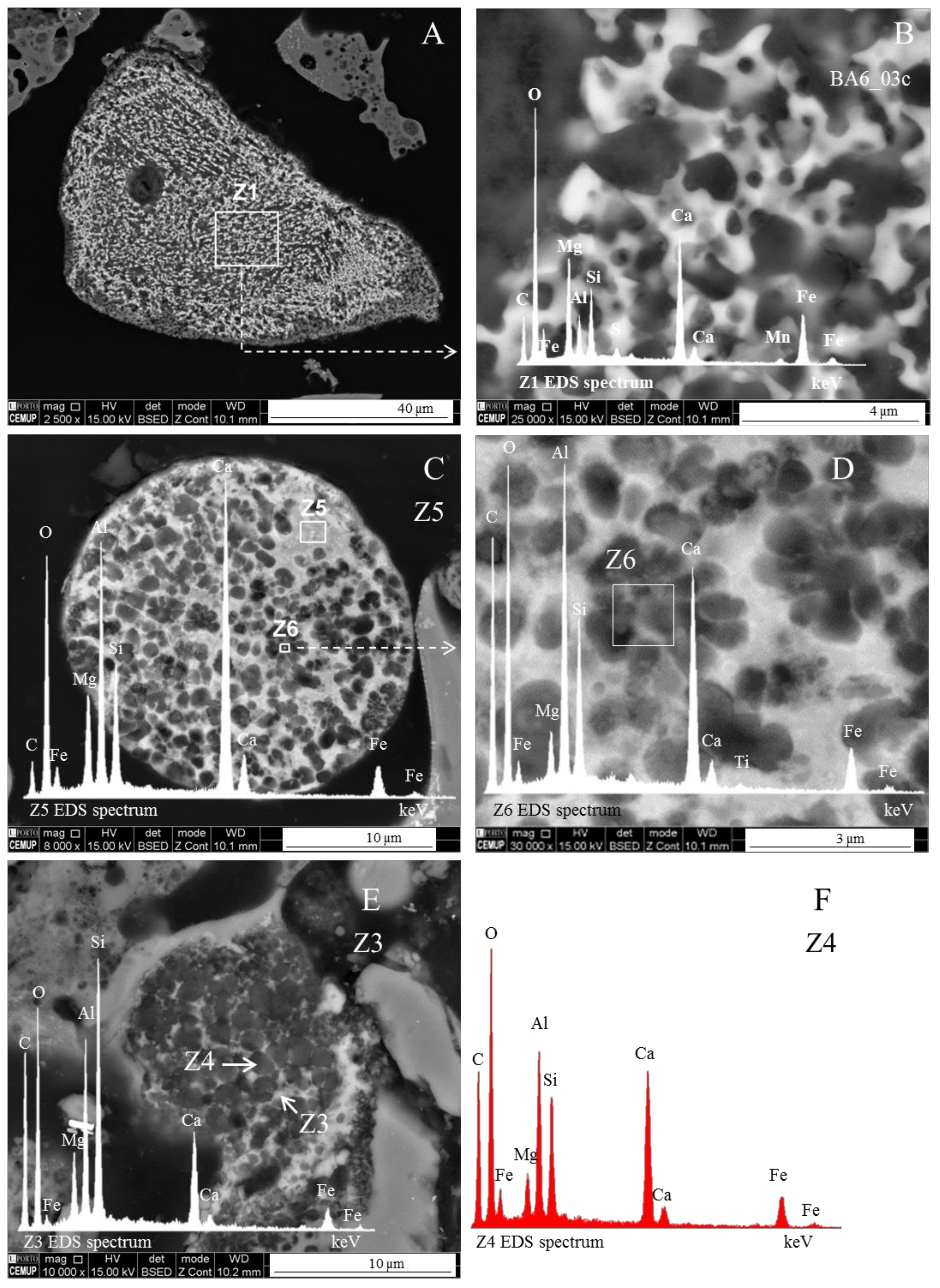
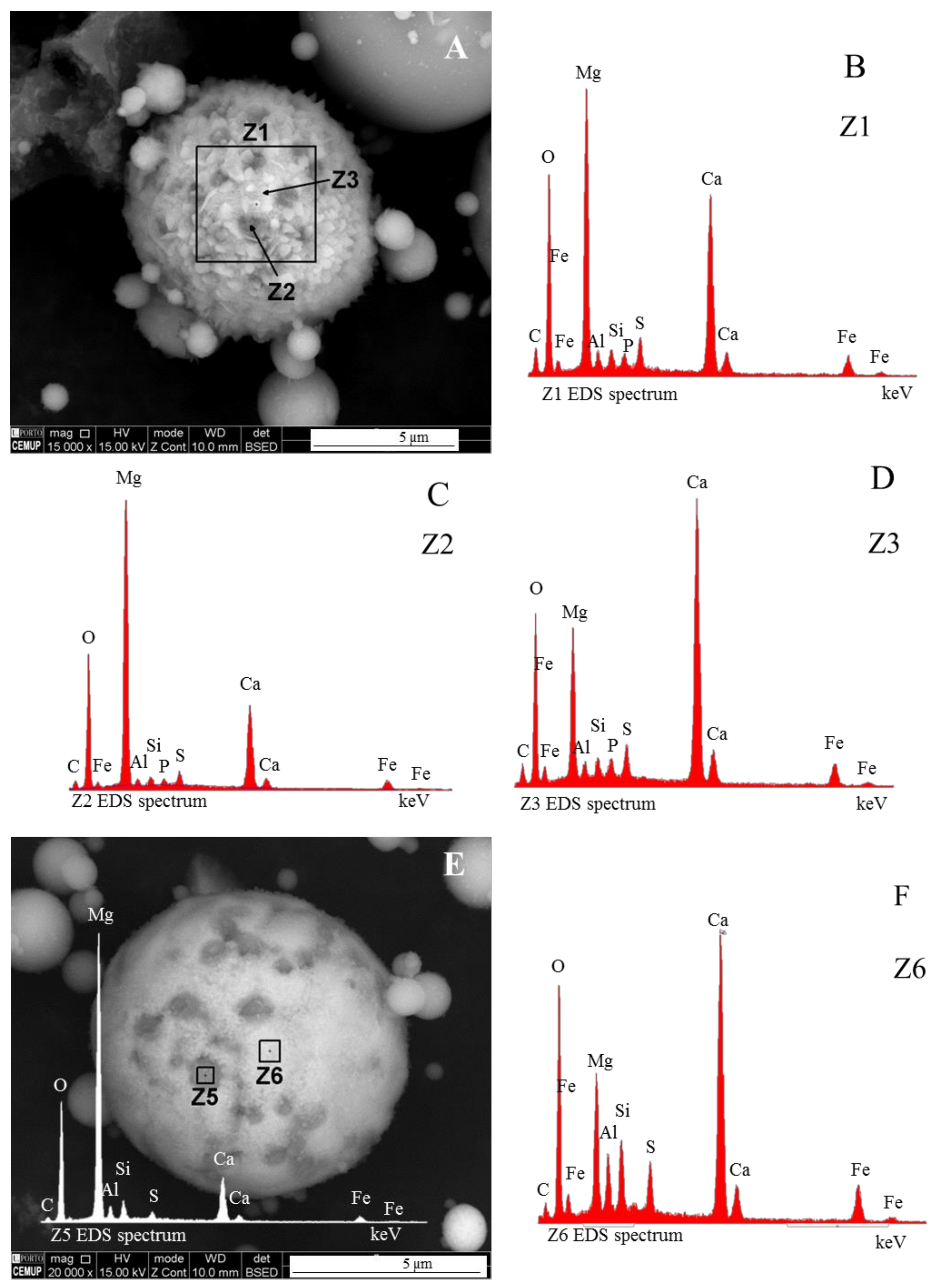
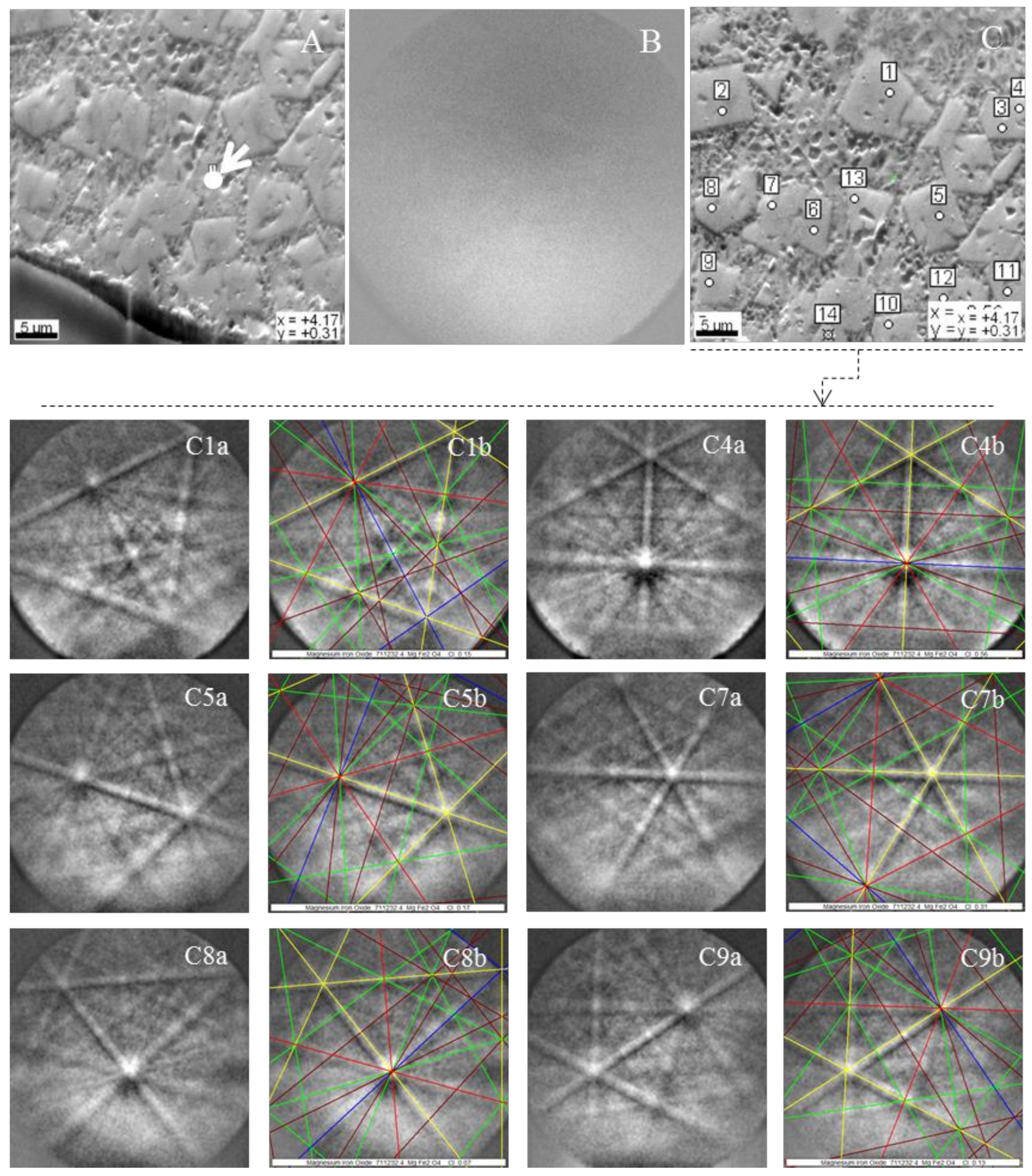
Magnesioferrite in Coal FA
- (i)
- when, in relation to magnetite and hematite, magnesioferrite octahedra are residual in ferrospheres, the morphotype is classified as “ferrosphere”. In this case, Anshits et al. [44] considered that magnesioferrites are impurities occurring on the ferrosphere. Nevertheless, “ferromagnesiasphere” may be used to designate this ferrosphere variety.
- (ii)
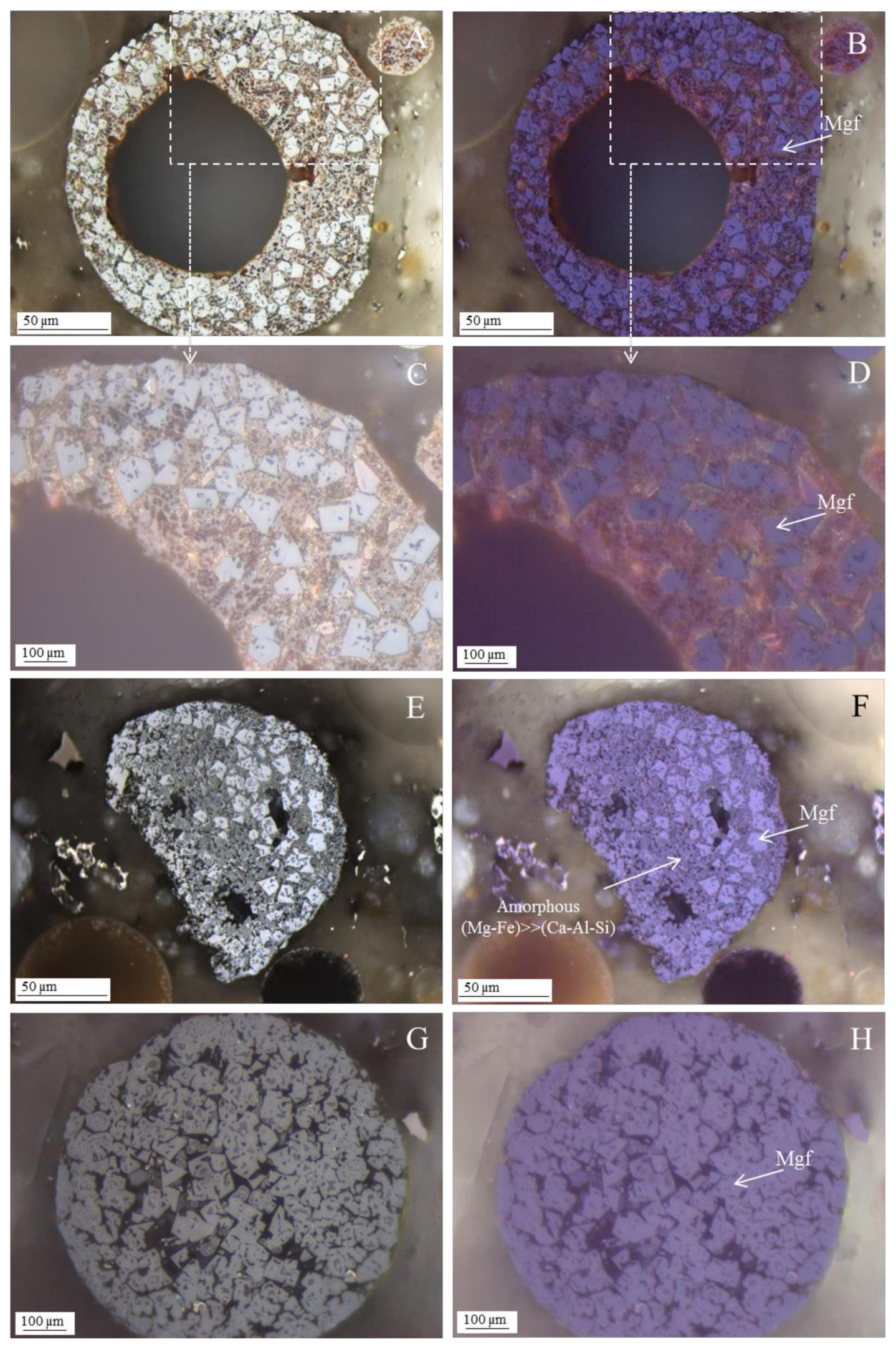
- The morphotypes should be classified in the “magnesiaspheres” group and designated as “magnesiaferrospheres” when euhedral magnesioferrite (MgFe2O4) crystals are the only distinctive pattern, and magnetite dendrites and a Ca spongy structure are residual or absent. In the EDS spectrum from the magnesioferrite crystals, Mg may be the dominant peak together with Fe, and the surrounding matrix may be composed of Si, Al, Ca, and Fe associations with higher or small amounts of Mg. This seems to be the case in Choo et al. [43] when they describe round morphotypes with a crystalline ash matrix, mainly in the form of magnesioferrite (MgFe2O4) and maghemite (γ-Fe2O3) occurring in a fly ash rich in magnesioferrite.
