Rare Earth Elements (La, Ce, Pr, Nd, and Sm) from a Carbonatite Deposit: Mineralogical Characterization and Geochemical Behavior
Abstract
:1. Introduction
1.1. REE Background
1.2. Geochemical Behavior of REE
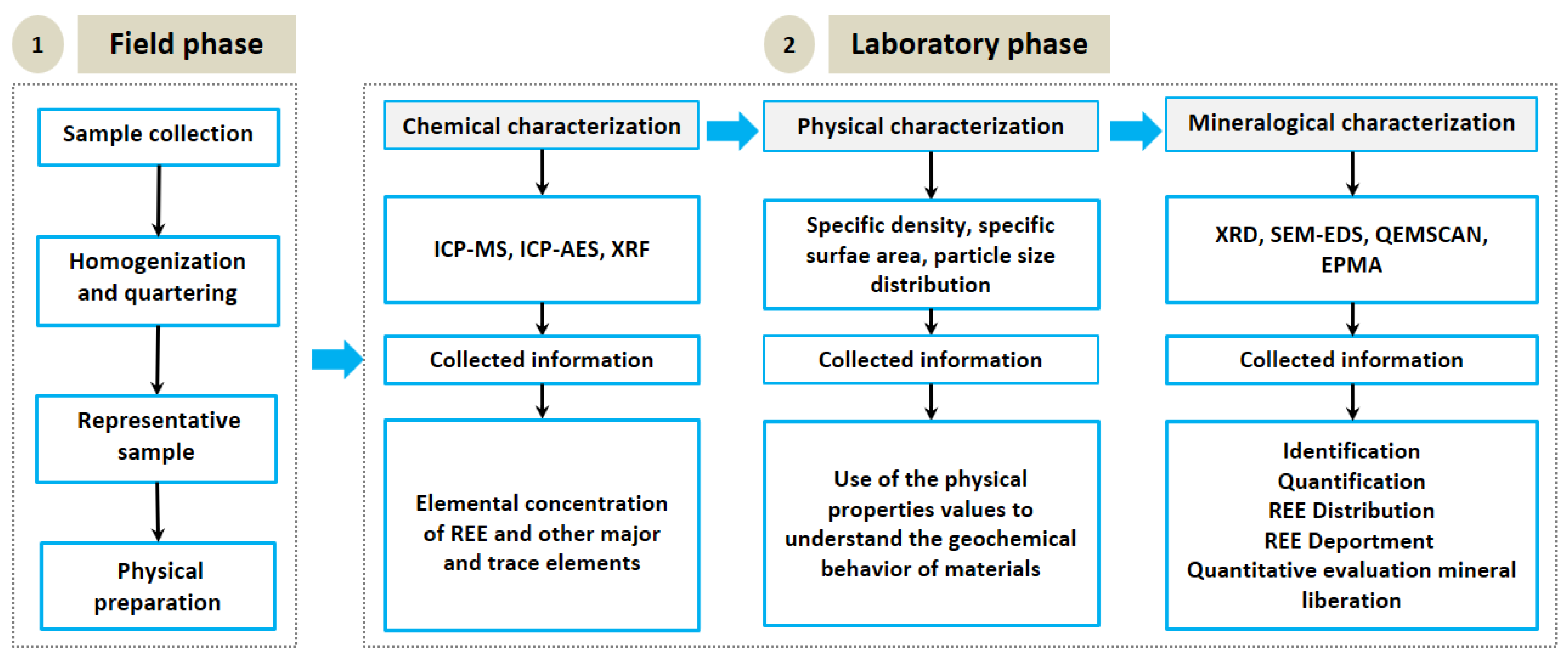
2. Materials and Methods
2.1. Location and Geological Setting of Montviel Deposit
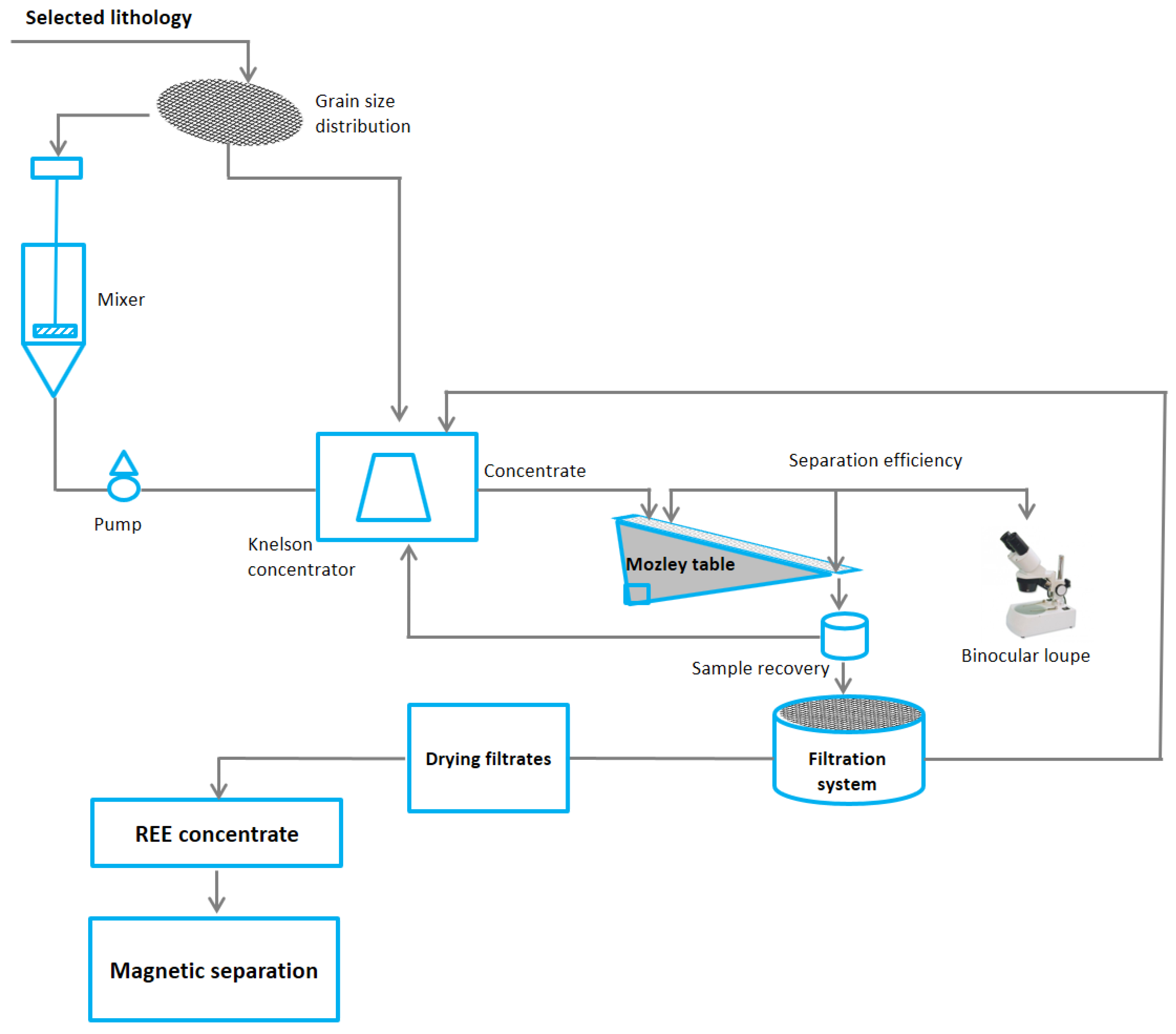
2.2. Sampling and Preparation
2.3. Physical and Chemical Analysis
