Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece)
Abstract
:1. Introduction
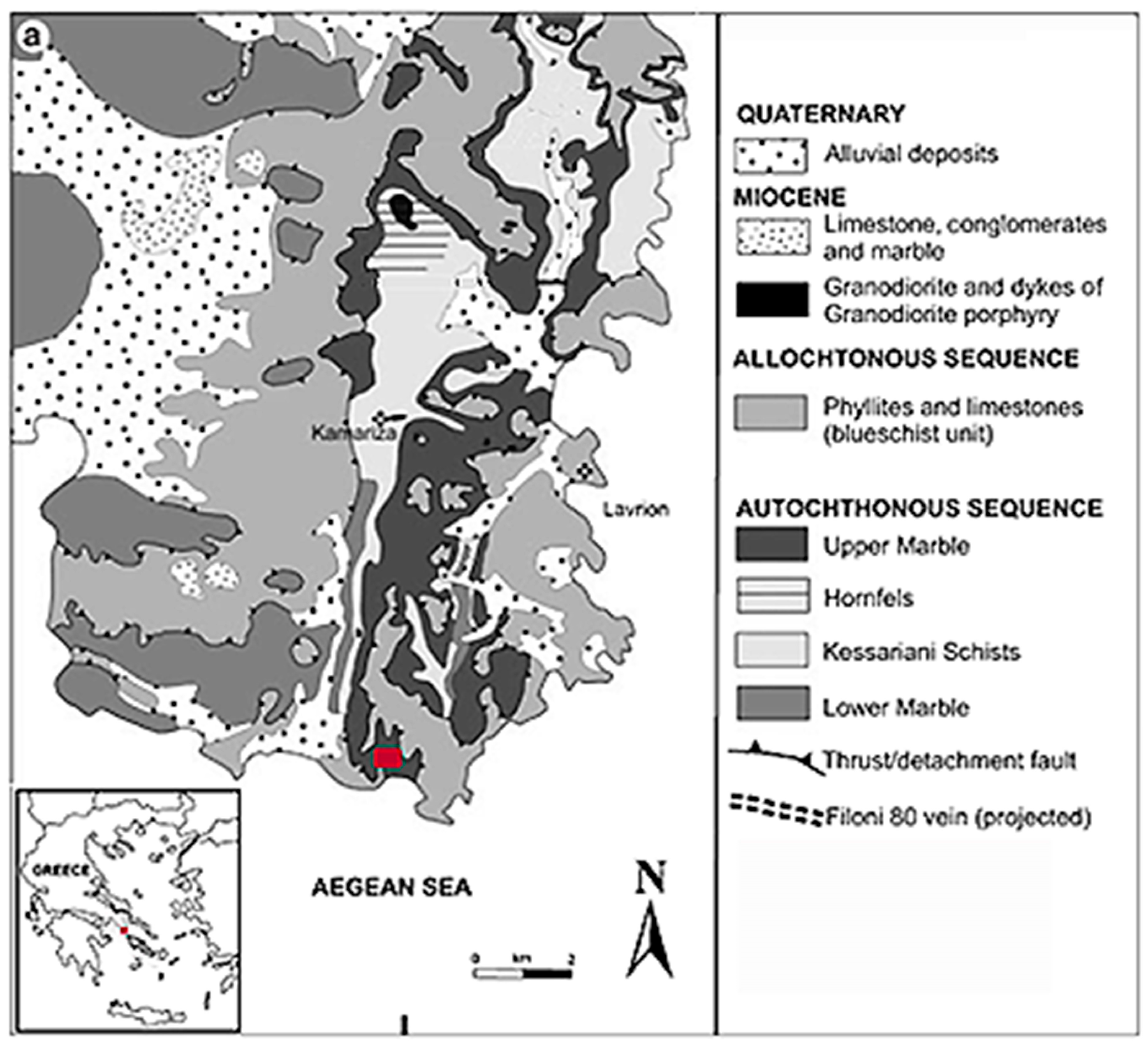
2. Geological Outline
3. Materials and Methods
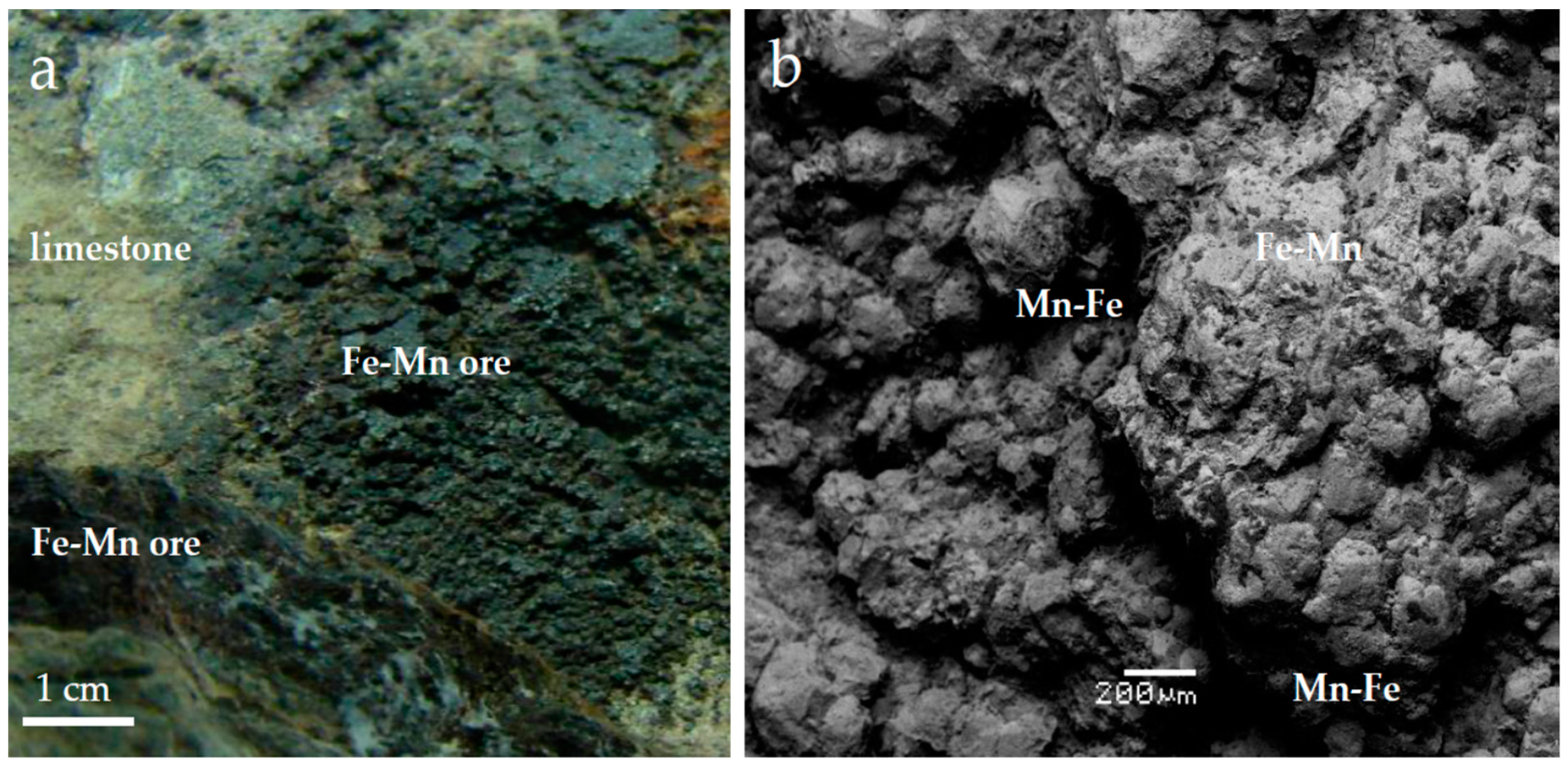
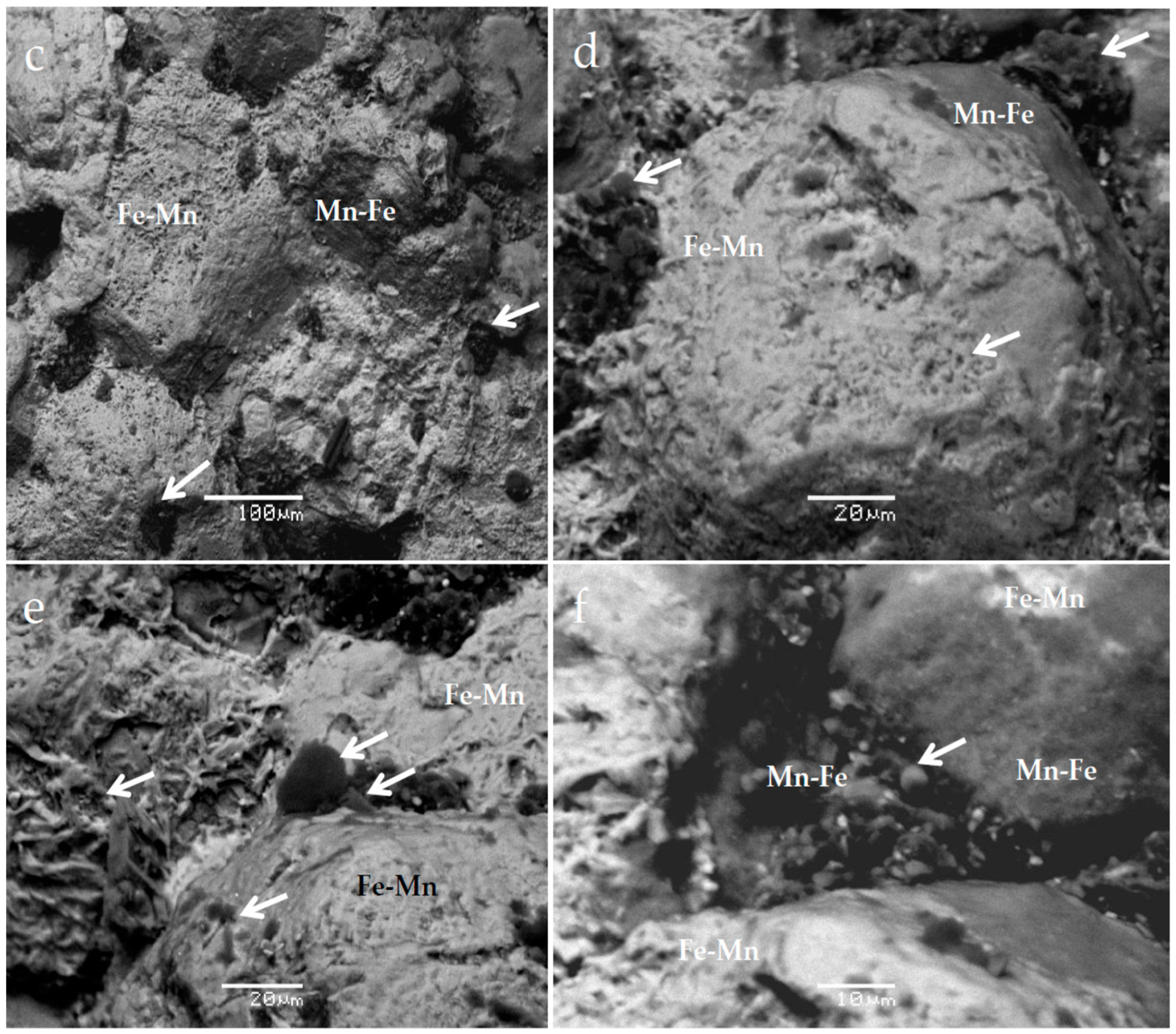
4. Results
4.1. Mineralogical Characteristics
4.2. Geochemical Characteristics
5. Discussion
5.1. The Potential Role of Microorganisms in the Redox Reactions
5.2. Multstage Fe-Mn Mineralization
6. Conclusions
- The occurrence of abundant bacteriomophic Fe-Mn-oxides/hydroxides on Fe-Mn-mineralization from the Legrena valley indicates the catalytic role of microorganisms to the redox reactions during ore formation.
