Fossilization History of Fossil Resin from Jambi Province (Sumatra, Indonesia) Based on Physico-Chemical Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Microscopic Methods
2.3. Microhardness Measurements
2.4. Spectroscopic Methods
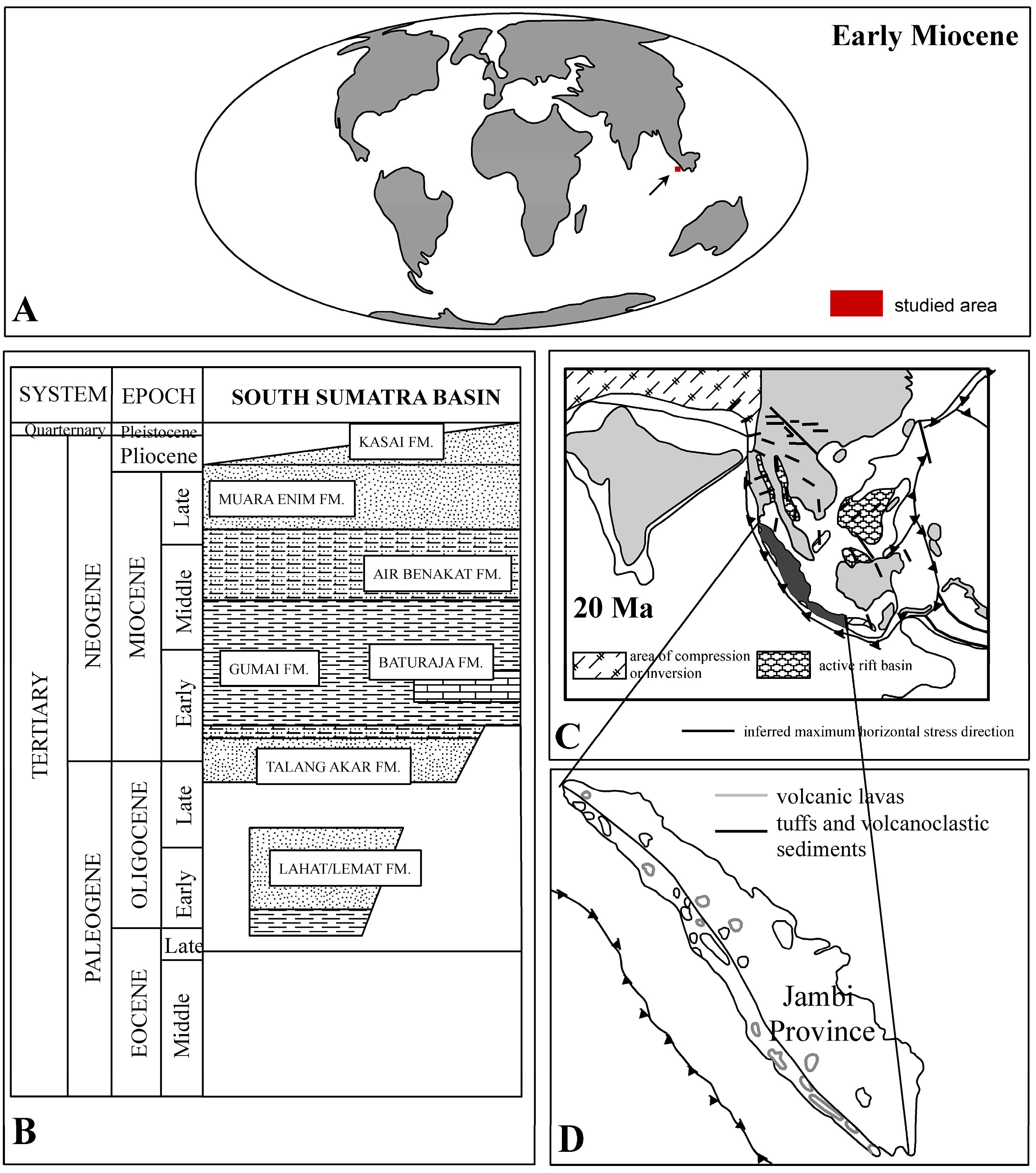
3. Geological Settings
4. Results