2.4. Mineralogical Characterization
2.5. Weathering Cells
3. Results
3.1. Physical and Chemical Characteristics of the Three Montviel Lithologies and the REE Concentrate
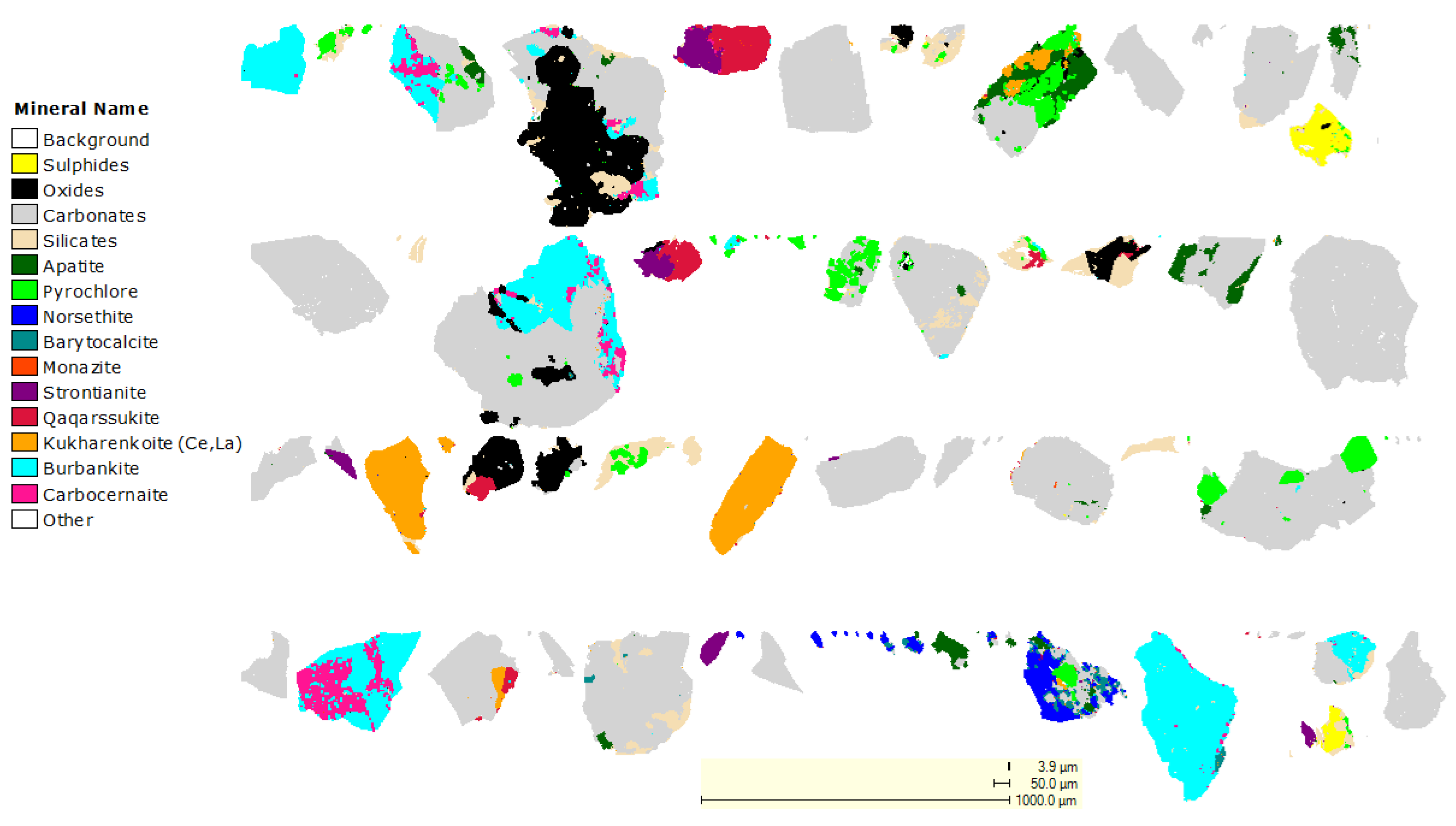
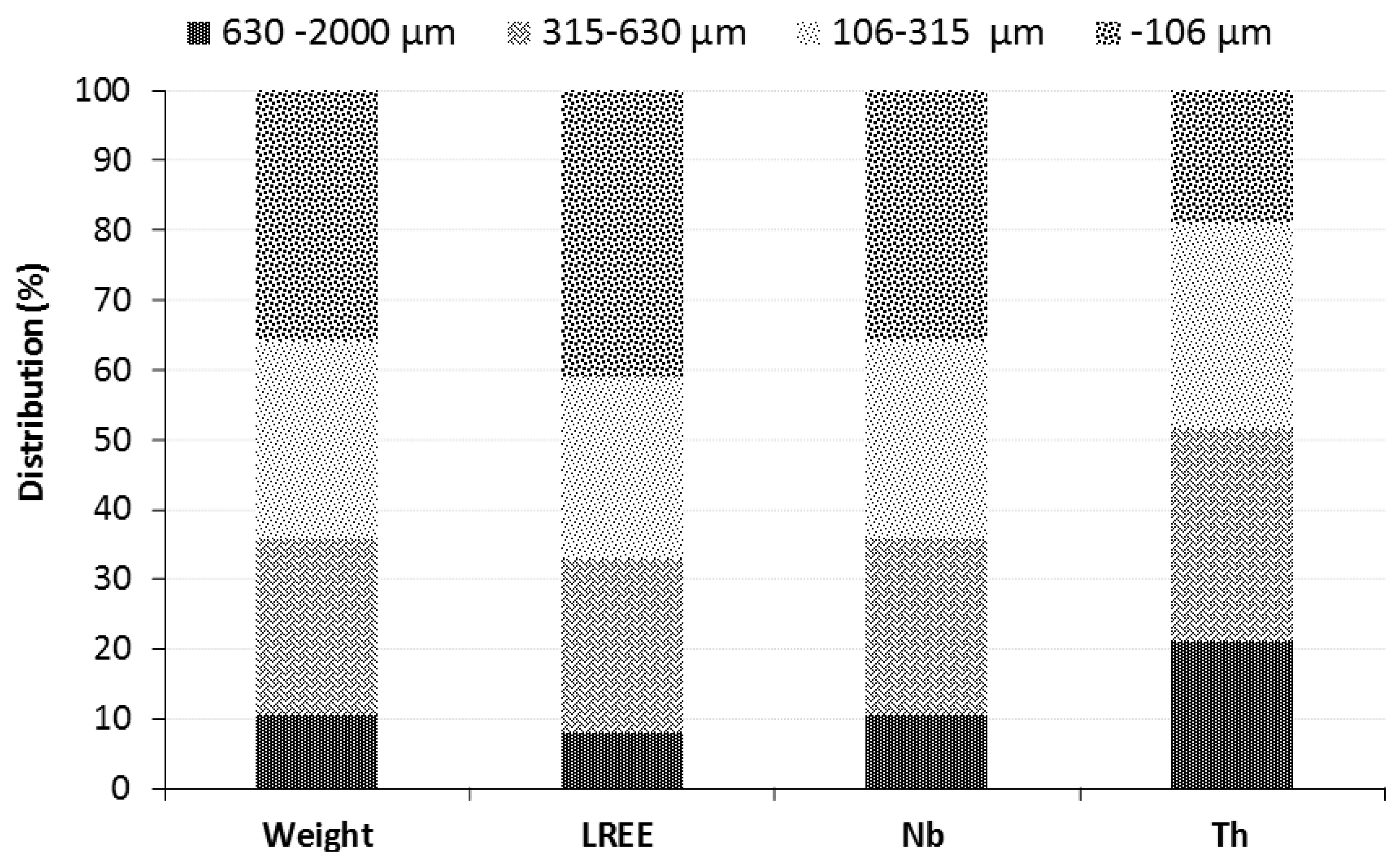
3.2. Mineralogical Characterization
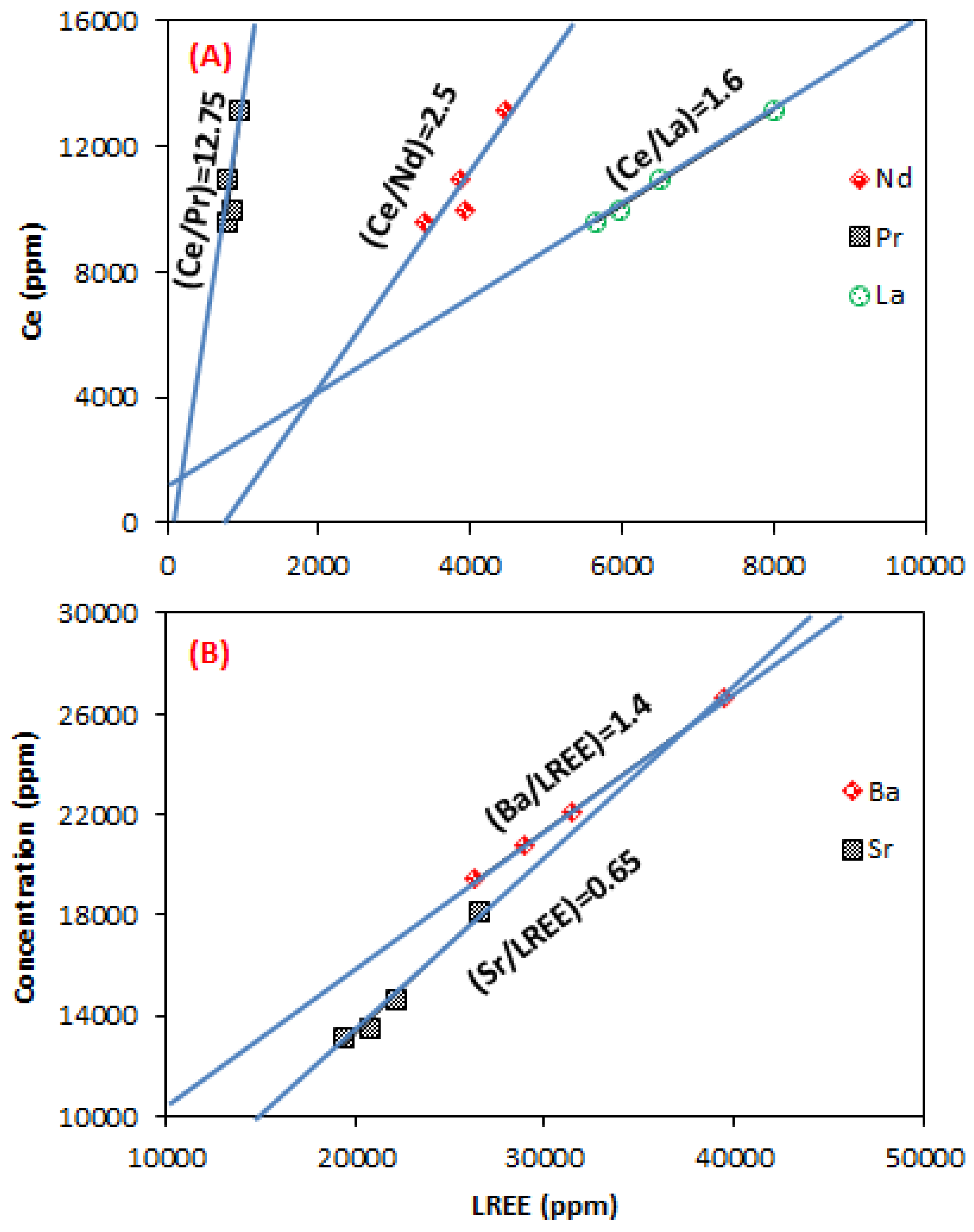
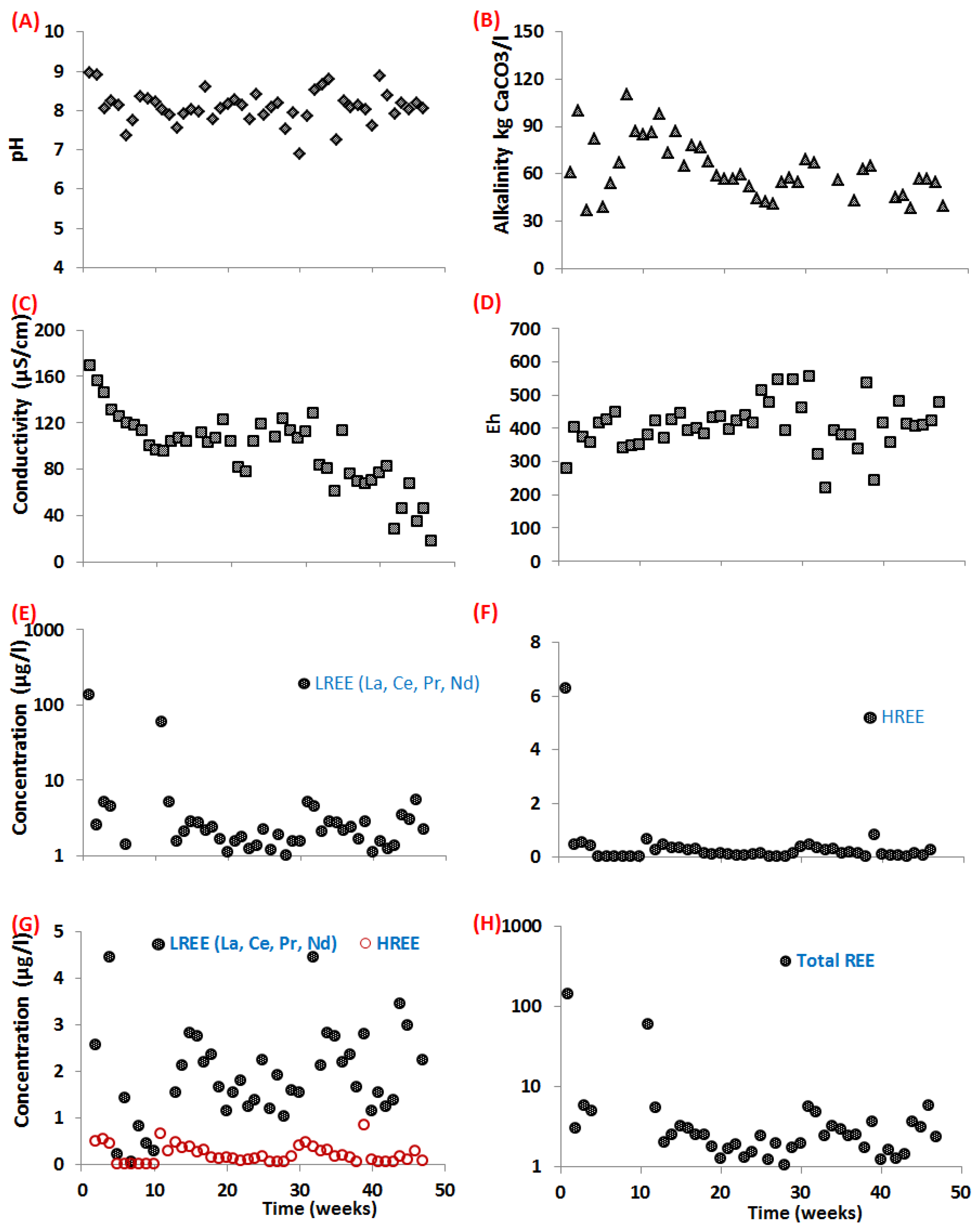
3.3. Geochemical Behavior
4. Discussion
4.1. Separation Efficiency and Environmental Challenges