- The presence of microorganisms in Fe-Mn-hydroxides, combined with their chemical features (Na, Cl, K, P, S, Ca, and As), in particular the relatively high Na and Cl contents, may point to a saline environment.
- The relatively high background and broad peak shape in the X-ray powder diffraction (XRD) patterns of Fe-Mn samples suggest a low grade crystallinity of the major mineral phases.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Marinos, G.P.; Petrascheck, W.E. Laurium. Geol. Geophys. Res. 1956, 4, 1–247. [Google Scholar]
- Skarpelis, N. The Lavrion deposit (SE Attica, Greece): Geology, mineralogy and minor elements chemistry. Neues Jahrb. Mineral. Abh. 2007, 183, 227–249. [Google Scholar] [CrossRef]
- Voudouris, P.; Economou-Eliopoulos, M. Mineralogy and chemistry of Cu-rich ores from the Kamariza carbonate hosted deposit (Lavrion), Greece. In Mineral Exploration and Sustainable Development; Eliopoulos, D., Ed.; Millpress: Rotterdam, The Netherlands, 2003; pp. 499–502. [Google Scholar]
- Bonsall, T.A.; Spry, P.G.; Voudouris, P.; St Seymour, K.; Tombros, S.; Melfos, V. Fluid inclusion and stable isotope characteristics of carbonate replacement Pb-Zn-Ag deposits in the Lavrion district, Greece. In Mineral Exploration and Research: Digging Deeper; Irish Association for Economic Geology: Dublin, Ireland, 2007; pp. 283–286. [Google Scholar]
- Skarpelis, N.; Ardyraki, A. Geology and origin of supergene ore at the Lavrion Pb-Ag-Zn deposit, Attica, Greece. Resour. Geol. 2008, 59, 1–14. [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, V.; Spry, P.; Bonsall, T.; Tarkian, M.; Economou-Eliopoulos, M. Mineralogical and fluid inclusion constraints on the evolution of the Plaka intrusion-related ore system, Lavrion, Greece. Miner. Petrol. 2008, 93, 79–110. [Google Scholar] [CrossRef]
- Berger, A.; Schneider, D.; Grasemann, B.; Stockli, D. Footwall mineralization during Late Miocene extension along the West Cycladic Detachment System, Lavrion, Greece. Terra Nova 2012, 25, 181–191. [Google Scholar] [CrossRef]
- Dando, P.; Aliani, S.; Arab, H.; Bianchi, C.; Brehmer, M.; Cocito, S.; Fowlers, S.W.; Gundersen, J.; Hooper, L.E.; Kölbh, R.; et al. Hydrothermal studies in the Aegean Sea. Phys. Chem. Earth B Hydrol. Oceans Atmos. 2000, 25, 1–8. [Google Scholar] [CrossRef]
- Kampouroglou, E.; Economou-Eliopoulos, M. Natural contamination by as and heavy metals in soil, their bio-accumulation and potential sources: The case of a travertine limestone quarry, Greece. Cent. Eur. J. Geosci. 2013, 5, 174–188. [Google Scholar] [CrossRef]