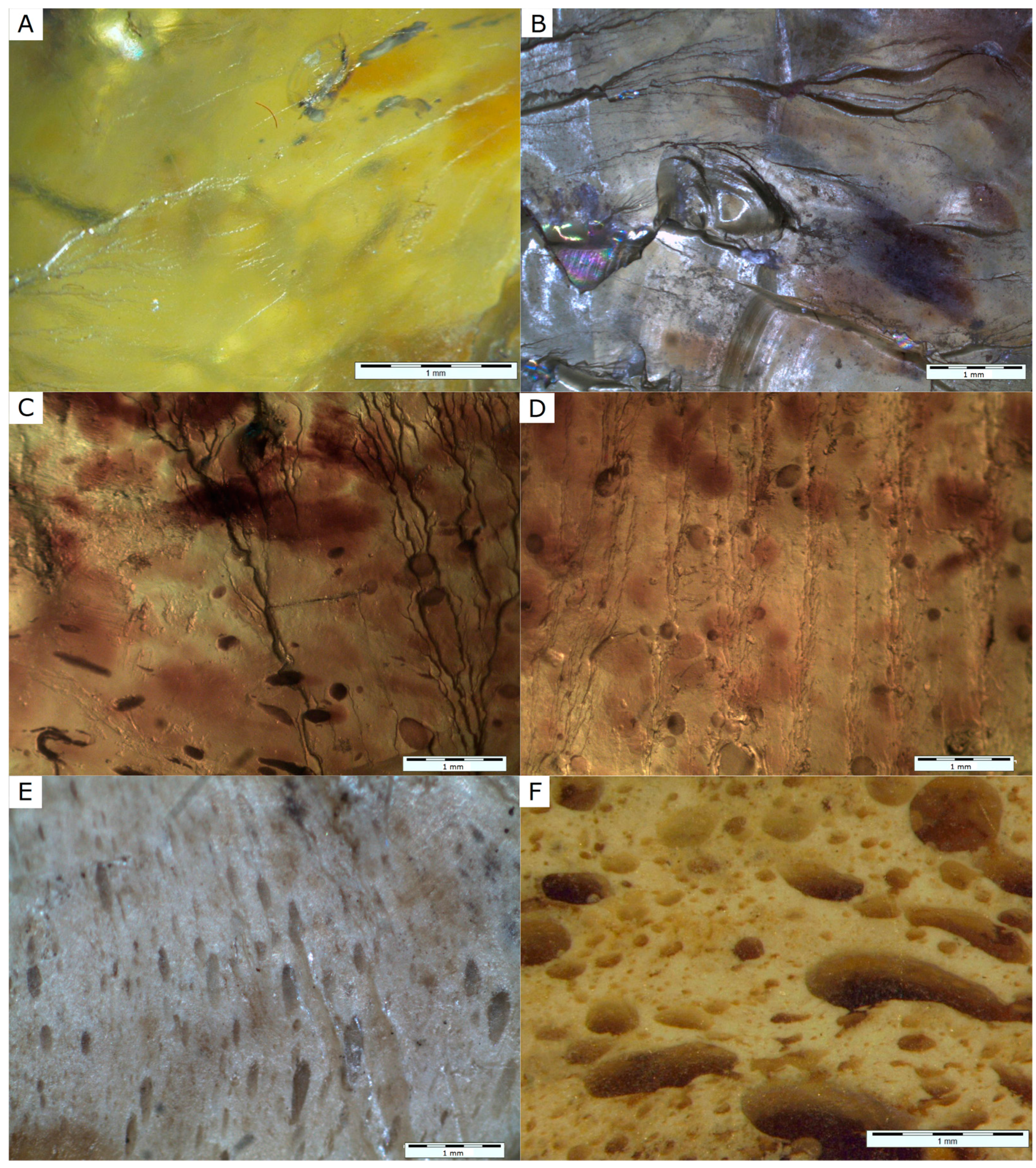
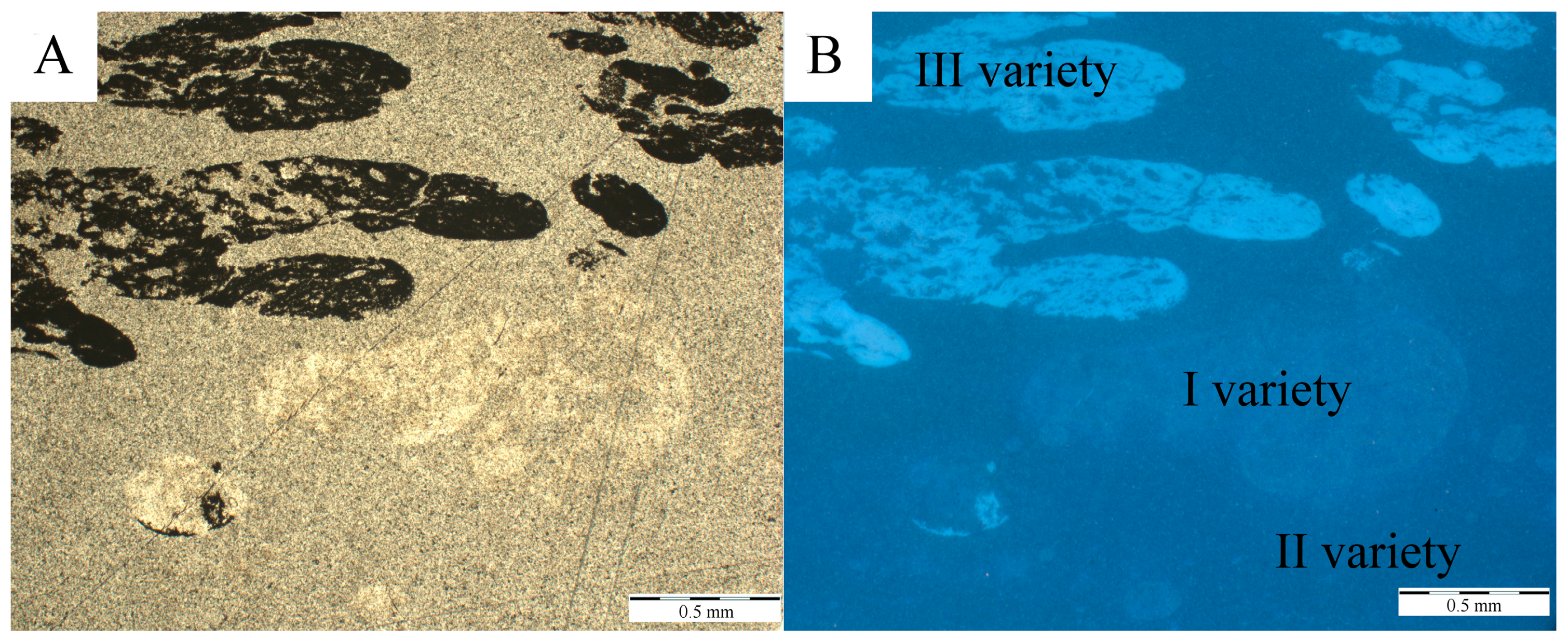
4.1. Physical Investigations
4.2. Spectroscopic Studies
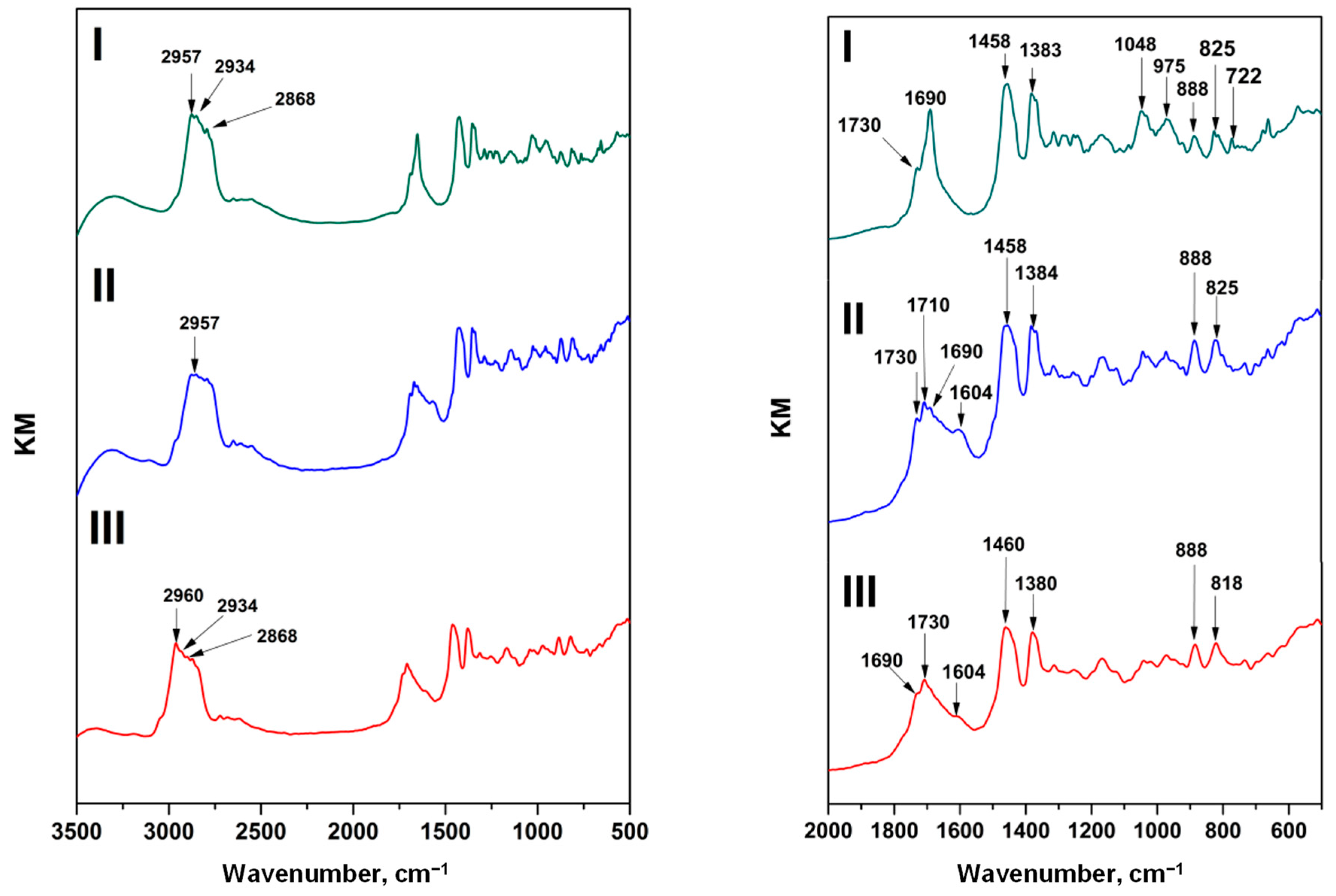
4.2.1. FT-IR Analysis
4.2.2. RS Analysis
5. Discussion
6. Conclusions
- Fossil resin from the island of Sumatra (Jambi Province, Sarolangun mine) occurs in three varieties, which differ macroscopically in their colour and transparency.
- Resin specimens were formed from exudates from different times and locations, and thus they varied in clarity, inclusion content, maturation degree and fluorescence properties.
- Aromatization of the cyclohexane ring was found to be part of the fossilization mechanism. This is supported by consideration of the reducing environment of the swamp where the resins were deposited and buried.
- The maturation grade of fossil resins assessed via Raman spectroscopy was higher than expected for resins of Miocene age.
