Promote Alumina Leaching through Re-Pelleting: Effects of Directional Coating of Calcite on Silicate Grains
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Materials
2.2. Experimental Instruments
2.3. Experimental Methods
- η, the leaching yield of Al2O3, SiO2, or Na2O, %;
- c, the concentration of Al2O3, SiO2, or Na2O in lixivium, g/L;
- Vol, the initial addition of water for leaching, L;
- M, the mass of the mixture before pregrinding, g;
- ω, the mass fraction of Al2O3, SiO2, or Na2O in the mixture before pregrinding, %.
3. Results and Discussion
3.1. Methods of Re-Pelleting
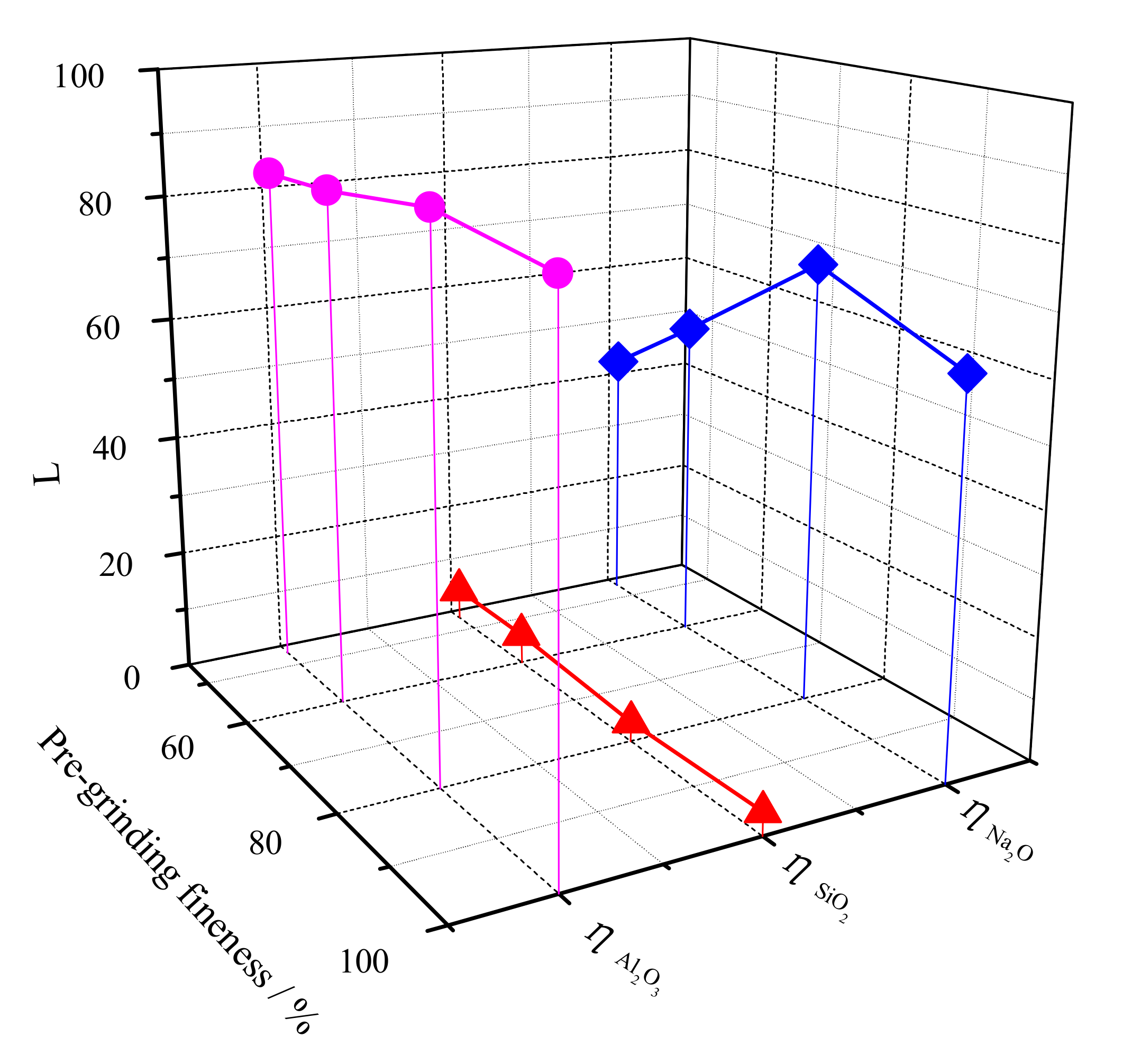
3.2. Pregrinding Fineness
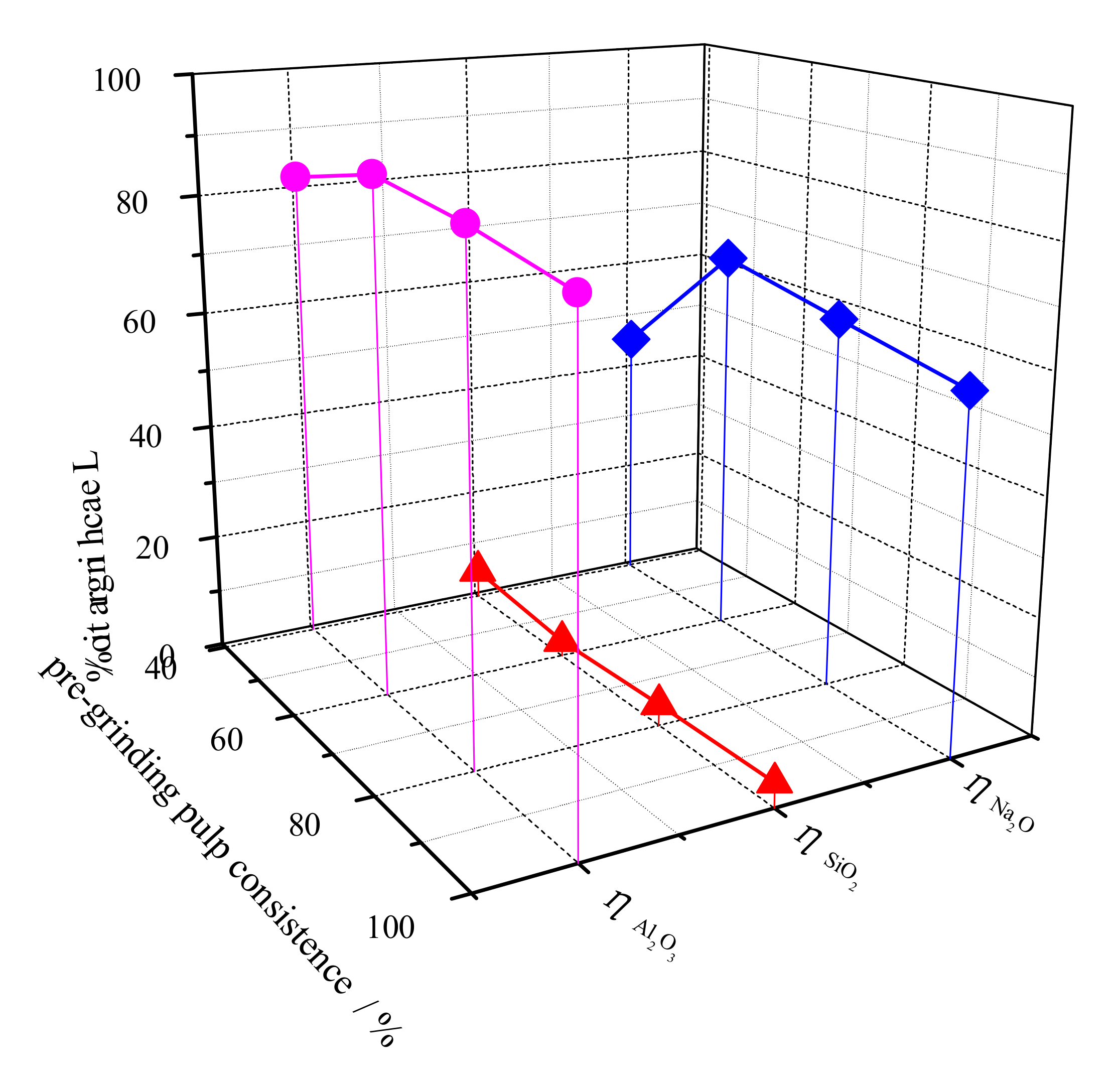
3.3. Pulp Consistence of Pregrinding
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Deng, J.; Wang, Q.F.; Yang, S.J.; Liu, X.F.; Zhang, Q.Z.; Yang, L.Q.; Yang, Y.H. Genetic relationship between the Emeishan plume and the bauxite deposits in Western Guangxi, China: Constraints from U–Pb and Lu–Hf isotopes of the detrital zircons in bauxite ores. J. Asian Earth Sci. 2010, 37, 412–424. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Zhou, L.J.; Li, Y.J.; Wu, C.Q.; Zheng, C.F. The “coal-bauxite-iron” structure in the ore-bearing rock series as a prospecting indicator for southeastern Guizhou bauxite mines. Ore Geol. Rev. 2013, 53, 145–158. [Google Scholar] [CrossRef]
- Cheng, G.; Gao, G.M.; Chen, S.L. Geological characteristics and mineralization regularity of bauxite in Boloven Plateau Laos. J. Cent. South Univ. (Sci. Technol.) 2008, 39, 380–386. [Google Scholar]
- Kahn, H.; Ml, M.; Tassinari, G.; Ratti, G. Characterization of bauxite fines aiming to minimine their iron content. Miner. Eng. 2003, 16, 1313–1315. [Google Scholar] [CrossRef]
- Solymár, K.; Mádai, F.; Papanastassiou, D. Effect of bauxite microstructure on beneficiation and processing. In Essential Readings in Light Metals; Springer: Berlin, Germany, 2016; Volume 1, pp. 37–42. [Google Scholar]
- Thair, A.A. Mineralogy, geochemistry, and ore formation of Nuwaifa bauxite deposit, western desert of Iraq. Arab J. Geosci. 2017, 10, 1–15. [Google Scholar]
- Peng, Z.; Hwang, J.Y. Microwave-assisted metallurgy. Int. Mater. Rev. 2015, 60, 30–63. [Google Scholar] [CrossRef]