- Kampouroglou, E.; Economou-Eliopoulos, M. Assessment of the environmental impact by as and heavy metals in lacustrine travertine limestone and soil in Attica, Greece: Mapping of potentially contaminated sites. Catena 2016, 139, 137–166. [Google Scholar] [CrossRef]
- Stouraiti, C.; Lekkas, S.; Lozios, S.; Kanellopoulos, C. Iron-oxide mineralization of Sesi, Koropi (S. Hymittos, Greece): Mineralization within a detachment zone. Bull. Geol. Soc. Greece 2016, 50, 2025–2036. [Google Scholar] [CrossRef]
- Conofagos, K. The Ancient Laurium and the Greek Technique for Silver Production; Ekdotiki Athinon: Athens, Greece, 1980; 458p. [Google Scholar]
- Papanikolaou, D.J.; Syskakis, D. Geometry of acid intrusives in Plaka, Laurium and relation between magmatism and deformation. Bull. Geol. Soc. Greece 1991, 25, 355–368. [Google Scholar]
- Photiades, A.; Carras, N. Stratigraphy and geological structure of the Lavrion area (Attica, Greece). Bull. Geol. Soc. Greece 2001, 34, 103–109. [Google Scholar]
- Marakis, G. Remaks on the age of sulfide mineralization in Cyclades area. Ann. Geol. Pays. Hell. 1968, 19, 695–700. [Google Scholar]
- Altherr, R.; Kreuzer, H.; Wendt, I.; Lenz, H.; Wagner, G.A.; Keller, J.; Harre, W.; Hohndorf, A. A late Oligocene/early Miocene high temperature belt in the Attic-Cycladic crystalline complex (SE Pelagonian, Greece). Geol. Jahrb. 1982, E23, 97–164. [Google Scholar]
- Pe-Piper, G.; Piper, D. The Igneous Rocks of Greece: Anatomy of an Orogen, 1st ed.; Beiträge zur Regionalen Geologie der Erde: Stuttgart, Germany, 2002; 588p, ISBN 978-3-443-11030-7. [Google Scholar]
- Tsikouras, B.; Karipi, S.; Grammatikopoulos, T.A.; Hatzipanagiotou, K. Listwaenite evolution in the ophiolite mélange of Iti mountain (Continental Central Greece). Eur. J. Mineral. 2006, 18, 243–255. [Google Scholar] [CrossRef]
- Skarpelis, N.; Tsikouris, B.; Pe-Piper, G. The Miocene igneous rocks in the Basal Unit of Lavrion (SE Attica, Greece): Petrology and geodynamic implications. Geol. Mag. 2007, 145, 1–15. [Google Scholar] [CrossRef]
- Skarpelis, N. Geodynamics and evolution of the Miocene mineralization in the Cycladic-Pelagonian Belt, Hellenides. Bull. Geol. Soc. Greece 2002, 34, 2191–2209. [Google Scholar]
- Economou, M.; Skounakis, S.; Papathanasiou, C. Magnetite deposits of skarn type from the Plaka area of Laurium, Greece. Chem. Erde 1981, 40, 241–252. [Google Scholar]