- Volcanism, which was previously documented as a factor determining the increased resin production during the Miocene epoch, was also responsible for the relatively high maturation grade of the fossil resins.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tomkeieff, S.I. Coals and Bitumens and Related Fossil Carbonaceous Substances: Nomenclature and Classification; Pergamon Press: Oxford, UK, 1954. [Google Scholar]
- Ragazzi, E.; Alexander, R.S. (Eds.) Amber. In Encyclopedia of Geobiology; Springer: Dordrecht, The Netherlands, 2011; pp. 24–36. [Google Scholar]
- Kosmowska-Ceranowicz, B.; Sachanbiński, M.; Łydżba-Kopczyńska, B. Analytical characterization of “Indonesian amber” deposits: Evidence of formation from volcanic activity. Baltica 2017, 30, 55–60. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B. Bursztyn Bałtycki i Inne Żywice Kopalne [Baltic Amber and Other Fossil Resins]; The Museum of the Earth of the Polish Academy of Sciences: Warsaw, Poland, 1993; 141p. (In Polish) [Google Scholar]
- Jehlička, J.; Villar, S.E.J.; Edwards, H.G.M. Fourier transform Raman spectra of Czech and Moravian fossil resins from freshwater sediments. J. Raman Spectrosc. 2004, 35, 761–767. [Google Scholar] [CrossRef]
- Winkler, W.A. Raman spectroscopic approach to the maturation process of fossil resins. J. Raman Spectrosc. 2001, 32, 59–63. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B. Bursztyn z Borneo—największe na świecie złoże żywicy kopalnej (The greatest fossil resin deposit in the world). Prz. Geol. 1994, 7, 576–578, (In Polish with English Summary). [Google Scholar]
- Brackman, W.; Spaargaren, K.; Van Dongen, J.P.C.M.; Couperus, P.A.; Bakker, F. Origin and structure of the fossil resin from an Indonesian Miocene coal. Geochim. Cosmochim. Acta 1984, 48, 2483–2487. [Google Scholar] [CrossRef]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.B.; Levy, A.J.; Santiago-Blay, J.A.; Wu, Y. Nuclear Magnetic Resonance Characterization of Indonesian Amber. Life: Excitement Biol. 2013, 1, 136–155. [Google Scholar] [CrossRef]
- Leelawatanasuk, T.; Wathanakul, P.; Paramita, S.; Sutthirat, C.; Sriprasert, B.; Bupparenoo, P. The characteristic of amber from Indonesia. Aust. Gemmol. 2013, 25, 142–145. [Google Scholar]
- Schmidt, A.R.; Jancke, S.; Lindquist, E.E.; Ragazzi, E.; Roghi, G.; Nascimbene, P.C.; Schmidt, K.; Wappler, T.; Grimaldi, D.A. Arthropods in amber from the Triassic Period. Proc. Nat. Acad. Sci. 2012, 109, 14796–14801. [Google Scholar] [CrossRef] [PubMed]
- Saint Martin, S.; Saint Martin, J.P.; Girard, V.; Grosheny, D.; Néraudeau, D. Filamentous micro-organisms in Upper Cretaceous amber (Martigues, France). Cretac. Res. 2012, 35, 217–229. [Google Scholar] [CrossRef]
- Kosmowska-Ceranowicz, B.; Vávra, N. Infrared Spectra of Fossil Resins, Subfossil Resins and Selected Imitation of Amber; Polish Academy of Science Museum of Earth: Warsaw, Poland, 2015. [Google Scholar]