4.2. Geochemical Behavior of REE
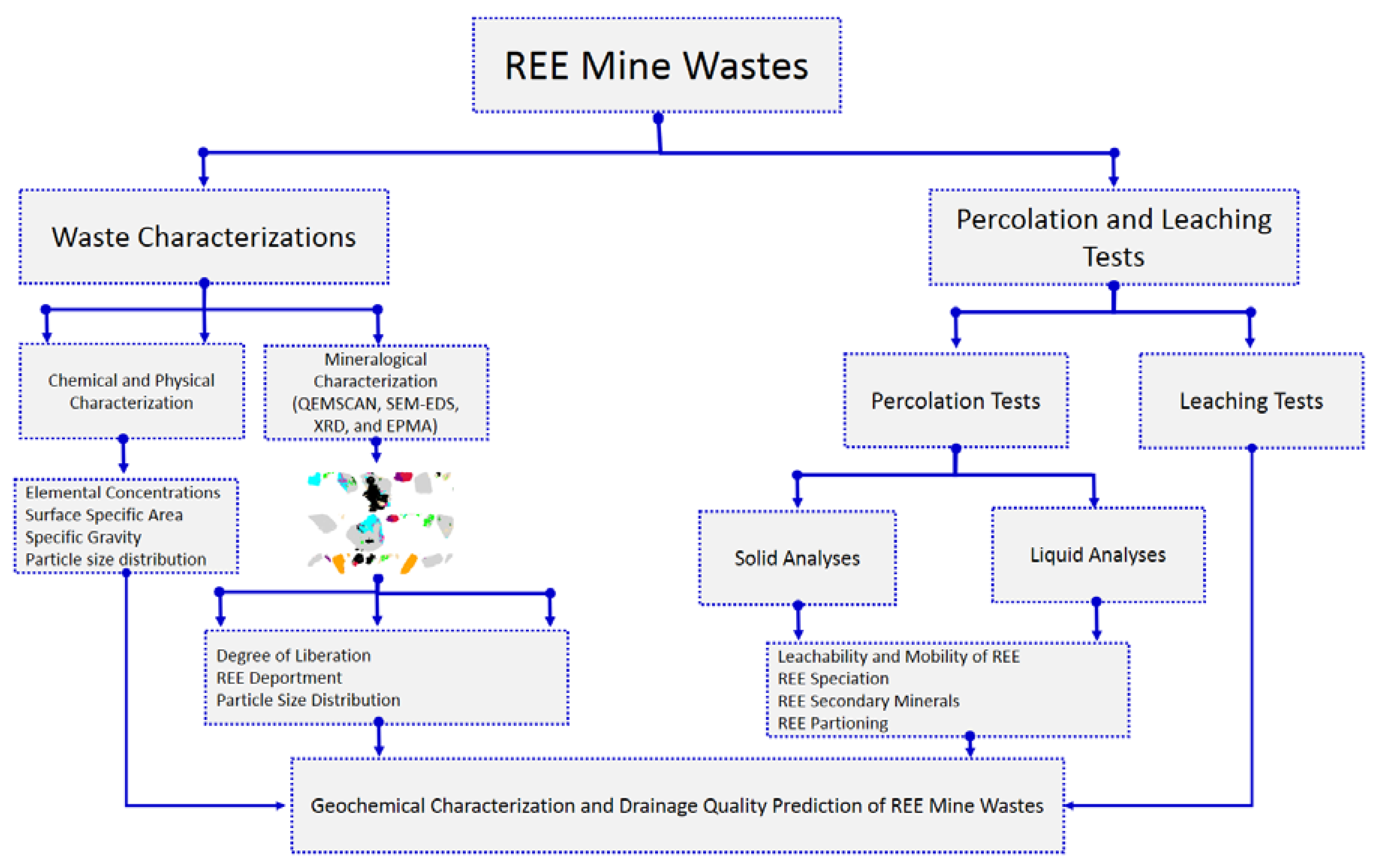
4.3. Implications to the Prediction of the Geochemical Behavior and Water Quality of REE Mine Wastes
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| REE | rare earth elements |
| QEMSCAN | quantitative evaluation of minerals by scanning electron microscopy |
| XRD | X-ray diffraction |
| EPMA | electron probe micro-analyzer |
| SEM-EDS | scanning electron microscopy with X-ray microanalysis |
References
- Catinat, M. Critical Raw Materials for the EU—Report of the Ad-Hoc Working Group on Defining Critical Raw Materials; European Commission, Enterprise and Industry: Brussels, Belgium, 2010. [Google Scholar]
- Humphries, M. Rare Earth Elements: The Global Supply Chain. Congressional Research Service, 7-5700. 2013. Available online: http://www.fas.org/sgp/crs/natsec/R41347.pdf (accessed on 6 February 2018).