- Gelaude, P.; van Kalmthout, P.; Rewitzer, C. Laurion: The Minerals in the Ancient Slags; Janssen Print: Nijmegen, The Netherlands, 1996; 195p. [Google Scholar]
- Dando, P.R.; Thomm, M.; Arab, H.; Brehmer, M.; Hooper, L.; Jochimsen, B.; Schlesner, H.; Stohr, R.; Miquel, J.-C.; Fowler, S. Microbiology of shallow hydrothermal sites off Palaeochori Bay, Milos (Hellenic Volcanic Arc). Cah. Biol. Mar. 1999, 39, 369–372. [Google Scholar]
- Hein, J.R.; Stamatakis, G.M.; Dowling, S.J. Trace metal-rich Quaternary hydrothermal manganese oxide and barite deposit, Milos Island, Greece. Appl. Earth Sci. 2000, 109, 67–76. [Google Scholar] [CrossRef]
- Hein, J.R.; Schulz, M.S.; Dunham, R.E.; Stern, R.J.; Bloomer, S.H. Diffuse flow hydrothermal manganese mineralization along the active Mariana and southern Izu-Bonin arc system, western Pacific. J. Geophys. Res. 2008, 113, B08S14. [Google Scholar] [CrossRef]
- Kilias, S.P.; Naden, J.; Cheliotis, I.; Shepherd, T.J.; Constandinidou, H.; Crossing, J.; Simos, I. Epithermal gold mineralization in the active Aegean volcanic arc: The Profitis Ilias deposit, Milos Island, Greece. Miner. Deposita 2001, 36, 32–44. [Google Scholar] [CrossRef]
- Alfieris, D.; Voudouris, P.; Spry, P.G. Shallow submarine epithermal Pb-Zn-Cu-Au-Ag-Te mineralization on western Milos Island, Aegean Volcanic Arc, Greece: Mineralogical, geological and geochemical constraints. Ore Geol. Rev. 2013, 53, 159–180. [Google Scholar] [CrossRef]
- Petersen, S.; Monecke, T.; Westhues, A.; Hannington, M.D.; Gemmell, J.B.; Sharpe, R.; Peters, M.; Strauss, H.; Lackschewitz, K.; Augustin, N.; et al. Drilling shallow-water massive sulfides at the Palinuro Volcanic Complex, Aeolian Island Arc, Italy. Econ. Geol. 2014, 109, 2129–2158. [Google Scholar] [CrossRef]
- Giovannelli, D.; d’ Errico, G.; Manini, E.; Yakimov, M.; Vetriani, C. Diversity and phylogenetic analyses of bacteria from a shallow-water hydrothermal vent in Milos island (Greece). Front. Microbiol. 2013, 4, 184. [Google Scholar] [CrossRef] [PubMed]
- Sievert, S.M.; Brinkhoff, T.; Muyzer, G.; Ziebis, W.; Kuever, J. Spatial heterogeneity of bacterial populations along an environmental gradient at a shallow submarine hydrothermal vent near Milos Island (Greece). Appl. Environ. Microbiol. 1999, 65, 3834–3842. [Google Scholar] [PubMed]
- Chi Fru, E.; Ivarsson, M.; Kilias, S.P.; Bengtson, S.; Belivanova, V.; Marone, F.; Fortin, D.; Broman, C.; Stampanoni, M. Fossilized iron bacteria reveal a pathway to the biological origin of banded iron formation. Nat. Commun. 2013, 4, 2050. [Google Scholar] [CrossRef] [PubMed]