Magnesiaferrosphere Case Study
- (1)
- (2)
- (3)
- (4)
- (5)
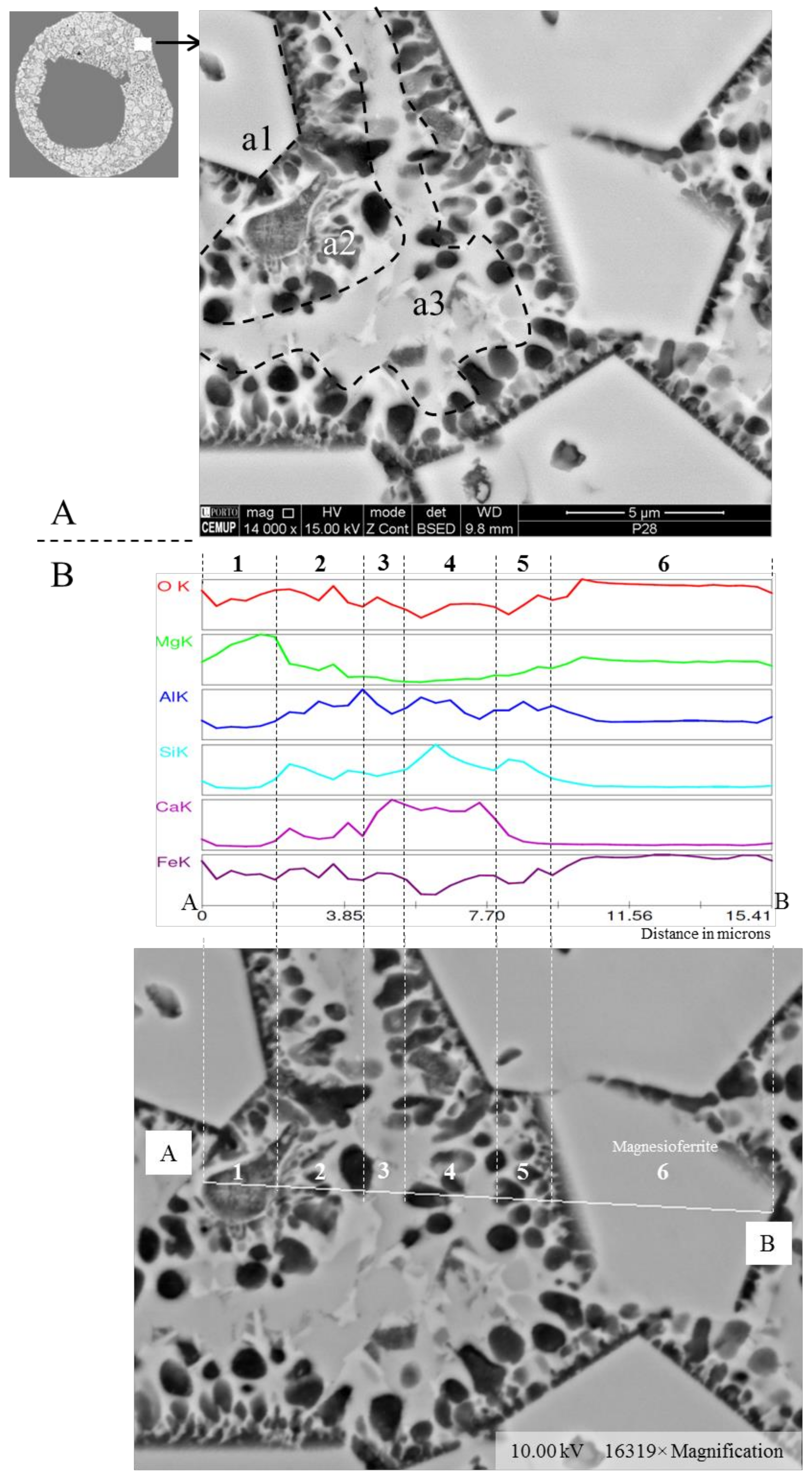
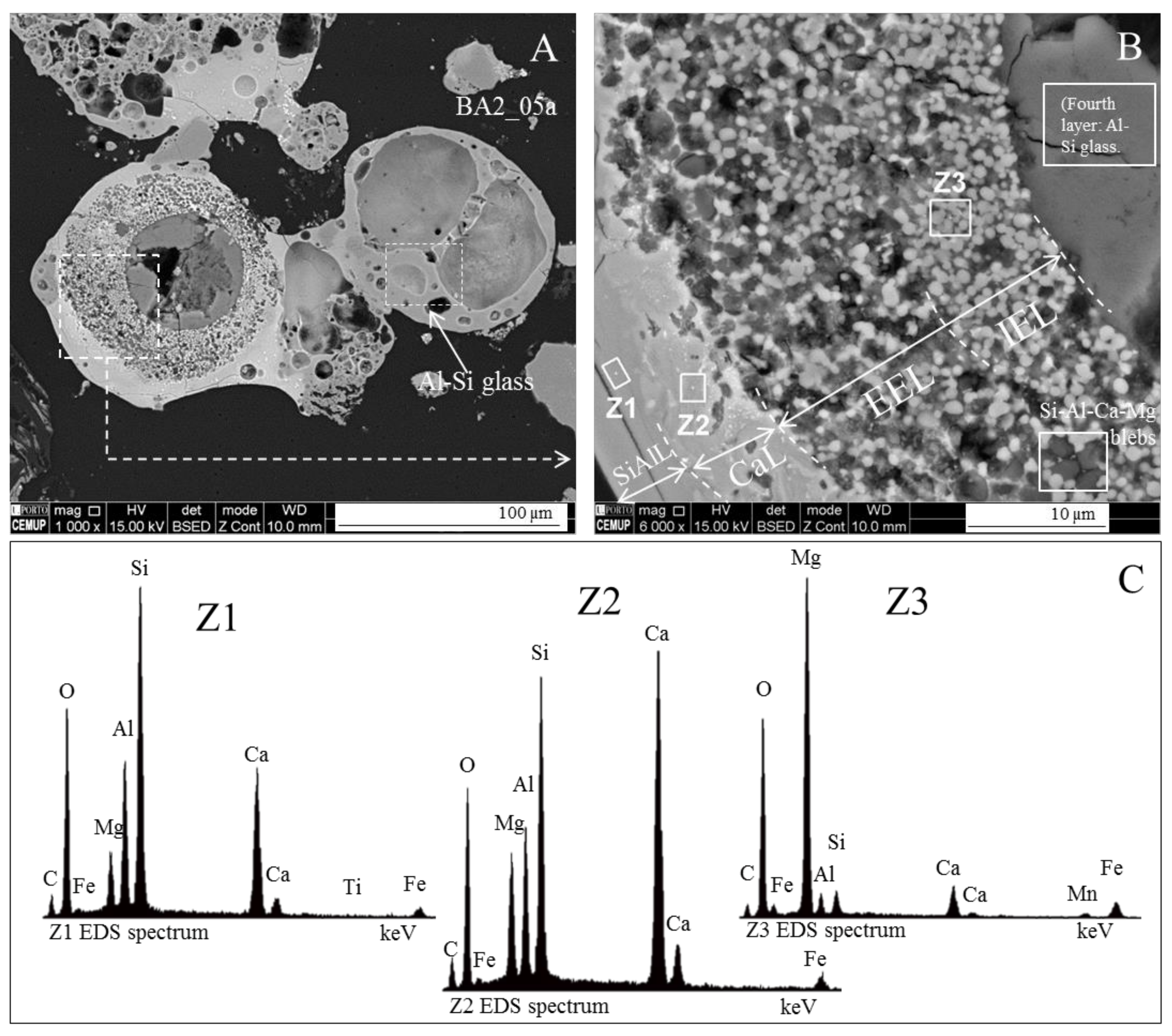
- (area a1) Magnesioferrite crystals with Al, and residual amounts of Ca and Si. Near the edge of the crystals the amount of Al increases and the Mg and Fe decrease. This means that as the melt became depleted in Fe and Mg, more Al was incorporated into the crystal lattice;
- (area a2) Adjacent to the magnesioferrite crystals, a spongy texture reflects the reactions that occurred on a complex Ca-aluminate glass (FeO–SiO2–Al2O3–CaO–MgO) system from which several amorphous phases (e.g., periclase and (Ca–Al–Si)·(Mg,Fe) nodules) exsolved and from which magnesioferrite crystallized;
- (area a3) In the middle, more or less equidistant to the magnesioferrite crystals, the materials show a substantial Ca, Al, and Si concentration, and the Mg is residual.