- Kosmowska-Ceranowicz, B. Glessite from Malesia and Indonesia. In Bursztyn w Polsce i na Świecie (Amber in Poland and in the World), 2nd ed.; Wydawnictwo Uniwersytetu Warszawskiego: Warsaw, Poland, 2017; pp. 1–310. (In Polish) [Google Scholar]
- Krumbiegiel, G.; Kosmowska-Ceranowicz, B. Vorkommen von Glessit, Siegburgit (?) und Krantzit in Tertiär Mitteldeutschlands (Bitterfeld, Niederlausitz). Fundgrube 1990, 26, 78–81. (In German) [Google Scholar]
- Krumbiegiel, G. Glessit, ein tertiäres Harz von Bedecktsamern. Fossilien 1993, 10, 83–90. (In German) [Google Scholar]
- Kosmowska-Ceranowicz, B.; Krumbiegel, G.; Vávra, N. Glessit, ein tertiäres Harz von Angiospermen der Familie Burseraceae. N. Jb. Geol. Paläont. Abh. 1993, 187, 299–324. (In German) [Google Scholar]
- Lambert, J.B.; Johnson, S.C.; Shawl, C.E.; Poinar, G.O. Fossil resin from Asia. Anc. Biomol. 1999, 3, 29–35. [Google Scholar]
- Adiwidjaja, P.; De Coster, G.L. Pre-Tertiary paleotopography and related sedimentation in South Sumatra. In 2nd Annual Convention Proceedings; Indonesian Petroleum Association: Jakarta, Indonesia, 1973; pp. 89–103. [Google Scholar]
- Young, R.; Atkinson, C.D. A review of Talang Akar Formation (Oligo-Miocene) reservoirs in the offshore areas of Southeast Sumatra and Northwest Java. In Clastic Rocks and Reservoirs of Indonesia: A Core Workshop; Indonesian Petroleum Association: Jakarta, Indonesia, 1993; pp. 177–210. [Google Scholar]
- Friederich, M.C.; Moore, T.A.; Flores, R.M. A regional review and new insights into SE Asian Cenozoic coal-bearing sediments: Why does Indonesia have such extensive coal deposits? Int. J. Coal Geol. 2016, 166, 2–35. [Google Scholar] [CrossRef]
- Barber, A.J.; Crow, M.J.; Milsom, J.S. (Eds.) Sumatra: Geology, Resources and Tectonic Evolution; Geological Society of London: London, UK, 2005; Memoirs No. 31. [Google Scholar]
- Guiliano, M.; Asia, L.; Onoratini, G.; Mille, G. Applications of diamond crystal ATR FTIR spectroscopy to the characterization of ambers. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Truică, G.I.; Teodor, E.D.; Litescu, S.C.; Radu, G.L. LC-MS and FT-IR characterization of amber artefacts. Cent. Eur. J. Chem. 2012, 10, 1882–1889. [Google Scholar]
- Beck, C.; Wilbur, E.; Meret, S.; Kossove, D.; Kermani, K. The infrared spectra of amber and the identification of Baltic amber. Archaeometry 1965, 8, 96–109. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2000. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Farwell, D.W. Fourier transform-Raman spectroscopy of amber. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1996, 52, 1119–1125. [Google Scholar] [CrossRef]
- Moreno, Y.M.; Christensen, D.H.; Nielsen, O.F. An NIR-FT Raman spectroscopic study of amber. Asian J. Spectrosc. 2000, 4, 49–56. [Google Scholar]
- Brody, R.H.; Edwards, H.G.M.; Pollard, A.M. A study of amber and copal samples using FT-Raman spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2001, 57, 1325–1338. [Google Scholar] [CrossRef]