- Post, F.J. The microbial ecology of the Great Salt Lake. Microb. Ecol. 1977, 3, 143–165. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, L.E. Geomicrobiology, 4th ed.; Marcel Decker: New York, NY, USA, 2002; 768p, ISBN 0-8247-0764-8. [Google Scholar]
- Navrotsky, A.; Mazeina, L.; Majzslan, J. Size driven structural and thermodynamic complexity in iron oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Lowenstam, A.H.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; 336p, ISBN 0-19-504977-2. [Google Scholar]
- Bazylinski, D.A.; Schübbe, S. Controlled biomineralization by and applications of magnetotactic bacteria. Adv. Appl. Microbiol. 2007, 62, 21–62. [Google Scholar] [CrossRef] [PubMed]
- Haferburg, G.; Kothe, E. Microbes and metals: Interactions in the environment. J. Basic Microbiol. 2007, 47, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Heim, C. An Integrated Approach to the Study of Biosignatures in Mineralizing Biofilms and Microbial Mats. Ph.D. Thesis, Georg-August-Universität Göttingen, Göttingen, Germany, 2010. [Google Scholar]
- Konhauser, K. Bacterial iron biomineralization in nature. FEMS Microbiol. Rev. 1997, 20, 315–326. [Google Scholar] [CrossRef]
- Pósfai, M.; Buseck, P.R.; Bazylinski, D.A.; Frankel, R.B. Reaction sequence of iron sulfide minerals in bacteria and their use as biomarkers. Science 1998, 280, 880–883. [Google Scholar] [PubMed]
- Pósfai, M.; Buseck, P.R.; Bazylinski, D.A.; Frankel, R.B. Iron sulfides from magnetotactic bacteria: Structure, compositions, and phase transitions. Am. Mineral. 1998, 83, 1469–1481. [Google Scholar] [CrossRef]
- Texier, A.C.; Andres, Y.; le Cloirec, P. Selective biosorption of lanthanide (La, Eu, Yb) ions by Pseudomonas aeruginosa. Environ. Sci. Technol. 1999, 33, 489–495. [Google Scholar] [CrossRef]
- Ferris, G.F.; Hallberg, O.R.; Lyvén, B.; Pedersen, K. Retention of strontium, cesium, lead and uranium by bacterial iron oxides from a subterranean environment. Appl. Geochem. 2000, 15, 1035–1042. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Bio-mineralization and potential biogeochemical processes in bauxite deposits: Genetic and ore quality significance. Miner. Petrol. 2013, 107, 471. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Tazaki, K. Biomineralization of layer silicates and hydrated Fe/Mn oxides in microbial mats: An electron microscopical study. Clays Clay Miner. 1997, 45, 203–212. [Google Scholar] [CrossRef]