- Mariano, A.N.; Mariano, A. Rare Earth Mining and Exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Cox, C.; Kynicky, J. The rapid evolution of speculative investment in the REE market before, during, and after the rare earth crisis of 2010–2012. Extr. Ind. Soc. 2017, in press. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Smith, M.P.; Kynicky, J. From “strategic” tungsten to “green” neodymium: A century of critical metals at a glance. Ore Geol. Rev. 2015, 64, 455–458. [Google Scholar] [CrossRef]
- Massari, S.; Ruberti, M. Rare earth elements as critical raw materials: Focus on international markets and future strategies. Resour. Policy 2013, 38, 36–43. [Google Scholar] [CrossRef]
- Schlinkert, D.; van den Boogaart, K.G. The development of the market for rare earth elements: Insights from economic theory. Resour. Policy 2015, 46, 272–280. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Grammatikopoulos, T.; Waters, K.E. Understanding the effect of mineralogy on muscovite flotation using QEMSCAN. Int. J. Miner. Process. 2016, 155, 6–12. [Google Scholar] [CrossRef]
- Dehaine, Q.; Filippov, L.O. Rare earth (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-product of kaolin production, Cornwall: Part 1: Selection and characterisation of the valuable stream. Miner. Eng. 2015, 76, 141–153. [Google Scholar] [CrossRef]
- Filippov, L.O.; Dehaine, Q.; Filippova, I.V. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production—Part 3: Processing of fines using gravity and flotation. Miner. Eng. 2016, 95, 96–106. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Reguir, E.P.; Zaitsev, A.N.; Couëslan, C.; Xu, C.; Kynický, J.; Mumin, A.H.; Yang, P. Apatite in carbonatitic rocks: Compositional variation, zoning, element partitioning and petrogenetic significance. Lithos 2017, 274, 188–213. [Google Scholar] [CrossRef]
- Nadeau, O.; Cayer, A.; Pelletier, M.; Stevenson, R.; Jébrak, M. The Paleoproterozoic Montviel carbonatite-hosted REE–Nb deposit, Abitibi, Canada: Geology, mineralogy, geochemistry and genesis. Ore Geol. Rev. 2015, 67, 314–335. [Google Scholar] [CrossRef]
- Hurst, C. China’s Rare Earth Elements Industry: What Can the West Learn? Institute for the Analysis of Global Security: Washington, DC, USA, 2010. [Google Scholar]
- Weber, R.J.; Reisman, D.J. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; US EPA Region: Cincinnati, OH, USA, 2012. [Google Scholar]
- Pecht, M.; Kaczmarek, R.; Song, X.; Hazelwood, D.; Kavetsky, R.; Anand, D. Rare Earth Material: Insights and Concerns; CALCE EPSC Press, University of Maryland: College Park, MD, USA, 2011. [Google Scholar]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare Earth Mineralization in Igneous Rocks: Sources and Processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Diversity of rare earth deposits: The key example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Orris, G.J.; Grauch, R.I. Rare Earth Element Mine, Deposits, and Occurences; USGC (Science for a Changing World): Reston, VA, USA, 2002. [Google Scholar]