- Adar, F. Introduction to Interpretation of Raman Spectra Using Database Searching and Functional Group Detection and Identification. Spectroscopy 2016, 31, 16–23. [Google Scholar]
- Bąk, M.; Natkaniec-Nowak, L.; Drzewicz, P.; Czapla, D.; Bogdasarov, М. Ambrosia-like fungi in fossil resin from Jambi Province in Sumatra Island—Possible phoretic organisms interacted with invaded insects. In Proceedings of the 17th Czech-Slovak-Polish Palaeontological Conference, Krakow, Poland, 20–21 October 2016; Abstract Volume 23. [Google Scholar]
- Natkaniec-Nowak, L.; Drzewicz, P.; Bąk, M.; Matusik, J.; Kowalczyk, J.; Bogdasarov, M.; Ivanina, A.V. Fossil resins from Jambi province (Sumatra, Indonesia)—Proxy indicators of palaeonvironment and palaeoclimate of Sundaland. In Proceedings of the Third International Conference Viet-Pol, Hanoi, Vietnam, 12–15 November 2016; pp. 230–231. [Google Scholar]
- Matuszewska, A.; Gołąb, A. Próba wykorzystania parametru mikrotwardości żywic kopalnych i sztucznych, jako cechy klasyfikacyjnej (An attempt at using the parameter of the microhardness of fossil and artificial resins as a classification feature). Bursztynisko 2008, 31, 56–61. [Google Scholar]
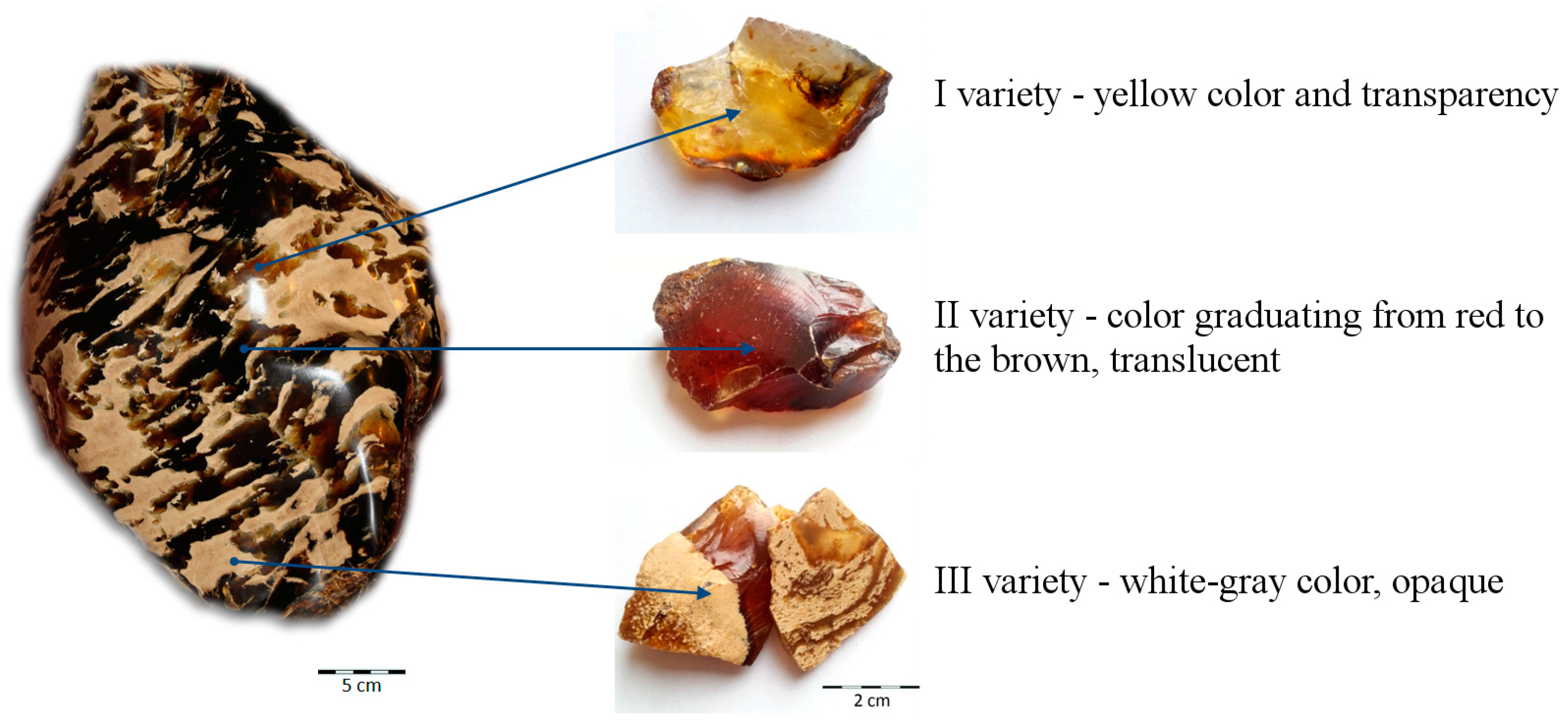




| Physical Property | Variety I | Variety II | Variety III |
|---|---|---|---|
| Color | yellow | red to brown | white-gray |
| Transparency | transparent | translucent | opaque |
| Structure | homogenous | non-homogenous with single droplets | foamy-like with numerous droplets filled with resin of I variety |
| Inclusions | only locally—spherical plant microspores or fragments of channels of strongly macerated wood | dispersed pigment of fungus spores or pollen plants | coalified plant remains |
| Fluorescence | low | low | intensive |
| FT-IR Wave Numbers (cm−1) | Assignment | ||
|---|---|---|---|
| I Variety | II Variety | III Variety | |
| 825 | 825 | 825 | Aromatic ring C–H—1,4-Disubstitution (para) |
| 888 | 888 | 888 | CH ethylenic bending [24] |
| 975 | 975 | 975 | C–O bonds [24] |
| 1033 | 1028 | 1027 | C–O bonds [24]; cyclohexane ring vibrations (1000–1055 cm−1) [27] |
| 1048 | 1048 | 1048 | Cyclohexane ring vibrations (1000–1055 cm−1); primary alcohol, C–O stretching [27] |
| 1167 | 1173 | 1174 | C–O simple bond stretching of esters [26]; tertiary alcohol, C–O stretching [27] |
| 1199 | 1200 | 1200 | Phenol, C–O stretching [27] |
| 1255 | 1255 | 1255 | Primary or secondary, OH in-plane bending [27] |
| 1315 | 1315 | 1317 | Primary or secondary, OH in-plane bending [27] |
| - | 1332 | 1339 | Methane C–H bending (1330–1350 cm−1); primary or secondary, OH in-plane bending [27] |
| 1367 | 1367 | 1367 | Due to CH3 bending [24] |
| 1377 | 1378 | 1377 | Due to CH3 bending [24] |
| 1383 | 1384 | 1380 | Due to CH3 bending [24] |
| 1456 | 1455 | 1456 | Involves CH2 and CH3 bending [24] |
| 1458 | 1458 | 1460 | Involves CH2 and CH3 bending [24] |
| - | 1604 | 1614 | Conjugated C=C (1600 cm−1); aryl-substituted C=C (1625 cm−1); alkenyl C=C stretching (1620–1680 cm−1) [27] |