- Li, G.H.; Gu, F.Q.; Luo, J.; Deng, B.N.; Peng, Z.W.; Jiang, T. Recovery of iron-titanium-bearing constituents from bauxite ore residue via magnetic separation followed by sulfuric acid leaching. In Light Metals; Springer: Berlin, Germany, 2017; pp. 75–81. [Google Scholar]
- Papassiopi, N.; Vaxevanidou, K.; Paspaliaris, I. Effectiveness of iron reducing bacteria for the removal of iron from bauxite ores. Miner. Eng. 2010, 23, 25–31. [Google Scholar] [CrossRef]
- Liu, Z.G.; Chu, M.S.; Wang, Z.; Zhao, W.; Tang, J. Study on metallized reduction and magnetic Separation of iron from fine particles of high iron bauxite ore. High Temp. Mater. Process. 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Li, Z.; Jin, J.; Wang, Y.; Wu, Y. Study on preparation of iron powder and nano-carbon black by reaction process of high-iron bauxite and natural gas. Integr. Ferroelectr. 2015, 160, 160–168. [Google Scholar] [CrossRef]
- Li, G.H.; Luo, J.; Jiang, T.; Li, Z.X.; Peng, Z.W.; Zhang, Y.B. Digestion of Alumina from Non-Magnetic Material Obtained from Magnetic Separation of Reduced Iron-Rich Diasporic Bauxite with Sodium Salts. Metals 2016, 6, 294. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y. Effect of nonmetallic additives on iron grain grindability. Int. J. Miner. Process. 2014, 130, 108–113. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y. Correlation between aggregation structure and tailing mineral crystallinity. Int. J. Miner. Metall. Mater. 2014, 21, 845–850. [Google Scholar] [CrossRef]
- Yeh, C.H.; Zhang, G.Q. Stepwise carbothermal reduction of bauxite ores. Int. J. Miner. Process. 2013, 124, 1–7. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y.; Duan, X.Q. Restraining sodium volatilization in the ferric bauxite direct reduction system. Minerals 2016, 6, 31. [Google Scholar] [CrossRef]
- Hu, W.T.; Liu, X.W.; Wang, H.J.; Dai, X.J.; Pan, D.L.; Li, J.; Sun, C.Y.; Xia, H.W.; Wang, B. Improvement of sodium leaching ratio of ferric bauxite sinter after direct reduction. Minerals 2017, 7, 10. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Liu, X.W.; Sun, C.Y. Effects of Minerals in Ferric Bauxite on Sodium Carbonate Decomposition and Volatilization. J. Cent. South Univ. 2015, 46, 2503–2507. [Google Scholar] [CrossRef]