- Chakhmouradian, A.R.; Wall, F. Rare Earth Elements: Minerals, Mines, Magnets (and More). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements a Tale of “Ceria” and “Yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Terry Williams, C.; Jeffries, T.E.; Strekopytov, S.; Moutte, J.; Ivashchenkova, O.V.; Spratt, J.; Petrov, S.V.; Wall, F.; Seltmann, R.; et al. Rare earth elements in phoscorites and carbonatites of the Devonian Kola Alkaline Province, Russia: Examples from Kovdor, Khibina, Vuoriyarvi and Turiy Mys complexes. Ore Geol. Rev. 2014, 61, 204–225. [Google Scholar] [CrossRef]
- Verplanck, P.L.; Gosen, B.S.V. Carbonatite and Alkaline Intrusion-Related Rare Earth Element Deposits—A Deposit Model; USGS: Reston, VA, USA, 2011. [Google Scholar]
- Kanazawa, Y.; Kamitani, M. Rare earth minerals and resources in the world. J. Alloys Compd. 2006, 408–412, 1339–1343. [Google Scholar] [CrossRef]
- Edahbi, M.; Benzaazoua, M.; Plante, B.; Doire, S.; Kormos, L. Mineralogical characterization using QEMSCAN® and leaching potential study of REE within silicate ores: A case study of the Matamec project, Québec, Canada. J. Geochem. Explor. 2017, 185, 64–73. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Schmid, R. Descriptive nomenclature and classification of pyroclastic deposits and fragments: Recommendations of the IUGS Subcommission on the Systematics of Igneous Rocks. Geology 1981, 9, 41–43. [Google Scholar] [CrossRef]
- Andrade, F.R.D.; Möller, P.; Lüders, V.; Dulski, P.; Gilg, H.A. Hydrothermal rare earth elements mineralization in the Barra do Itapirapuã carbonatite, southern Brazil: Behaviour of selected trace elements and stable isotopes (C, O). Chem. Geol. 1999, 155, 91–113. [Google Scholar] [CrossRef]
- Zaitsev, A.N.; Wall, F.; Bas, M.J.L. REE-Sr-Ba minerals from the Khibina carbonatites, Kola Peninsula, Russia: Their mineralogy, paragenesis and evolution. Mineral. Mag. 1998, 62, 225–250. [Google Scholar]
- Nadeau, O.; Stevenson, R.; Jébrak, M. Evolution of Montviel alkaline-carbonatite complex by coupled fractional crystallization, fluid mixing and metasomatism—Part II: Trace element and Sm–Nd isotope geochemistry of metasomatic rocks: Implications for REE-Nb mineralization. Ore Geol. Rev. 2016, 72, 1163–1173. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Censi, P.; Saiano, F.; Pisciotta, A.; Tuzzolino, N. Geochemical behaviour of rare earths in Vitis vinifera grafted onto different rootstocks and growing on several soils. Sci. Total Environ. 2014, 473–474, 597–608. [Google Scholar] [CrossRef] [PubMed]