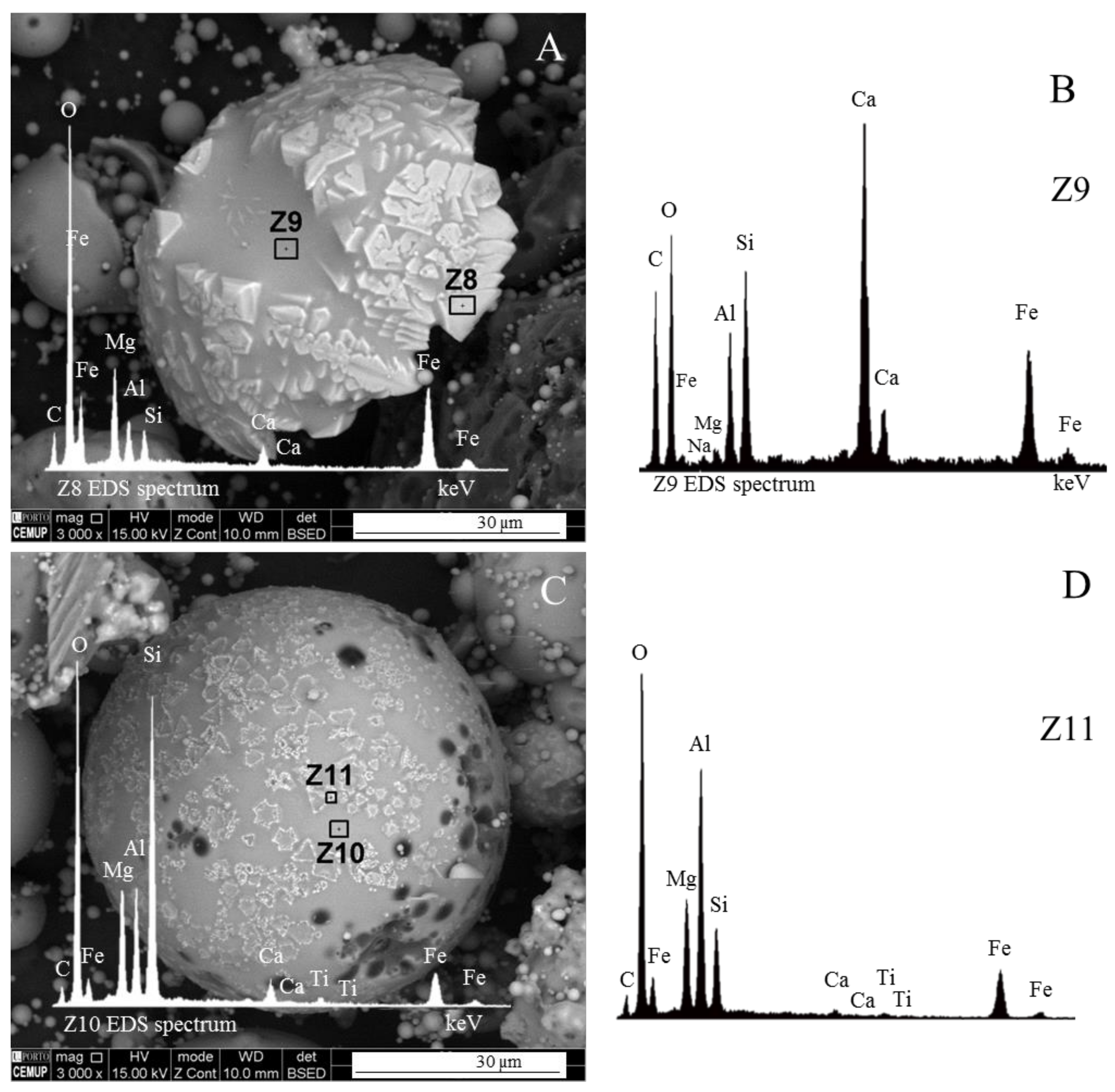
- (i)
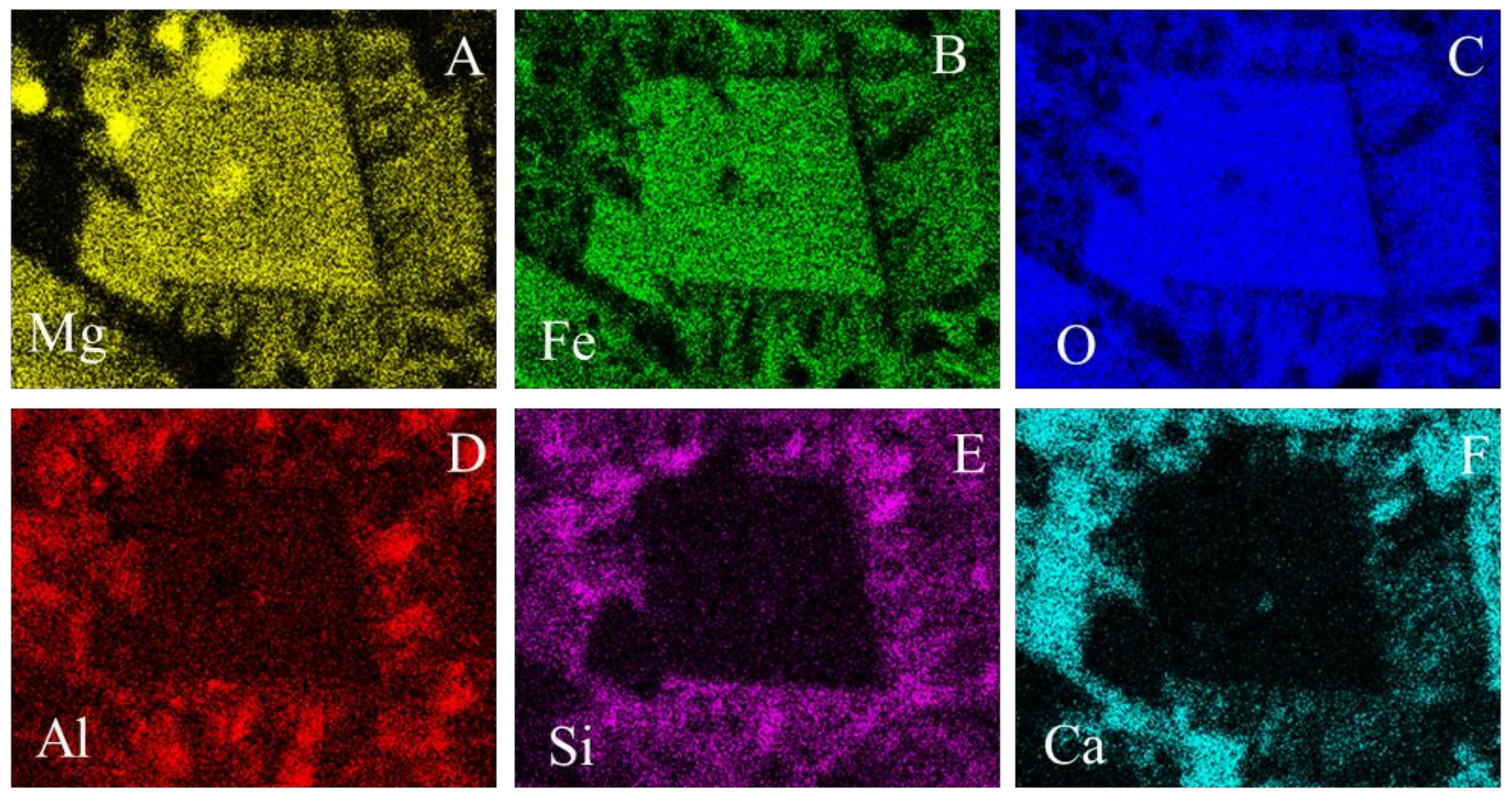
- The euhedral crystal is essentially composed of Mg, Fe, and residual Al. There is an Mg rim near the crystal edge, while the Fe is homogeneously distributed (Figure 11A,B,D).
- (ii)
- The right and bottom sides of the crystal are surrounded by a Ca-depleted material rich in Fe and O.
- (iii)
- (iv)
- A cloud of Al, along with Si, surrounds the crystal. However, several high-concentration spots are clear, probably corresponding to amorphous aluminate, Ca-aluminate, Ca-aluminosilicate, and silica (Figure 11D,E).