- Hu, W.T.; Wang, H.J.; Sun, C.Y.; Ji, C.L.; He, Y.; Wang, C.L. Alkali consumption mechanism on ferrous bauxite reduction process. J. Cent. South Univ. (Sci. Technol.) 2012, 43, 1595–1603. [Google Scholar]
- Hu, W.T.; Wang, H.J.; Sun, C.Y.; Tong, G.K.; Ji, C.L. Direct reduction-leaching process for high ferric bauxite. J. Univ. Sci. Technol. Beijing 2012, 34, 506–511. [Google Scholar]
- Hu, W.T.; Xia, H.W.; Pan, D.L.; Wei, X.L.; Li, J.; Dai, X.J.; Yang, F.H.; Lu, X.; Wang, H.J. Difference of zinc volatility in diverse carrier minerals: The critical limit of blast furnace dust recycle. Miner. Eng. 2018, 116, 24–31. [Google Scholar] [CrossRef]
- Tripathi, H.S.; Ghosh, A.; Halder, M.K.; Muktherjee, B.; Maiti, H.S. Microstructure and properties of sintered mullite developed from Indian bauxite. Bull. Mater. Sci. 2012, 35, 639–643. [Google Scholar] [CrossRef]
- Le, T.; Ju, S.H.; Lu, L.M.; Peng, J.H.; Zhou, L.X.; Wang, S.X. A novel process and its mechanism for recovering alumina from diasporic bauxite. Hydrometallurgy 2017, 169, 124–134. [Google Scholar] [CrossRef]