- Leal Filho, W. Chapter 17—An Analysis of the Environmental Impacts of the Exploitation of Rare Earth Metals. In Rare Earths Industry; Elsevier: Boston, MA, USA, 2016; pp. 269–277. [Google Scholar]
- Schüler, D.; Buchert, M.; Liu, R.; Dittrich, S.; Merz, C. Study on Rare Earths and Their Recycling; Öko-Institut e.V.: Darmstadt, Germany, 2011. [Google Scholar]
- Sapsford, D.J.; Bowell, R.J.; Geroni, J.N.; Penman, K.M.; Dey, M. Factors influencing the release rate of uranium, thorium, yttrium and rare earth elements from a low grade ore. Miner. Eng. 2012, 39, 165–172. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, C.-Q.; Wang, Z.L.; Liu, X.; Li, J. Rare earth elements concentrations and speciation in rainwater from Guiyang, an acid rain impacted zone of Southwest China. Chem. Geol. 2016, 442, 23–34. [Google Scholar] [CrossRef]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.E. Processing a rare earth mineral deposit using gravity and magnetic separation. Miner. Eng. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Goutier, J. Géologie de la Région du lac au Goéland (32F/15); Ressources Naturelles et Faune, Québec: Rouyn-Noranda, QC, Canada, 2006. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Young, R.A. The rietveld method. Int. Union Crystallogr. 1993, 5, 252–254. [Google Scholar]
- Smythe, D.M.; Lombard, A.; Coetzee, L.L. Rare Earth Element deportment studies utilising QEMSCAN technology. Miner. Eng. 2013, 52, 52–61. [Google Scholar] [CrossRef]
- Bouzahzah, H.; Benzaazoua, M.; Bussiere, B.; Plante, B. Prediction of acid mine drainage: Importance of mineralogy and the test protocols for static and kinetic tests. Mine Water Environ. 2014, 33, 54–65. [Google Scholar] [CrossRef]
- Purdy, C. The Geochemical and Mineralogical Controls on the Environmental Mobility of Rare Earth Elements from Tailings, Nechalacho Deposit, Northwest Territories. Master’s Thesis, Queen’s University, Kingston, ON, Canada, 2014. [Google Scholar]
- Janssen, R.P.T.; Verweij, W. Geochemistry of some rare earth elements in groundwater, Vierlingsbeek, The Netherlands. Water Res. 2003, 37, 1320–1350. [Google Scholar] [CrossRef]
- Leybourne, M.I.; Goodfellow, W.D.; Boyle, D.R.; Hall, G.M. Rapid development of negative Ce anomalies in surface waters and contrasting REE patterns in groundwaters associated with Zn–Pb massive sulphide deposits. Appl. Geochem. 2000, 15, 695–723. [Google Scholar] [CrossRef]
- Edahbi, M.; Plante, B.; Benzaazoua, M.; Pelletier, M. Geochemistry of rare earth elements within waste rocks from the Montviel carbonatite deposit, Québec, Canada. Environ. Sci. Pollut. Res. 2018. [CrossRef]