3.3.2. Magnesiapheres: Magnesiaoxyspheres Type
3.3.3. Borderline Magnesiaspheres
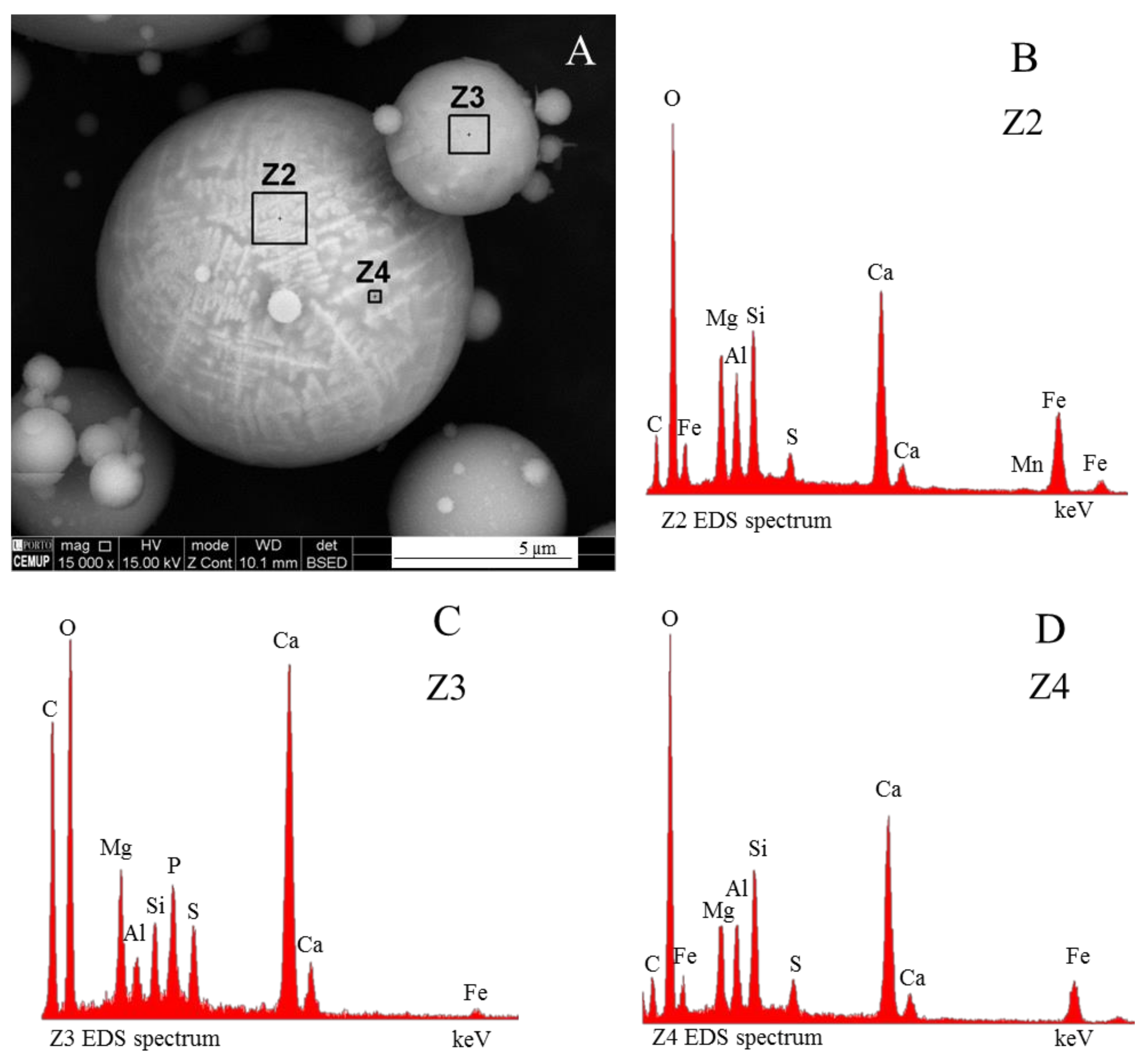
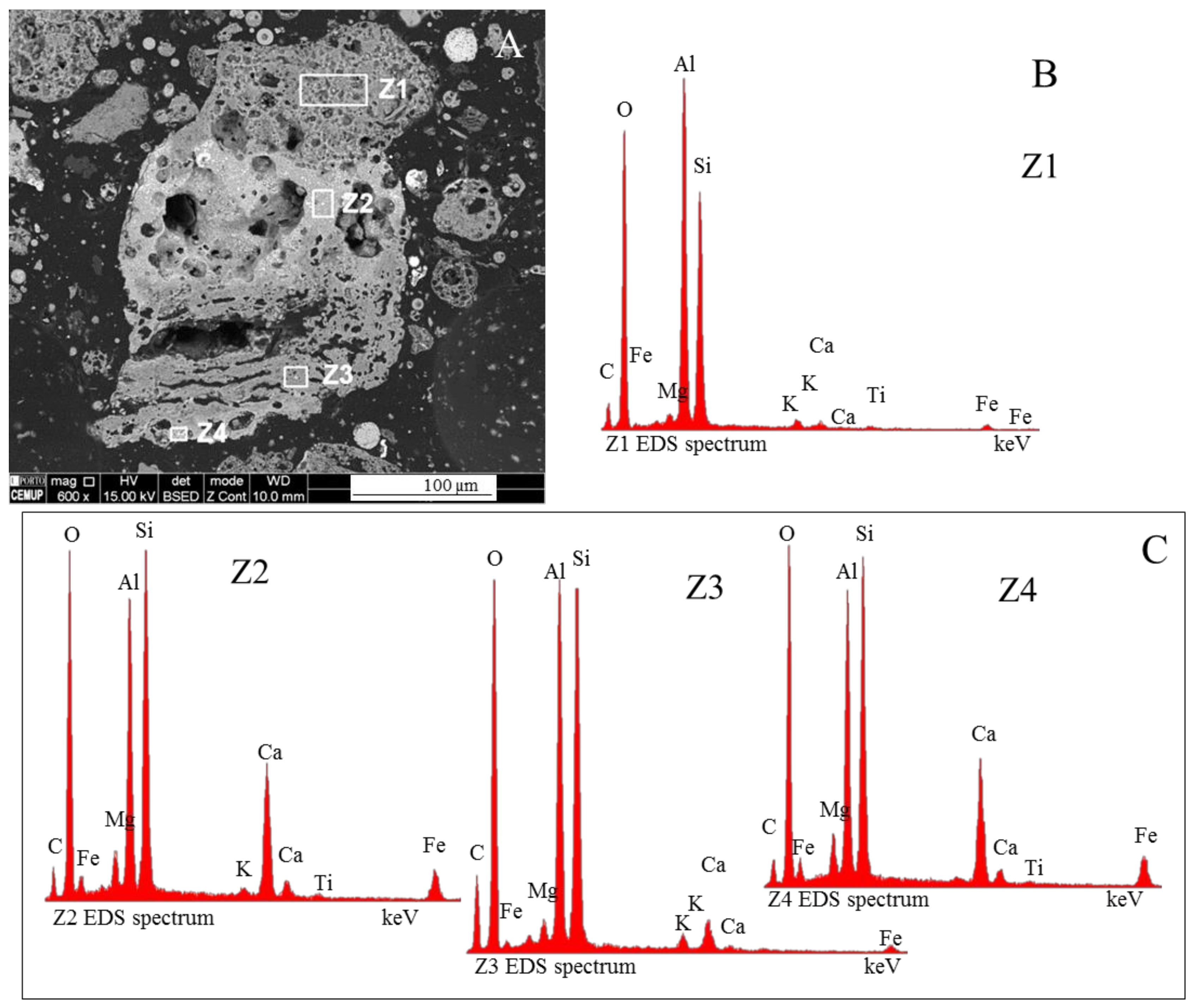
3.4. Calcimagnesiadermaspheres and Mixed Calcialuminosilicate Morphotypes
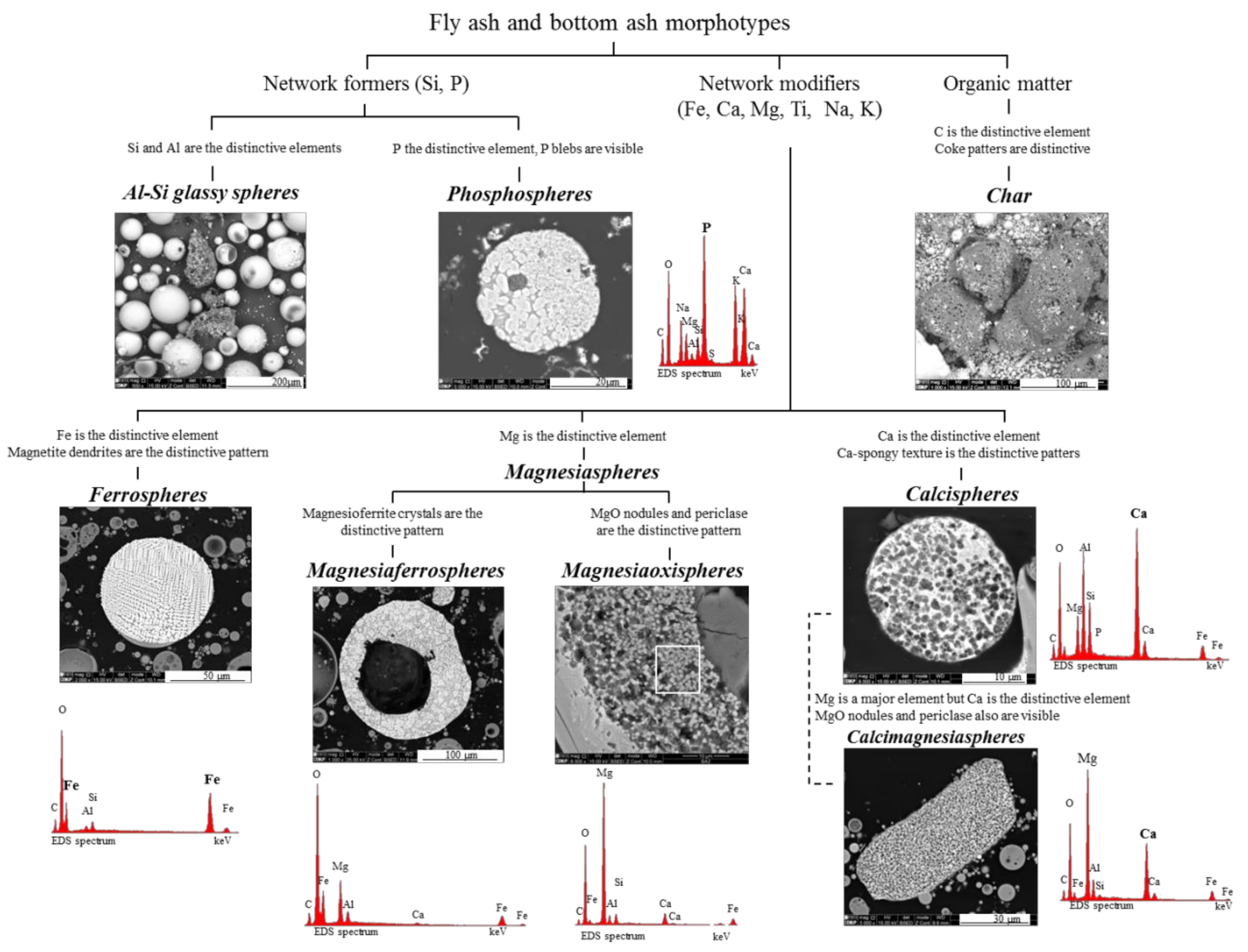
3.5. Systematization of Calcispheres and Magnesiaspheres in the Fly Ash Classification
- Network-forming elements Si, P, and C: “Al–Si glass”, “phosphospheres”, and “char”, respectively:Al–Si glass. The most abundant material in fly ash and bottom ash is partially baked clay, and amorphous and glassy aluminosilicate in the form of a vast array of irregular forms and glassy spheres, which reflect both the coal mineralogical composition (in general, mostly clays, carbonates and pyrite) and the combustion conditions, which influence the melt viscosity, trapped volatile matter, surface tension, and cooling rate. The glassy spheres or “Al–Si glassy spheres” are characterized by an aluminosilicate matrix embedding a skeleton of quartz and mullite [17,20,22,47,48,49,50,51];Phosphospheres. These morphotypes are described in Valentim et al. [28], and their “distinctive pattern” is the “pomegranate” texture caused by the phosphorus network;Char. A solid carbonaceous combustion residue—“char” is formed from coal organic matter during the combustion pyrolysis stage inside the furnace, where devolatilization is the main process that occurs and where oxidation is limited. During the oxidation stage, however, not all of the char burns, and coke-like particles composed of a C-rich network are either carried by the gas flow or fall to the furnace bottom, becoming a component of fly ash and bottom ash.The char results from the burning of low, medium and high rank coals with different macerals (vitrinite, inertinite, and liptinite). Therefore, char is a very heterogeneous material and classifications that focused on fly ash while including char were developed (e.g., [2,3]), as were detailed classifications (e.g., [5,23,52,53,54]).
- Network modifier elements Fe, Mg, and Ca: ferrospheres, magnesiaspheres, calcispheres, and “calcimagnesiaspheres. (Note: Na and K are also network modifiers, but examples of morphotypes with Na and K “distinctive patterns” were not found in this study.)Ferrospheres (magnetic microspheres or magnetite globules) are well-known iron-rich spheres with a mineralogical composition that includes quartz, mullite, magnetite, hematite, and anhydrite embedded with amorphous aluminosilicate. Fe is the distinctive element responsible for distinctively smooth, polygonal, dendritic, granular, and molten drop magnetite, hematite, and maghemite [22,45,55,56];Magnesiaspheres. Although it has an important Fe concentration, Mg is the “distinctive element” of the magnesiaspheres. However, these were divided into magnesiaferrospheres, if magnesioferrite crystals are not residual and are the “distinctive pattern”, and into magnesiaoxyspheres, when the morphotype is mainly formed by an (Mg–Fe)-rich amorphous material with MgO nodules and/or periclase.Calcispheres and calcimagnesiaspheres. The main “distinctive pattern” of “calcispheres” is a Ca-spongy structure. If a P-“pomegranate” texture is also visible, that texture is more relevant; the morphotype is then classified as a “phosphosphere”, not a “calcisphere”, because P is capable of forming its own network. However, when magnesioferrite crystals, MgO nodules or euhedral periclase are also visible without being abundant, the morphotype is a “calcimagnesiaphere”.
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Alvarez, D.; Borrego, A.G.; Menéndez, R. Unbiased methods for the morphological description of char structures. Fuel 1997, 76, 1241–1248. [Google Scholar] [CrossRef]
- Hower, J.C.; Mastalerz, M. An approach toward a combined scheme for the petrographic classification of fly ash. Energy Fuels 2001, 15, 1319–1321. [Google Scholar] [CrossRef]
- Hower, J.C.; Suárez-Ruiz, I.; Mastalerz, M. An approach toward a combined scheme for the petrographic classification of fly ash: Revision and clarification. Energy Fuels 2005, 19, 653–655. [Google Scholar] [CrossRef]