- Kłosek-Wawrzyn, E.; Małolepszy, J. The potential use of calcite wastes in the production of clay masonry units. Mater. Ceram. 2016, 68, 230–235. [Google Scholar]
- Mandal, A.K.; Sinha, O.P. Effect of Bottom ash fineness on properties of red mud geopolymer. J. Solid Waste Technol. Manag. 2017, 43, 26–35. [Google Scholar] [CrossRef]
- Bazhin, V.Y.; Brichkin, V.N.; Sizyakov, V.M.; Cherkasova, M.V. Pyrometallurgical treatment of a nepheline charge using additives of natural and technogenic origin. Metallurgist 2017, 61, 147–154. [Google Scholar] [CrossRef]
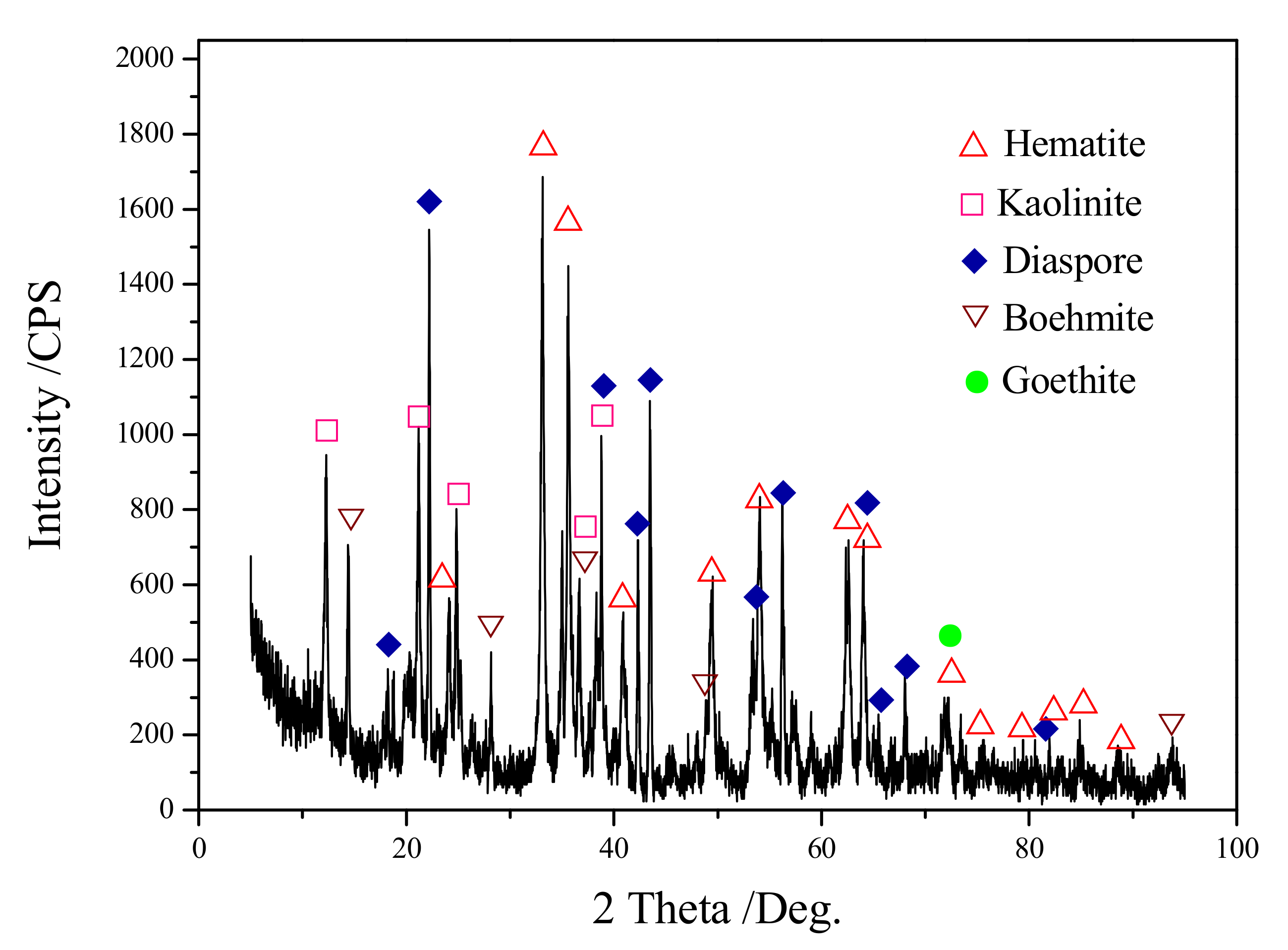

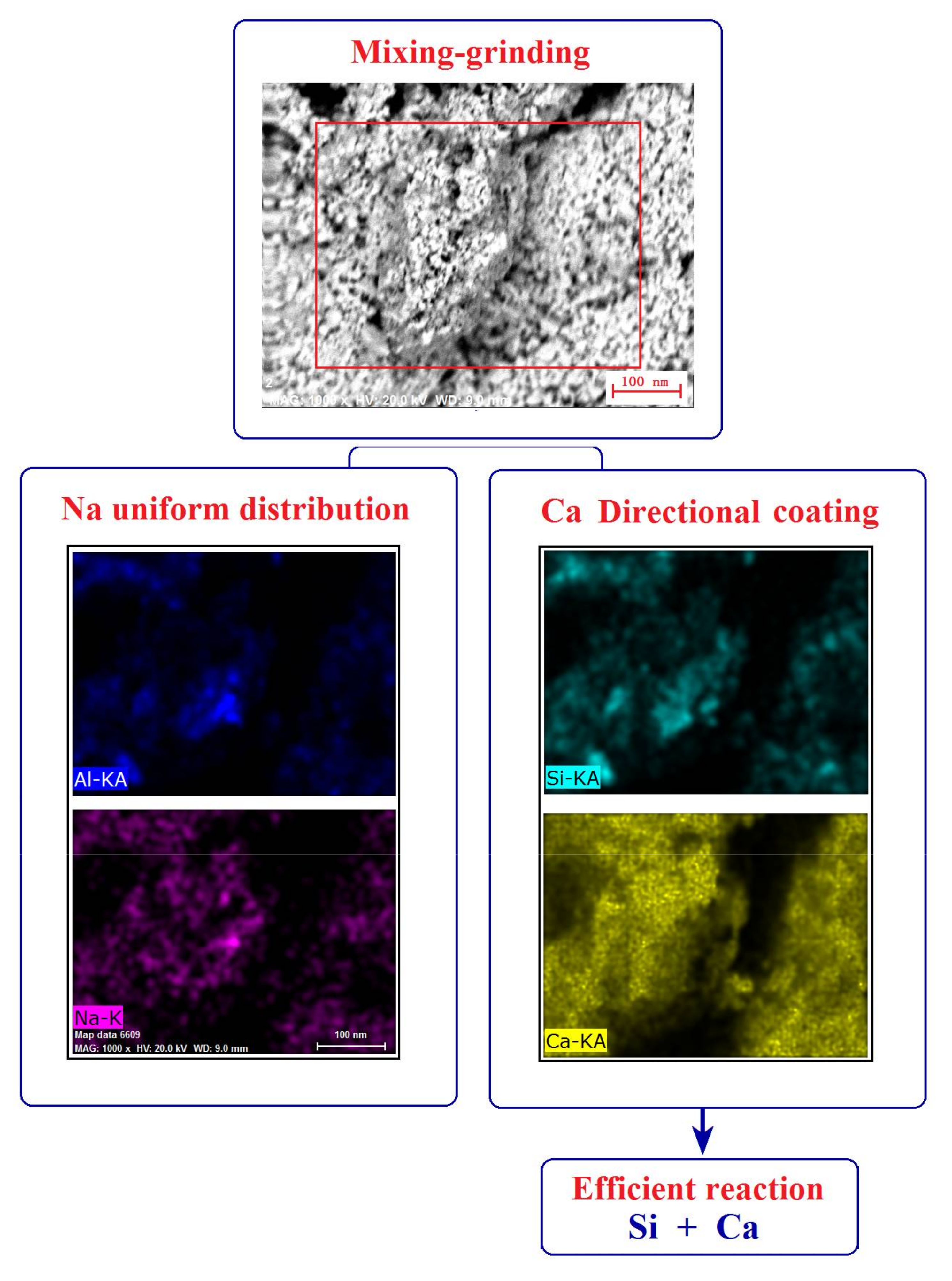
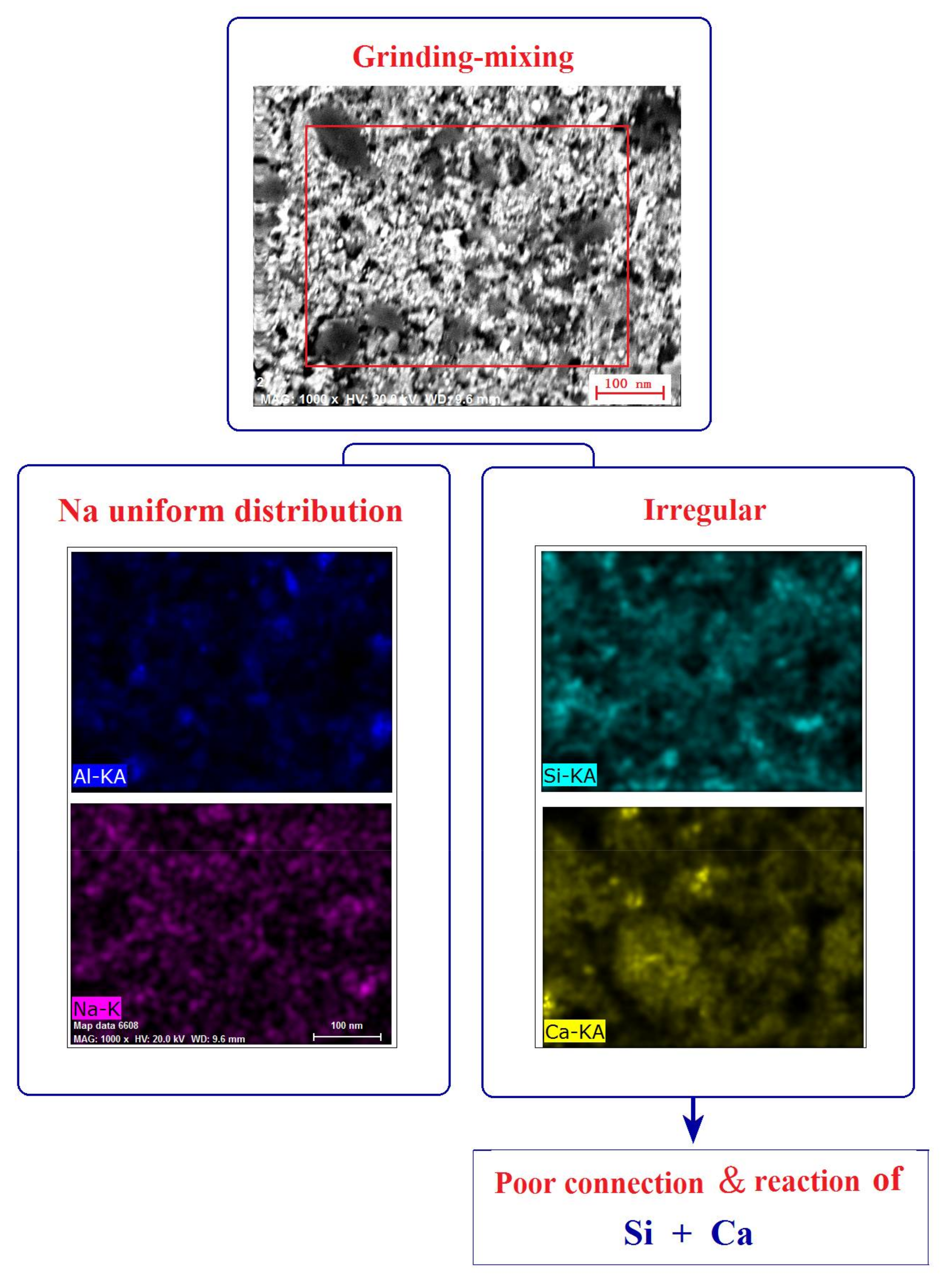



| Composition | Fe2O3 | Al2O3 | SiO2 | TiO2 | MgO | CaO | Na2O | K2O | P2O5 | LOI |
|---|---|---|---|---|---|---|---|---|---|---|
| Contents/% (w/w) | 41.13 | 33.02 | 12.22 | 1.49 | 0.68 | 0.63 | 0.32 | 0.06 | 0.04 | 8.97 |
| Component | Total Moisture (Mt) | Volatiles (Vad) | Ash (Aad)/% | Fixed Carbon (FCad) |
|---|---|---|---|---|
| Contents/% (w/w) | 9.16 | 39.42% | 5.07% | 46.35% |