- Tebo, B.M.; Bargar, J.R.; Clement, B.; Dick, G.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S. Manganese biooxides: Properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berlin, Germany, 1988; 176p. [Google Scholar]
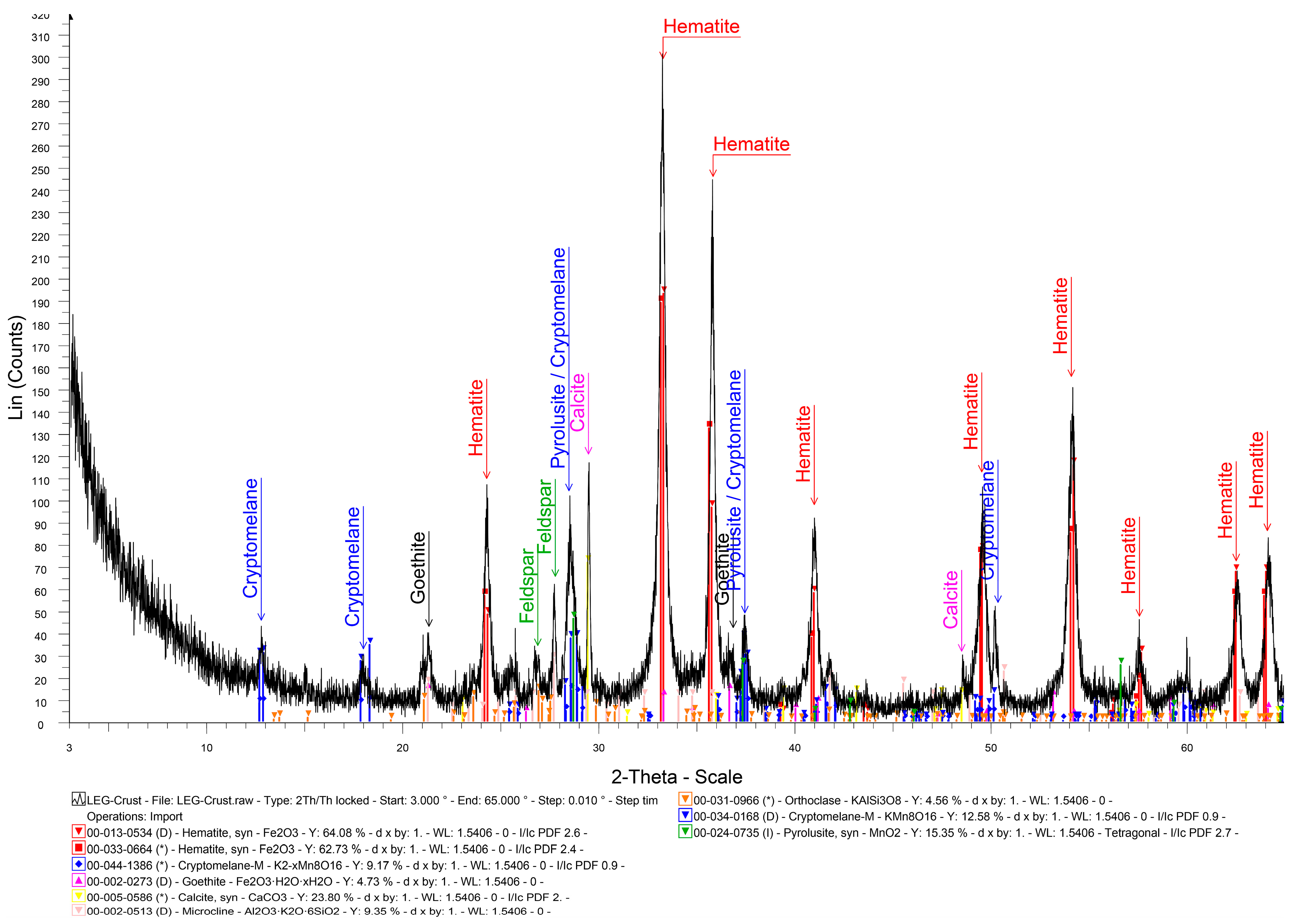
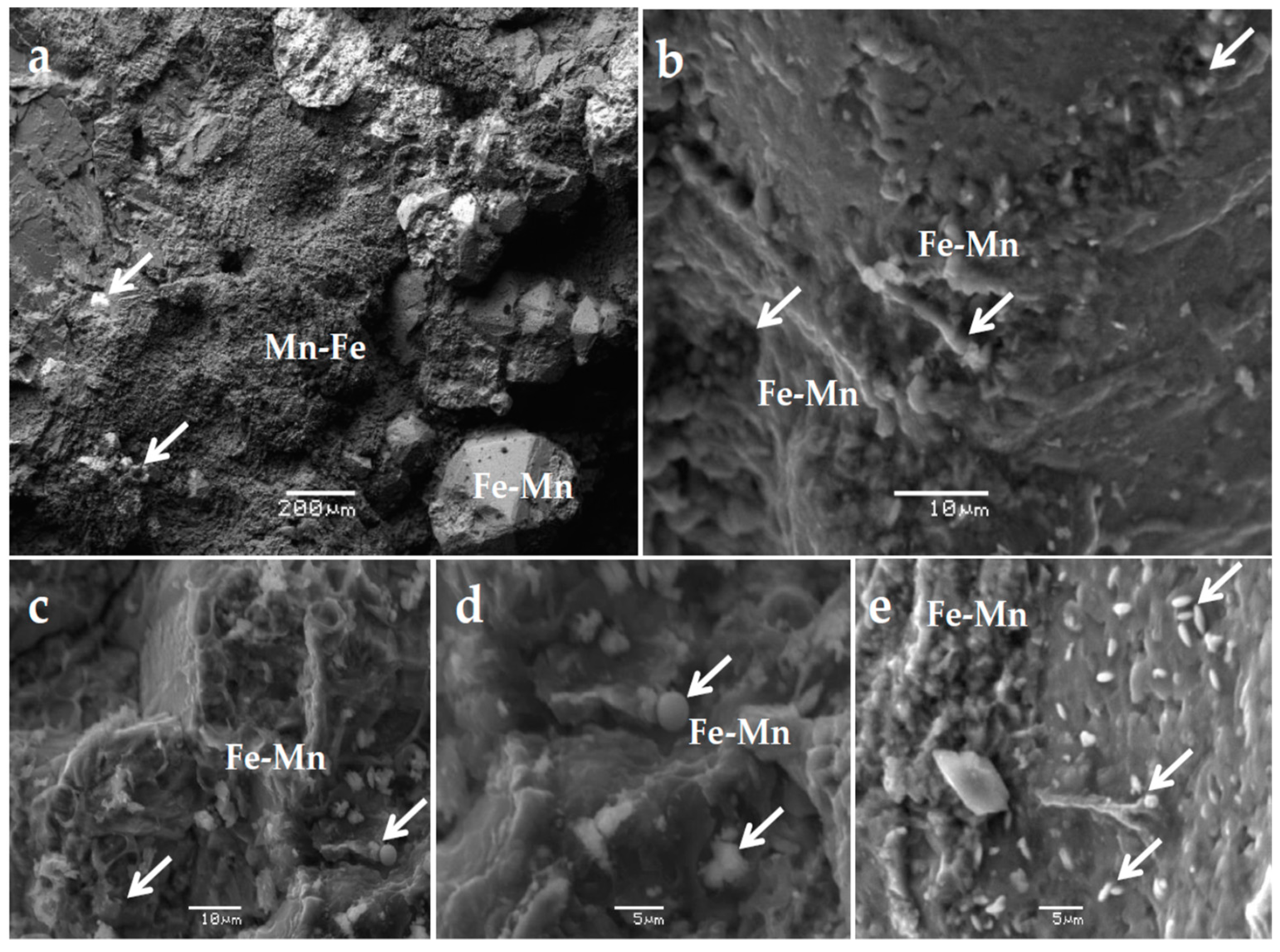
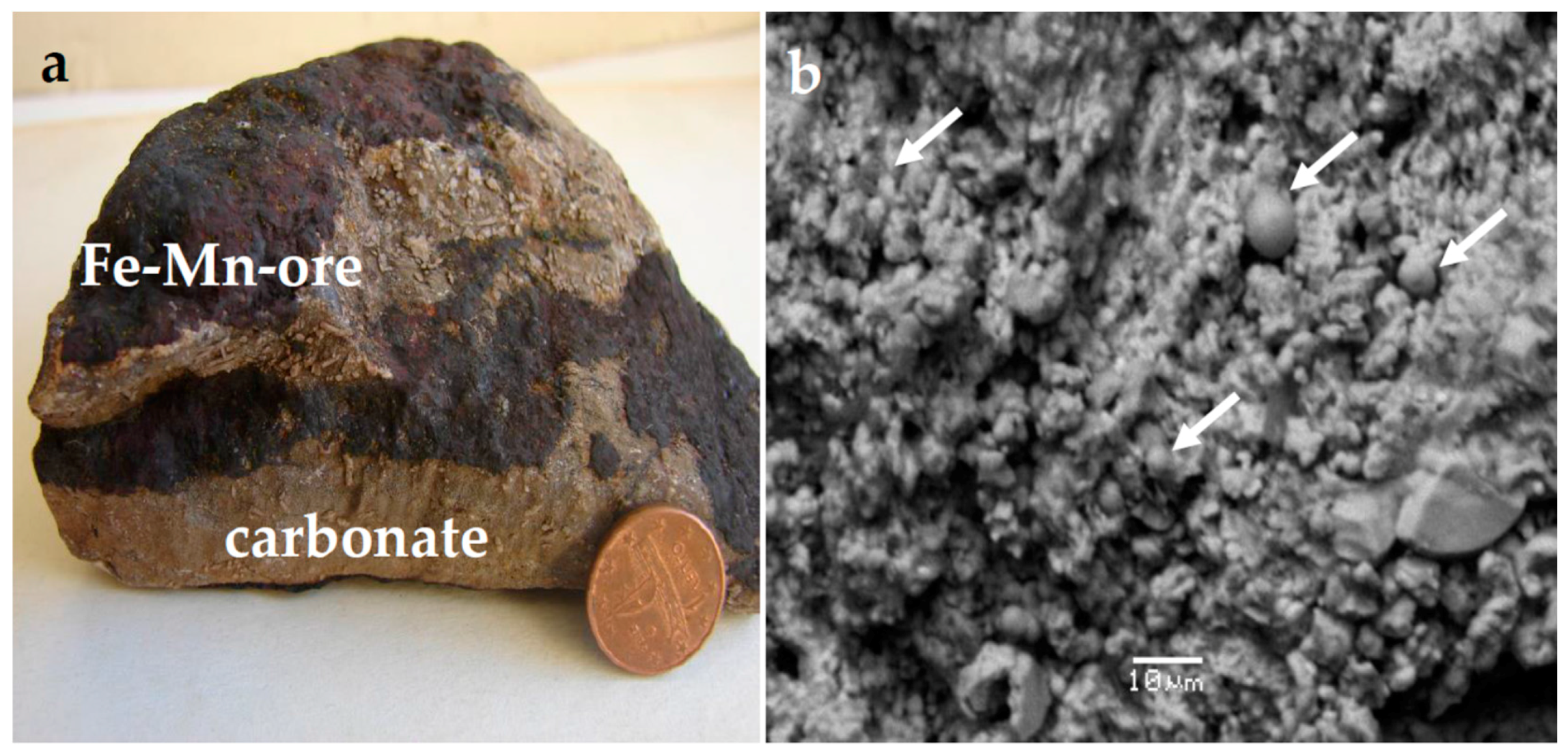
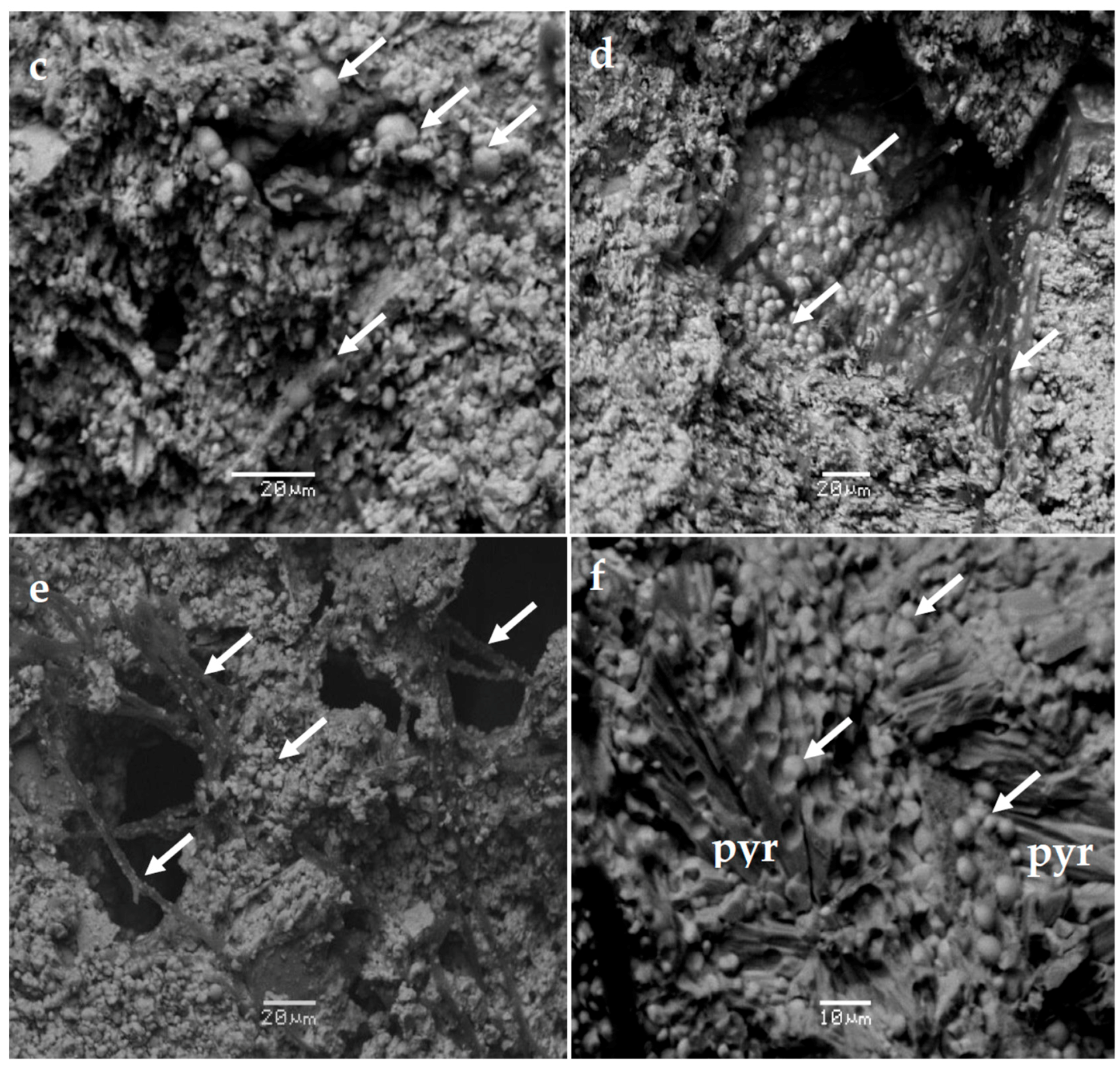
| Mineral | Massive Fe-Mn Ore | Fe-Mn Crust |
|---|---|---|
| Hematite | ++ | +++ |
| Goethite | ++ | ++ |
| Pyrolusite | ++ | ++ |
| Cryptomelane | + | ++ |
| Calcite | +++ | + |
| Feldspars | + | |
| Quartz | + |
| Oxidewt % | Small Nodules (Figure 3) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Goethite (FeOOH) | Hematite (Fe2O3) | Limonite FeO(OH)·nH2O | Figure 5c–f (Black-Spherical) | Matrix | ||||||||
| SiO2 | 5.2 | 2.4 | 6.1 | 2.2 | 3.9 | n.d. | 22.1 | 25.4 | 25.1 | 3.6 | 6.2 | 40.7 |
| Al2O3 | 3.9 | n.d. | 4.8 | n.d. | n.d. | 7.6 | 12.1 | 16.3 | 15.1 | 2.1 | 3.1 | 25.6 |
| Fe2O3 | 73.8 | 85.1 | 74.8 | 96.8 | 92.6 | 59.2 | 39.8 | 34.8 | 24.3 | 9.7 | 37 | 19.4 |
| TiO2 | n.d. * | n.d. | n.d. | n.d. | n.d. | n.d. | 1.4 | 0.4 | 0.5 | n.d. | 0.4 | 0.5 |
| MnO | 0.6 | 0.9 | n.d. | n.d. | n.d. | 0.6 | 5.9 | n.d. | n.d. | n.d. | n.d. | n.d. |
| MgO | n.d. | n.d. | n.d. | 0.6 | 1.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.5 |
| CaO | n.d. | n.d. | n.d. | n.d. | 0.5 | n.d. | 0.4 | 2.5 | 6.9 | 1.1 | 1.4 | 0.5 |
| K2O | n.d. | n.d. | n.d. | n.d. | n.d. | 0.4 | 3.4 | 1.2 | 2.3 | 9.8 | 4.5 | 2.1 |
| Na2O | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.5 | 3.3 | 10.9 | 7.1 | n.d. |
| SO4 | n.d. | n.d. | n.d. | n.d. | 0.8 | 0.7 | 0.8 | 5.3 | 6.1 | 1.6 | n.d. | n.d. |
| As2O3 | n.d. | n.d. | 2.8 | n.d. | n.d. | n.d. | 2.2 | 4.9 | 3.2 | 2.1 | 1.4 | n.d. |
| P2O5 | n.d. | n.d. | n.d. | n.d. | 1.1 | n.d. | n.d. | 3.7 | 0.8 | 1.8 | n.d. | 1.3 |
| Cl | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 1.4 | 8.6 | 4.1 | n.d. |
| Total | 83.5 | 88.4 | 88.5 | 99.6 | 101 | 68.5 | 88.1 | 98.0 | 89.0 | 42.7 | 62.0 | 93.6 |
| Oxidewt % | Figure 5b–d (Spherical) | Figure 3(Fillaments) | Figure 3f (Pyrolusite, MnO2) | |||||||||
| SiO2 | 6.6 | 5.7 | 49.2 | 3.9 | 0.2 | 1.2 | 1.4 | 1.6 | 9.9 | 1.2 | n.d. | n.d. |
| Al2O3 | 2.9 | 3.1 | 18.9 | 2.1 | n.d. | 0.5 | n.d. | n.d. | 6.1 | n.d. | n.d. | n.d. |
| Fe2O3 | 15.2 | 6.5 | 8.3 | 72.5 | 76.8 | 74.6 | 76.3 | 77.9 | 8.9 | 87.6 | 95 | 1.3 |
| TiO2 | n.d | n.d. | 0.8 | n.d. | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| MnO | 43.3 | 55 | 0.5 | 2.8 | 6.2 | 1.6 | 4.4 | 4.1 | 54.7 | 3.1 | 3.7 | 76.3 |