- Hower, J.C.; Groppo, J.G.; Graham, U.M.; Ward, C.R.; Kostova, I.J.; Maroto-Valer, M.M.; Dai, S. Coal-derived unburned carbons in fly ash: A review. Int. J. Coal Geol. 2017, 179, 11–27. [Google Scholar] [CrossRef]
- Suárez-Ruiz, I.; Valentim, B.; Borrego, A.G.; Bouzinos, A.; Flores, D.; Kalaitzidis, S.; Malinconico, M.L.; Marques, M.; Misz-Kenan, M.; Predeanu, G.; et al. Petrographic Classification of Fly Ash Components; International Committee for Coal and Organic Petrology (ICCP; www.iccop.org); Open File (www.iccop.org); International Committee for Coal and Organic Petrology (ICCP): Keiraville, NSW, Australia, 2015; ISBN 978-84-608-1416-0. [Google Scholar]
- Tucker, M.E. Sedimentary Petrology, 3rd ed.; Blackwell Science: Oxford, UK, 2001; p. 262. ISBN 0-632-05735-1. [Google Scholar]
- Ward, C.R.; French, D. Determination of glass content and estimation of glass composition in fly ash using quantitative X-ray diffractometry. Fuel 2006, 85, 2268–2277. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis and significance of mineral matter in coal seams. Int. J. Coal Geol. 2002, 50, 135–168. [Google Scholar] [CrossRef]
- Ward, C.R. Analysis, origin and significance of mineral matter in coal: An updated review. Int. J. Coal Geol. 2016, 165, 1–27. [Google Scholar] [CrossRef]
- Dai, S.; Liu, J.; Ward, C.R.; Hower, J.C.; Xie, P.; Jiang, Y.; Hood, M.M.; O’Keefe, J.M.K.; Song, H. Petrological, geochemical, and mineralogical compositions of the low-Ge coals from the Shengli Coalfield, China: A comparative study with Ge-rich coals and a formation model for coal-hosted Ge ore deposit. Ore Geol. Rev. 2015, 71, 318–349. [Google Scholar] [CrossRef]
- Spears, A. The origin of tonsteins, an overview, and links with seatearths, fireclays and fragmental clay rocks. Int. J. Coal Geol. 2012, 94, 22–31. [Google Scholar] [CrossRef]
- Spears, A. Clay mineralogy of onshore UK Carboniferous mudrocks. Clay Miner. 2006, 41, 395–416. [Google Scholar] [CrossRef]
- Zachariasen, W.J. The atomic arrangement in glass. J. Am. Ceram. Soc. 1932, 54, 3841–3851. [Google Scholar] [CrossRef]
- Dietzel, A. Die kationenfeldstärken und ihre beziehungen zu entglasungsvorgängen, zur verbindungsbildung und zu den schmelzpunkten von silicaten. Elektrochemische 1942, 48, 9–23. [Google Scholar]
- MacDowell, J.F.; Beall, G.H. Immiscibility and crystallization in Al2O3-SiO2 glasses. J. Am. Ceram. Soc. 1969, 52, 17–25. [Google Scholar] [CrossRef]
- Vorres, K.S. Melting behavior of coal ash materials from coal ash composition. Div. Fuel Chem. 1977, 22, 118–142. [Google Scholar]
- Pietersen, H.S. Reactivity of Fly Ash and Slag in Cement. Ph.D. Thesis, Delft University of Technology, Delft, The Netherlands, 1993. [Google Scholar]
- Brindle, J.H.; McCarthy, M.J. Chemical Constraints on Fly Ash Glass Compositions. Energy Fuels 2006, 20, 2580–2585. [Google Scholar] [CrossRef]
- Creelman, R.A.; Ward, C.R.; Schumacher, G.; Juniper, L. Relation between coal mineral matter and deposit mineralogy in pulverized fuel furnaces. Energy Fuels 2013, 27, 5714–5724. [Google Scholar] [CrossRef]
- Hemmings, R.T.; Berry, E.E. On the glass in coal fly ashes: Recent advances. Mater. Res. Soc. Symp. Proc. 1988, 113, 3–38. [Google Scholar] [CrossRef]
- Hower, J.C.; Rathbone, R.F.; Graham, U.M.; Groppo, J.G.; Brooks, S.M.; Robl, T.L.; Medina, S.S. Approaches to the petrographic characterization of the fly ash. In Proceedings of the 11th International Coal Testing Conference, Lexington, KY, USA, 15–16 March 2018; pp. 49–54. [Google Scholar]
- Hower, J.C. Petrographic examination of coal-combustion fly ash. Int. J. Coal Geol. 2012, 92, 90–97. [Google Scholar] [CrossRef]
- Suárez-Ruiz, I.; Valentim, B.; Borrego, A.G.; Kalaitzidis, S.; Flores, D.; Malinconico, M.L.; Marques, M.; Misz-Kennan, M.; Predeanu, G.; Montes, J.R.; et al. Development of a petrographic classification of fly-ash components from coal combustion and co-combustion. (An ICCP Classification System, Fly-Ash Working Group—Commission III). Int. J. Coal Geol. 2017, 183, 188–203. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Hower, J.C.; Johnston, M.N.; Song, W.; Wang, P.; Zhang, S. Petrology, Mineralogy, and Chemistry of Size-Fractioned Fly Ash from the Jungar Power Plant, Inner Mongolia, China, with Emphasis on the Distribution of Rare Earth Elements. Energy Fuels 2014, 28, 1502–1514. [Google Scholar] [CrossRef]