| Component. | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|
| Contents/% (w/w) | 38.0 | 21.37 | 36.19 | 1.9 | 7.15 | 0.43 | 1.38 | 0.84 | 0.41 |
| Component | Al2O3 | SiO2 | Na2O |
|---|---|---|---|
| Mixing-grinding/% (w/w) | 86.78 | 3.21 | 72.13 |
| Grinding-mixing/% (w/w) | 79.88 | 9.72 | 65.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, W.; Qiu, Q.; Wei, X.; Wei, H.; Zhao, Q.; Yang, F.; Liu, X. Promote Alumina Leaching through Re-Pelleting: Effects of Directional Coating of Calcite on Silicate Grains. Minerals 2018, 8, 129. https://doi.org/10.3390/min8040129
Hu W, Qiu Q, Wei X, Wei H, Zhao Q, Yang F, Liu X. Promote Alumina Leaching through Re-Pelleting: Effects of Directional Coating of Calcite on Silicate Grains. Minerals. 2018; 8(4):129. https://doi.org/10.3390/min8040129
Chicago/Turabian StyleHu, Wentao, Qun Qiu, Xinlei Wei, Hong Wei, Qin Zhao, Feihua Yang, and Xinwei Liu. 2018. "Promote Alumina Leaching through Re-Pelleting: Effects of Directional Coating of Calcite on Silicate Grains" Minerals 8, no. 4: 129. https://doi.org/10.3390/min8040129