| 1650 | 1659 | 1649 | C=C double bond stretching [22]; alkenyl C=C stretching (1620–1680 cm−1) [27] |
| - | 1675 | 1674 | Alkenyl C=C stretch (1620–1680 cm−1) [27] |
| 1690 | 1690 | 1690 | Due to carbonyl group (C=O) stretching vibrations and, respectively, to carboxylic acid group [24] |
| - | 1710 | - | Due to carbonyl group (C=O) stretching vibrations and, respectively, to ketone and/or carboxylic acid group [24] |
| 1730 | 1729 | 1730 | Due to carbonyl group (C=O) stretching vibrations and, respectively, to ester [24] |
| 2868 | - | 2868 | Alkyl stretching and an important level of methyl groups [24] |
| 2934 | - | 2934 | Alkyl stretching; for the CH groups; an important level of methyl groups [24] |
| 2957 | 2957 | 2960 | Methyl C–H asymmetric/symmetric stretching [27]; corresponding to the alkyl stretching; for the methyl groups; an important level of methyl groups [24] |
| 3410 | 3404 | 3410 | OH stretching of alcohols and/or carboxylic acids [24] |
| - | - | 3544 | Hydroxy group, H-bonded OH stretch; internally bonded OH stretching [27] |
| - | - | 3566 | Hydroxy group, H-bonded OH stretch; internally bonded OH stretching [27] |
| Raman Shift (cm−1) | Proposed Assignment | ||
|---|---|---|---|
| Variety I | Variety II | Variety III | |
| - | 600 | - | (?) C–C modes of the alicyclic rings [30] |
| 680 | 680 | 680 | aromatic out-of-plane deformation [29] |
| 730 | 730 | 730 | aromatic out-of-plane deformation; bands related to the degree of maturation (600–800 cm−1) [5] |
| 800 | 800 | 800 | C–C functional group—highly mixed in complex molecule (700–1260 cm−1) [31], bands related to the degree of maturation (600–800 cm−1) [5] |
| 1360 | 1366 | 1360 | CH2 deformation [28] |
| 1315 | 1315 | 1315 | CH3 umbrella mode (~1375 cm−1) [28]; δCH2, δCH3 [30] |
| 1450 | 1450 | 1450 | an asymmetric deformation of CH3 and CH2 groups [6,31] |
| 1658 | 1658 | 1658 | C=C bonds (unsaturated hydrocarbons) [6,31] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglik, B.; Kosmowska-Ceranowicz, B.; Natkaniec-Nowak, L.; Drzewicz, P.; Dumańska-Słowik, M.; Matusik, J.; Wagner, M.; Milovsky, R.; Stach, P.; Szyszka, A. Fossilization History of Fossil Resin from Jambi Province (Sumatra, Indonesia) Based on Physico-Chemical Studies. Minerals 2018, 8, 95. https://doi.org/10.3390/min8030095
Naglik B, Kosmowska-Ceranowicz B, Natkaniec-Nowak L, Drzewicz P, Dumańska-Słowik M, Matusik J, Wagner M, Milovsky R, Stach P, Szyszka A. Fossilization History of Fossil Resin from Jambi Province (Sumatra, Indonesia) Based on Physico-Chemical Studies. Minerals. 2018; 8(3):95. https://doi.org/10.3390/min8030095
Chicago/Turabian StyleNaglik, Beata, Barbara Kosmowska-Ceranowicz, Lucyna Natkaniec-Nowak, Przemysław Drzewicz, Magdalena Dumańska-Słowik, Jakub Matusik, Marian Wagner, Rastislav Milovsky, Paweł Stach, and Arkadiusz Szyszka. 2018. "Fossilization History of Fossil Resin from Jambi Province (Sumatra, Indonesia) Based on Physico-Chemical Studies" Minerals 8, no. 3: 95. https://doi.org/10.3390/min8030095