- O’Connor, J.T. Silicate fly ash classification based on exsolution mineralogy. In Proceedings of the 1997 International Ash Utilization Symposium, Lexington, KY, USA, 20–22 October 1997; pp. 692–699. [Google Scholar]
- O’Connor, J.T. U- and F-bearing phosphate-silicate fly ash grains. In Proceedings of the 1997 International Ash Utilization Symposium, Center for Applied Energy Research, Lexington, KY, USA, 20–22 October 1997; pp. 716–721. [Google Scholar]
- O’Connor, J.T.; Meeker, G.M. Liquidus (Ca+Mg)-rich exsolution phases in low-sulfur fly ash. In Proceedings of the 24th International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, 8–11 March 1999; pp. 333–342. [Google Scholar]
- Valentim, B.; Flores, D.; Guedes, A.; Guimarães, R.; Shreya, N.; Paul, B.; Ward, C.R. Notes on the occurrence of phosphate mineral relics and spheres (phosphospheres) in coal and biomass fly ash. Int. J. Coal Geol. 2016, 154–155, 43–56. [Google Scholar] [CrossRef]
- ISO 7404-2. Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- McCarthy, G.J.; Solem, J.K.; Manz, O.E.; Hassett, D.J. Use of a database of chemical, mineralogical, and physical properties of North American fly qsh to study the nature of fly ash and its utilization as a mineral admixture in concrete. In Materials Research Society Symposium Proceedings; Materials Research Society: Boston, MA, USA, 1989; Volume 178. [Google Scholar]
- Sokol, E.V.; Kalugin, V.M.; Nigmatulina, E.N.; Volkova, N.I.; Frenkel, A.E.; Maksimova, N.V. Ferrospheres from fly ashes of Chelyabinsk coals: Chemical composition, morphology and formation conditions. Fuel 2002, 81, 867–876. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Vassileva, C.G.; Karayigit, A.I.; Bulut, Y.; Alastuey, A.; Querol, X. Phase-mineral and chemical composition of fractions separated from composite fly ahses at the Soma power station, Turkey. Int. J. Coal Geol. 2005, 61, 65–85. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part I. Phase-mineral transformations of organic and inorganic matter. Fuel 2013, 112, 391–449. [Google Scholar] [CrossRef]
- Brownfield, M.E.; Affolter, R.H.; Cathcart, J.D.; Brownfield, I.K.; Hower, J.C.; Stricker, G.D. Dispersed volcanic ash in feed coal and its influence on coal combustion products. In International Ash Utilization Symposium; Center for Applied Energy Research, University of Kentucky: Lexington, KY, USA, 1999; p. 61. [Google Scholar]
- Brownfield, M.E.; Affolter, R.H.; Cathcart, J.D.; O’Connor, J.T.; Brownfield, I.K. Characterization of feed coal and coal combustion products from power plants in Indiana and Kentucky. In Proceedings of the 24th International Technical Conference on Coal Utilization and Fuel Systems, Clearwater, FL, USA, 8–11 March 1999; pp. 989–1000. [Google Scholar]
- Hulett, L.D., Jr.; Weinberger, A.J.; Northcutt, K.J.; Ferguson, M. Chemical species in fly ash from coal-burning power plants. Science 1980, 210, 1356–1358. [Google Scholar] [CrossRef] [PubMed]
- Norton, G.A.; Markuszewski, R.; Shanks, H.R. Morphological and chemical characterization of iron-rich fly ash fractions. Environ. Sci. Technol. 1986, 20, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Franklin, F.F.; Hooper, R.L.; Rosenberg, P.L. An unusual pyroxene, melilite, and iron oxide mineral assemblage in a coal-fire buchite from Buffalo, Wyoming. Am. Mineral. 1980, 72, 137–147. [Google Scholar]
- Brownfield, M.E.; Cathcart, J.D.; Affolter, R.H.; Brownfield, I.K.; Rice, C.A.; O’Connor, J.T.; Zielinski, R.A.; Bullock, J.H., Jr.; Hower, J.C.; Meeker, G.P. Characterization and Modes of Occurrence of Elements in Feed Coal and Coal Combustion Products from a Power Plant Utilizing Low-Sulfur Coal from the Powder River Basin, Wyoming: USGS Scientific Investigations Report 2004-5271. 2005. Available online: http://pubs.usgs.gov/sir/2004/5271/ (accessed on 28 March 2018).
- Anthony, J.W.; Bideaux, R.A.; Bladh, K.W.; Nichols, M.C. Handbook of Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 2018; Available online: http://www.handbookofmineralogy.org/ (accessed on 11 January 2018).