| MgO | 1.4 | 2.5 | 5.3 | 2.4 | n.d. | 0.9 | 1.3 | 0.6 | 4.2 | 0.9 | 0.5 | n.d. |
| CaO | n.d | 1.5 | 1.5 | n.d. | n.d. | 1.1 | n.d. | 0.4 | 2.1 | 0.3 | n.d. | n.d. |
| K2O | n.d | 1.3 | 3.1 | n.d. | n.d. | 1.8 | n.d. | n.d. | 1.3 | 0.4 | n.d. | n.d. |
| Na2O | 2.8 | 7.3 | n.d. | n.d. | n.d. | n.d | n.d. | 1.5 | 7.1 | n.d. | n.d. | n.d. |
| SO4 | 0.9 | 1.9 | n.d. | n.d. | n.d. | 3.2 | n.d. | n.d. | 2.3 | 1.4 | n.d. | n.d. |
| As2O3 | n.d | n.d. | n.d. | n.d. | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| P2O5 | n.d | n.d. | 1.4 | n.d. | n.d. | 1.1 | n.d. | n.d. | 0.8 | n.d. | n.d. | n.d. |
| Cl | n.d | n.d. | n.d. | n.d. | n.d. | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Total | 73.1 | 87.2 | 89.0 | 83.7 | 83.2 | 86.0 | 83.4 | 86.1 | 99.9 | 94.9 | 100.0 | 77.6 |
| Element (ppm) | L.B1 | L.B2 | L.B.3 | L.B4 | Detection Limit |
|---|---|---|---|---|---|
| Mo | 0.1 | ||||
| Cu | 9.2 | 2.5 | 16 | 21 | 0.5 |
| Ni | 46 | 1.8 | 164 | 121 | 0.5 |
| Co | 21 | 9 | 17 | 12 | 1 |
| Pb | 9 | 100 | 6.6 | 3.5 | 0.5 |
| Zn | 7 | 197 | 23 | 12 | 5 |
| Cr | 1 | 3 | 1 | 1.7 | 1 |
| Mn | 1220 | 4270 | 6320 | 6700 | 5 |
| As | 130 | 77 | 16 | 10 | 5 |
| Sr | 200 | 17 | 20 | 16 | 5 |
| Cd | 0.2 | 0.5 | <0.5 | 0.8 | 0.5 |
| Sb | 0.7 | 1.8 | 13 | 6.5 | 0.5 |
| V | <2 | <2 | <2 | 2 | 10 |
| La | 5 | 6 | 7 | 3 | 0.5 |
| Ba | 22 | 238 | 36 | 18 | 5 |
| W | 70 | 60 | 86 | 45 | 0.5 |
| Zr | <0.5 | <0.5 | <0.5 | <0.5 | 0.5 |
| Y | 16 | 18 | 20 | 20 | 0.5 |
| % | |||||
| Fe | 8.29 | 3.28 | 53.4 | 48.4 | 0.01 |
| Al | <0.001 | 0.03 | 0.3 | 0.02 | 0.01 |
| Ti | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 |
| Mg | 7.12 | 0.13 | 0.06 | 0.02 | 0.01 |
| Ca | 20.31 | 34.15 | 0.05 | 0.04 | 0.01 |
| P | 0.03 | 0.02 | 0.03 | 0.02 | 0.01 |
| Na | 0.01 | 0.02 | 0.01 | 0.005 | 0.01 |
| K | 0.01 | 0.01 | 0.16 | 0.03 | 0.01 |
| S | 0.05 | 0.06 | <0.05 | 0.09 | 0.05 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasilatos, C.; Economou-Eliopoulos, M. Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece). Minerals 2018, 8, 107. https://doi.org/10.3390/min8030107
Vasilatos C, Economou-Eliopoulos M. Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece). Minerals. 2018; 8(3):107. https://doi.org/10.3390/min8030107
Chicago/Turabian StyleVasilatos, Charalampos, and Maria Economou-Eliopoulos. 2018. "Fossilized Bacteria in Fe-Mn-Mineralization: Evidence from the Legrena Valley, W. Lavrion Mine (Greece)" Minerals 8, no. 3: 107. https://doi.org/10.3390/min8030107