- Magiera, T.; Jabłonska, M.; Strzyszcz, Z.; Rachwał, M. Morphological and mineralogical forms of technogenic magnetic particles in industrial dusts. Atmos. Environ. 2011, 45, 281–290. [Google Scholar] [CrossRef]
- Magiera, T.; Mendakiewicz, M.; Szuszkiewicz, M.; Jabłonska, M.; Chróst, L. Technogenic magnetic particles in soils as evidence of historical mining and smelting activity: A case of the Brynica River Valley, Polland. Sci. Total Environ. 2016, 566–567, 536–551. [Google Scholar] [CrossRef] [PubMed]
- Choo, T.K.; Song, Y.; Zhang, L.; Selomulya, C.; Zhang, L. Mechanisms Underpinning the Mobilization of Iron and Magnesium Cations from Victorian Brown Coal Fly Ash. Energy Fuels 2014, 28, 4051–4061. [Google Scholar] [CrossRef]
- Anshits, A.G.; Sharonova, O.M.; Anshits, N.N.; Vereshchagin, S.N.; Rabchevskii, E.V.; Solovjev, A.V. Ferrospheres from fly ashes: Composition and catalytic properties in high-temperature oxidation of methane. In Proceedings of the Abstracts of the World of Coal Ash (WOCA) Conference, Denver, CO, USA, 9–12 May 2011. [Google Scholar]
- Lauf, R.J.; Harris, L.A.; Rawlston, S.S. Pyrite framboids as the source of magnetite spheres in fly ash. Environ. Sci. Technol. 1982, 16, 218–220. [Google Scholar] [CrossRef]
- Valentim, B.; Shreya, N.; Paul, B.; Gomes, C.S.; Sant’Ovaia, H.; Guedes, A.; Ribeiro, J.; Flores, D.; Pinho, S.; Ward, C.R. Characteristics of ferrospheres in fly ashes derived from Bokaro and Jharia (Jharkand, India) coals. Int. J. Coal Geol. 2016, 153, 52–74. [Google Scholar] [CrossRef]
- Fisher, G.L.; Prentice, B.A.; Silberman, D.; Ondov, J.M.; Biermann, A.H.; Ragaini, R.C.; McFarland, A.R. Physical and morphological studies of size-classified coal fly ash. Environ. Sci. Technol. 1978, 12, 447–451. [Google Scholar] [CrossRef]
- Hulett, L.D.; Weinberger, A.J. Some etching studies of the microstructure and composition of large aluminosilicate particles in fly ash from coal-burning power plants. Environ. Sci. Technol. 1980, 14, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Anshits, A.G.; Kondratenko, E.V.; Fomenko, E.V.; Kovalev, A.M.; Anshits, N.N.; Bajukov, O.A.; Sokol, E.V.; Salanov, A.N. Novel glass crystal catalysts for the processes of methane oxidation. Catal. Today 2001, 64, 59–67. [Google Scholar] [CrossRef]
- Matsunaga, T.; Kim, J.K.; Hardcastle, S.; Rohatgi, P.K. Crystallinity and selected properties of fly ash particles. Mater. Sci. Eng. 2002, 325, 333–343. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Menendez, R.; Diaz-Somoano, M.; Marinez-Tarazona, M.R. Phase-mineral and chemical composition of coal fly ashes as a basis for their multicomponent utilization. 2. Characterization of ceramic cenosphere and salt concentrates. Fuel 2004, 83, 585–603. [Google Scholar] [CrossRef]
- Bailey, J.G.; Tate, A.; Diessel, C.F.K.; Wall, T.F. A char morphology system with applications to coal combustion. Fuel 1990, 69, 225–239. [Google Scholar] [CrossRef]
- Lester, E.; Alvarez, D.; Gawronski, E.; Petersen, H.; Rosenberg, P.; Vleeskens, J.; Kwiecińska, B.; Misz, M.; Pusz, S.; Flores, D.; et al. Atlas of Char Occurrences of the Combustion Working Group-ICCP; CDrom; International Committee for Coal and Organic Petrology (ICCP): Keiraville, NSW, Australia, 2000. [Google Scholar]
- Lester, E.; Alvarez, D.; Borrego, A.G.; Valentim, B.; Flores, D.; Clift, D.A.; Rosenberg, P.; Kwiecińska, B.; Barranco, R.; Petersen, H.I.; et al. The procedure used to develop a coal char classification-commission III Combustion Working Group of the International Committee for Coal and Organic Petrology. Int. J. Coal Geol. 2000, 81, 333–342. [Google Scholar] [CrossRef]
- Sokol, E.V.; Maksimova, E.V.; Volkova, N.I.; Nigmatulina, E.N.; Frenkel, A.E. Hollow silicate microspheres from fly ashes of the chelyabinsk brown coals (the South Urals, Russia). Fuel Process. Technol. 2000, 67, 35–52. [Google Scholar] [CrossRef]
- Xue, Q.; Lu, S. Microstructure of ferrospheres in fly ashes: SEM, EDX and ESEM analysis. J. Zhejiang Univ. Sci. 2008, 9, 1595–1600. [Google Scholar] [CrossRef]
- Zhang, L.; Sato, A.; Ninomiya, Y. CCSEM analysis of ash from combustion of coal added with limestone. Fuel 2002, 81, 1499–1508. [Google Scholar] [CrossRef]
| Sample | SiO2 | Al2O3 | TiO2 | Fe2O3 | Mn3O4 | CaO | MgO | Na2O | K2O | P2O5 | SO3 | LOI * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | 47.27 | 20.92 | 0.83 | 8.15 | 0.06 | 7.63 | 2.53 | 0.52 | 1.77 | 0.17 | 0.37 | 9.24 |
| R4 | 47.48 | 20.56 | 0.82 | 8.68 | 0.07 | 8.22 | 2.60 | 0.49 | 1.79 | 0.18 | 0.47 | 7.59 |
| R7 | 46.57 | 20.66 | 0.82 | 8.21 | 0.06 | 7.68 | 2.52 | 0.48 | 1.76 | 0.17 | 0.35 | 9.17 |
| R8 | 47.35 | 20.56 | 0.81 | 8.58 | 0.07 | 8.15 | 2.61 | 0.54 | 1.81 | 0.18 | 0.46 | 8.30 |
| R10 | 47.78 | 20.76 | 0.84 | 8.64 | 0.07 | 8.19 | 2.64 | 0.53 | 1.82 | 0.18 | 0.53 | 7.51 |
| R16 | 44.82 | 19.48 | 0.80 | 12.17 | 0.08 | 12.10 | 2.97 | 0.50 | 1.45 | 0.21 | 0.77 | 4.89 |
| R24 | 48.46 | 20.81 | 0.82 | 8.64 | 0.07 | 8.25 | 2.69 | 0.53 | 1.84 | 0.18 | 0.45 | 8.60 |
| A6 | 48.99 | 26.17 | 1.07 | 9.55 | 0.09 | 3.90 | 2.45 | 1.50 | 2.65 | 0.33 | 0.71 | 1.90 |
| A20 | 51.62 | 28.14 | 1.21 | 6.34 | 0.07 | 3.02 | 2.37 | 1.32 | 3.35 | 0.63 | 0.28 | 2.13 |
| A28 | 51.04 | 21.92 | 0.98 | 6.08 | 0.11 | 3.32 | 2.53 | 1.04 | 2.67 | 0.17 | 0.10 | 8.82 |
| Z6 | 49.86 | 23.30 | 0.93 | 15.30 | 0.09 | 3.74 | 2.19 | 0.80 | 2.24 | 0.21 | 0.43 | 0.79 |
| Sample | R2 | R4 | R7 | R8 | R10 | R16 | R24 | A6 | A20 | A28 | Z6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | wt % | |||||||||||
| Quartz | 9.1 | 8.2 | 6.8 | 7.2 | 7.8 | 6.1 | 8.9 | 6.1 | 5.2 | 9.1 | 8.4 | |
| Cristobalite | 0.3 | 0.3 | 0.2 | 0.3 | 0.3 | 0.2 | ||||||
| Mullite | 5.0 | 6.1 | 4.4 | 4.4 | 5.1 | 4.6 | 5.6 | 13.2 | 19.1 | 13.6 | 14.3 | |
| Anorthite | 12.0 | 8.8 | 8.4 | 8.1 | 8.9 | 9.8 | 10.3 | 3.6 | ||||
| Melilite | 1.7 | 1.2 | 1.0 | 1.1 | 0.7 | 1.5 | 1.5 | |||||
| Diopside | 2.0 | 0.9 | 0.8 | 0.9 | 1.0 | 1.6 | 1.8 | |||||
| Lime | 0.2 | |||||||||||
| Calcite | 1.8 | 1.3 | 1.3 | 1.1 | 1.5 | 1.2 | 1.5 | 0.4 | ||||
| Anhydrite | 0.5 | 0.2 | 0.2 | |||||||||
| Gypsum | 1.5 | 1.5 | 1.8 | 1.7 | 4.0 | 1.5 | 2.5 | |||||
| Hematite | 0.8 | 0.6 | 0.7 | 0.6 | 0.6 | 0.4 | 0.8 | 0.8 | 0.3 | 0.1 | 1.6 | |
| Magnetite | 0.3 | 0.0 | 0.1 | 0.1 | 0.1 | 0.5 | 0.4 | 1.3 | 0.2 | 0.1 | 0.3 | |
| Maghemite | 0.8 | 0.9 | 0.7 | 0.7 | 0.9 | 1.3 | 0.9 | 1.1 | 1.6 | 0.6 | 0.8 | |
| Spinel, ferroan | 0.3 | 0.0 | 0.1 | 0.1 | 0.1 | 0.3 | 0.5 | 0.9 | ||||
| Periclase | 0.5 | 0.6 | ||||||||||
| Grossular | 0.1 | 0.3 | 0.2 | 0.2 | 0.2 | 0.4 | ||||||
| Amorphous | 64.7 | 70.1 | 73.2 | 73.6 | 69.0 | 70.8 | 64.7 | 76.9 | 72.7 | 75.8 | 69.8 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valentim, B.; Białecka, B.; Gonçalves, P.A.; Guedes, A.; Guimarães, R.; Cruceru, M.; Całus-Moszko, J.; Popescu, L.G.; Predeanu, G.; Santos, A.C. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals 2018, 8, 140. https://doi.org/10.3390/min8040140
Valentim B, Białecka B, Gonçalves PA, Guedes A, Guimarães R, Cruceru M, Całus-Moszko J, Popescu LG, Predeanu G, Santos AC. Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres. Minerals. 2018; 8(4):140. https://doi.org/10.3390/min8040140
Chicago/Turabian StyleValentim, Bruno, Barbara Białecka, Paula Alexandra Gonçalves, Alexandra Guedes, Renato Guimarães, Mihai Cruceru, Joanna Całus-Moszko, Luminiţa Georgeta Popescu, Georgeta Predeanu, and Ana Cláudia Santos. 2018. "Undifferentiated Inorganics in Coal Fly Ash and Bottom Ash: Calcispheres, Magnesiacalcispheres, and Magnesiaspheres" Minerals 8, no. 4: 140. https://doi.org/10.3390/min8040140