- Belzile Solutions Inc. NI 43-101 Technical Report Montviel Rare Earth Project Québec, Canada; Belzile Solutions Inc.: Rouyn-Noranda, QC, Canada, 2015. [Google Scholar]
- Chou, L.; Garrels, R.M.; Wollast, R. Comparative study of the kinetics and mechanisms of dissolution of carbonate minerals. Chem. Geol. 1989, 78, 269–282. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S. The dissolution kinetics of major sedimentary carbonate minerals. Earth Sci. Rev. 2002, 58, 51–84. [Google Scholar] [CrossRef]
- Morse, J.W.; Arvidson, R.S.; Lüttge, A. Calcium carbonate formation and dissolution. Chem. Rev. 2007, 107, 342–381. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, E.; Lawrence, R.; Poulin, R. On the neutralization of acid rock drainage by carbonate and silicate minerals. Environ. Geol. 1995, 25, 43–54. [Google Scholar] [CrossRef]
- Benzaazoua, M.; Bussiere, B.; Dagenais, A.M.; Archambault, M. Kinetic tests comparison and interpretation for prediction of the Joutel tailings acid generation potential. Environ. Geol. 2004, 46, 1086–1101. [Google Scholar] [CrossRef]
- Plante, B.; Benzaazoua, M.; Bussière, B. Predicting geochemical behaviour of waste rock with low acid generating potential using laboratory kinetic tests. Mine Water Environ. 2011, 30, 2–21. [Google Scholar] [CrossRef]
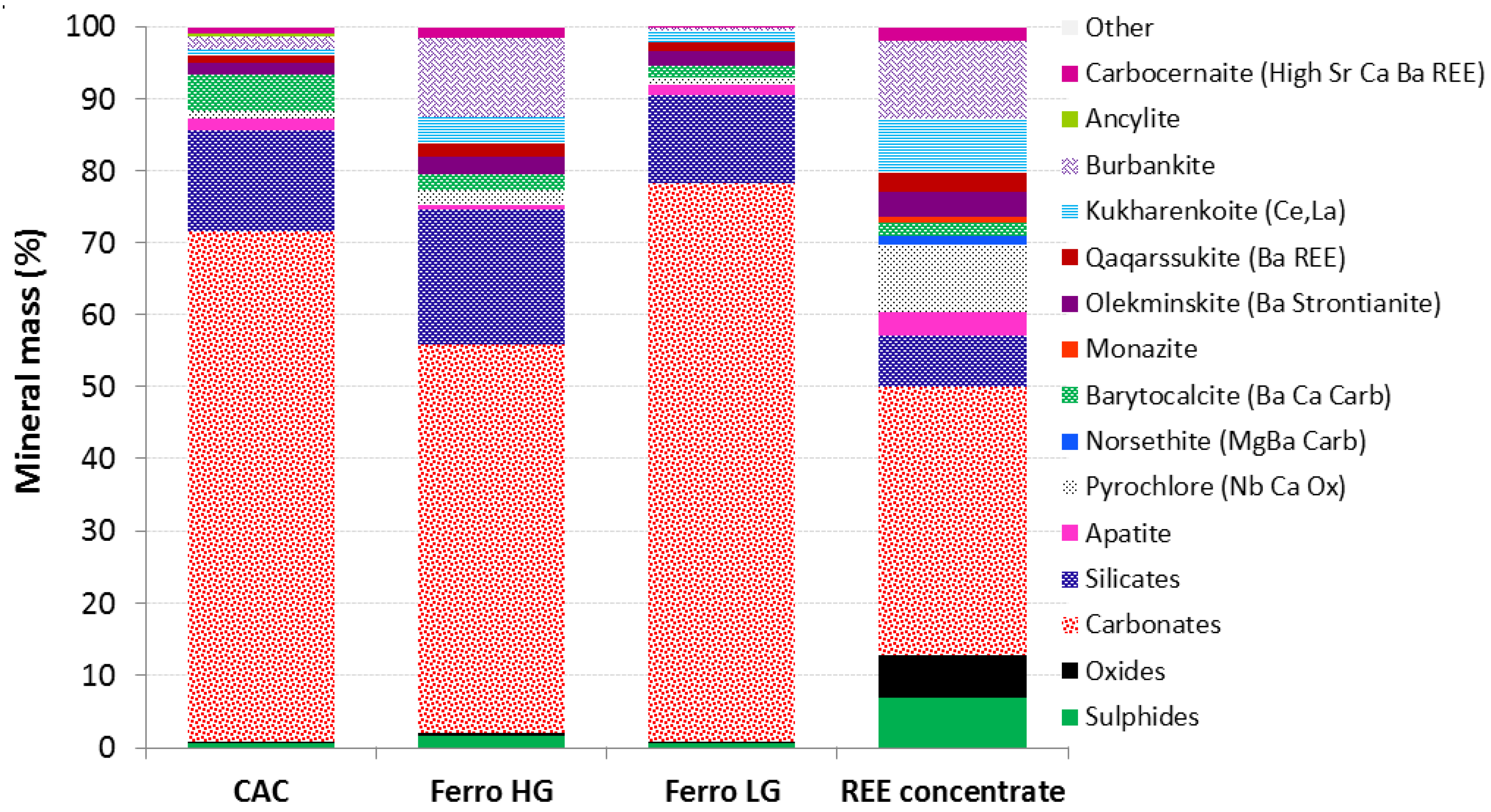
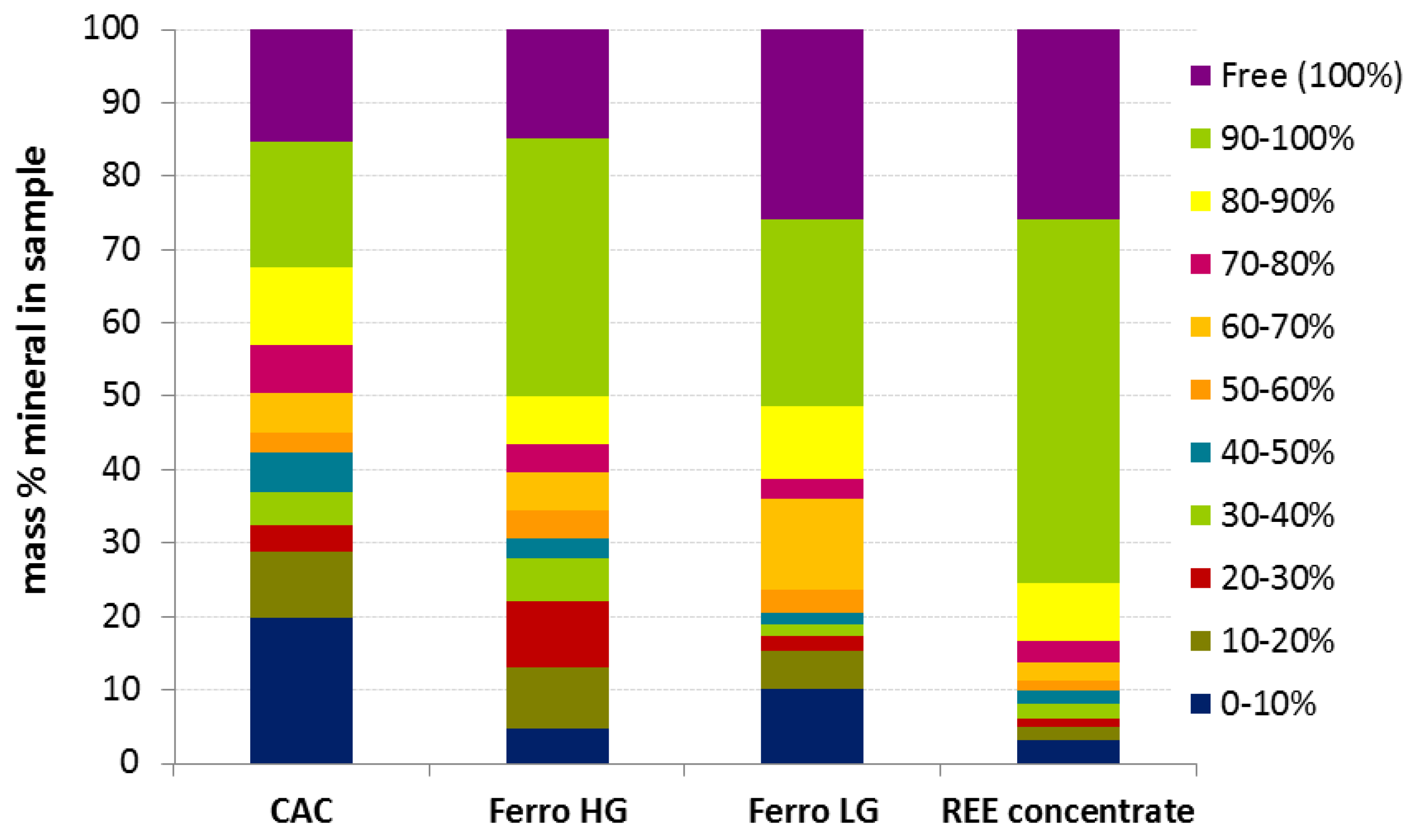
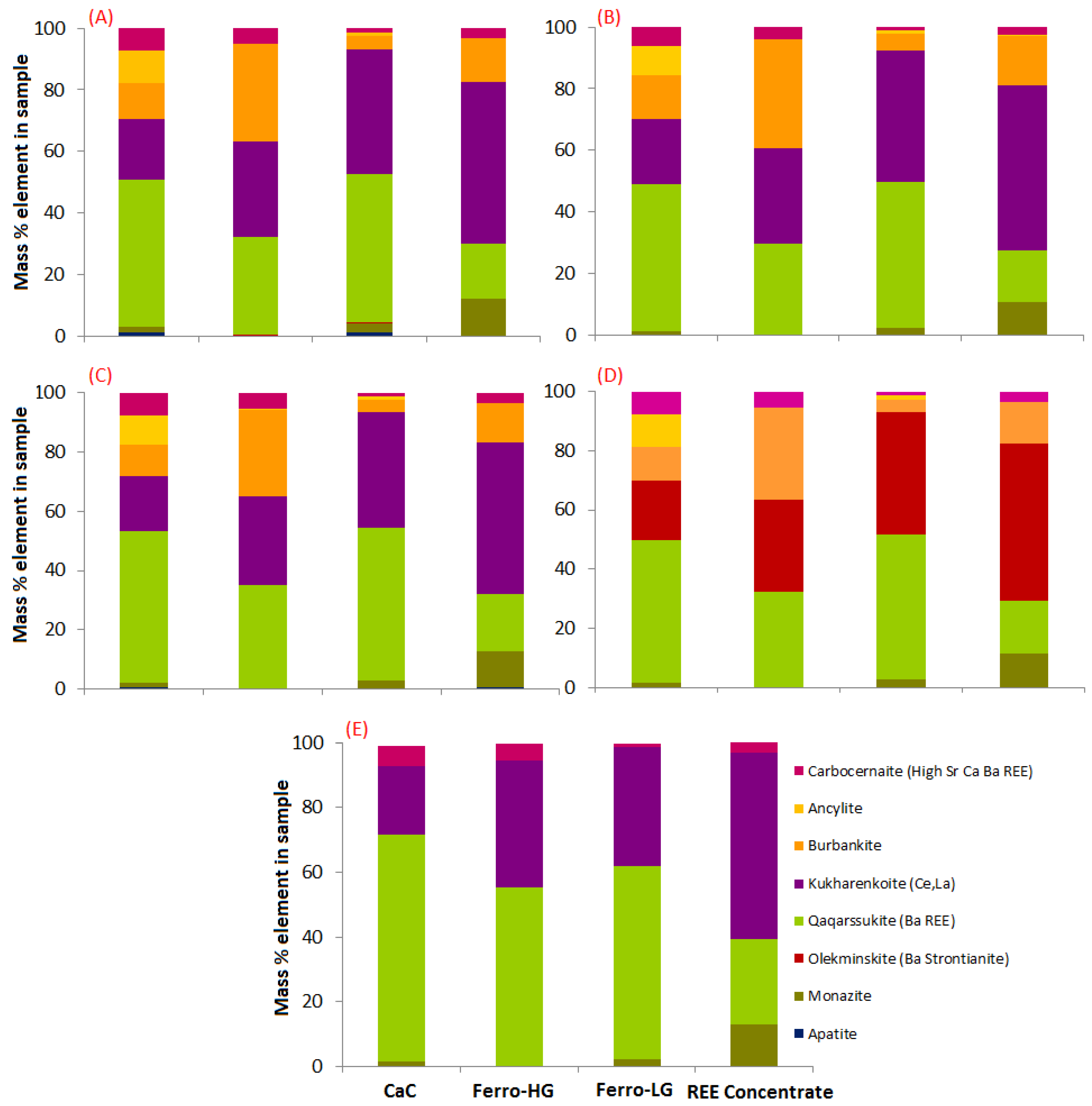
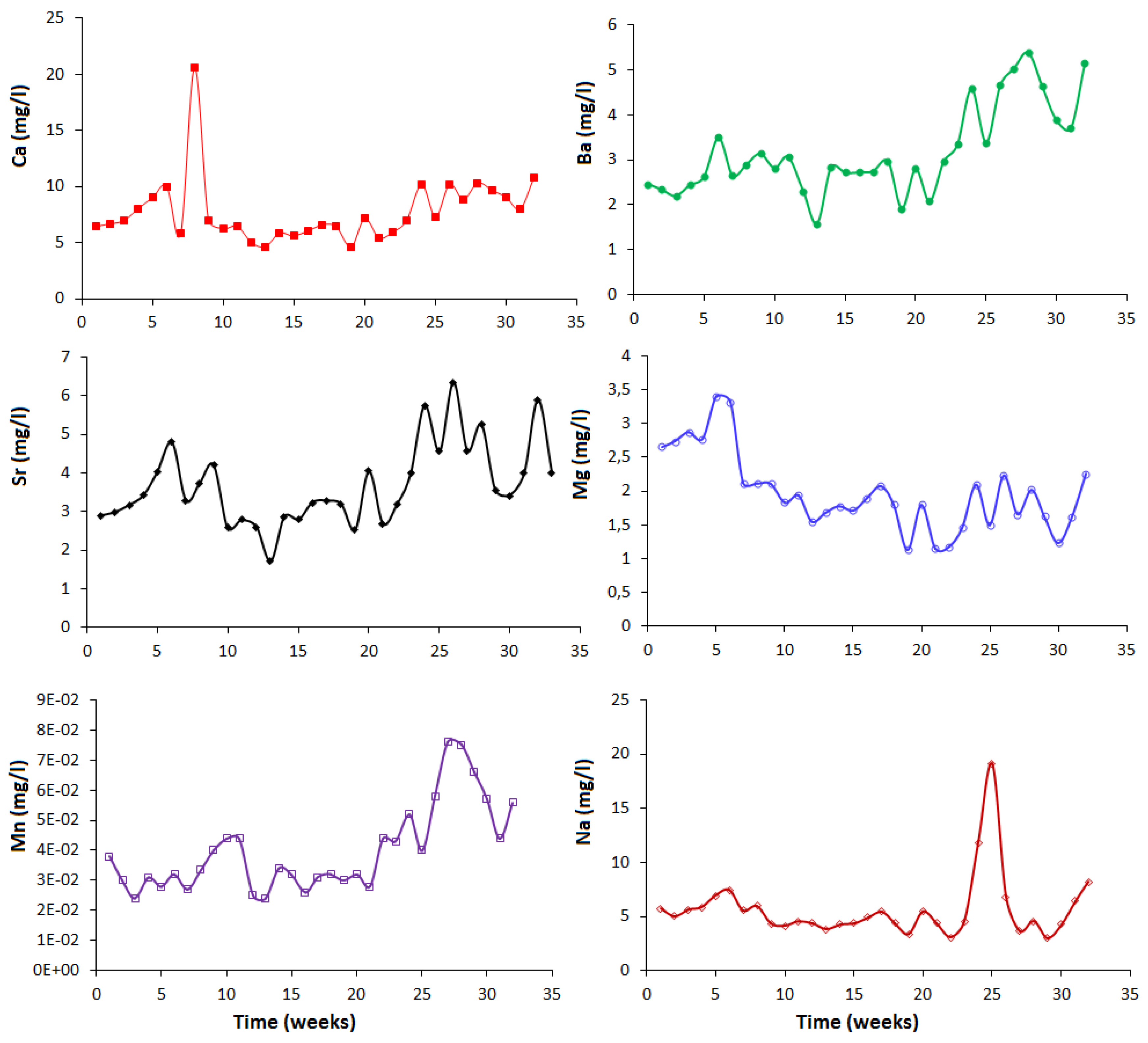

| Parameter | REE-ConC | CaC | FeC-LG | FeC-HG |
|---|---|---|---|---|
| Physical properties | ||||
| D50 | 124 | 2075 | 2775 | 2250 |
| D90 | 209 | 5000 | 5500 | 5200 |
| Specific gravity | 3.60 | 3.10 | 3.36 | 3.30 |
| Chemical composition | ||||
| Fe | 155,700 | 12,000 | 20,000 | 16,000 |
| Ca | 70,130 | 19,000 | 11,000 | 9200 |
| Mg | 26,990 | 2300 | 4200 | 3300 |
| Mn | 11,570 | 1300 | 2100 | 1700 |
| Zn | 1186 | 62 | 81 | 29 |
| Na | 11,900 | 450 | 120 | 900 |
| K | 3240 | 910 | 750 | 960 |
| Ba | 35,000 | 1800 | 1400 | 1900 |
| Pb | 6363 | 12 | 8 | 8 |
| Rare earth elements | ||||
| La | 10,881 | 2500 | 2100 | 8900 |
| Ce | 19,302 | 5400 | 4200 | 14,000 |
| Pr | 1414 | 680 | 450 | 1400 |
| Nd | 4629 | 2200 | 1300 | 4100 |
| Sm | 420 | 330 | 150 | 390 |
| Eu | 80 | 77 | 31 | 78 |
| Gd | 156 | 270 | 99 | 290 |
| Tb | 0.9 | 20 | 6.2 | 15 |
| Dy | 28 | 74 | 16 | 31 |
| Ho | 2.9 | 11 | 2.0 | 3.3 |
| Er | 6.4 | 18 | 3.7 | 5.5 |
| Tm | 0.3 | 1.5 | 0.39 | 0.49 |
| Yb | 2.7 | 6.9 | 2.3 | 2.9 |
| Lu | 0.3 | 0.75 | 0.29 | 0.35 |
| ΣLREE | 36,226 | 10,780 | 8050 | 28,400 |
| ΣHREE | 698 | 800 | 298 | 804 |
| Carbon | 6.98 | 9.31 | 9.77 | 8.73 |
| Sulfur | 2.77 | 0.41 | 0.22 | 0.84 |
| AP | 86.5 | 7.8 | 4.1 | 20.3 |
| NP | 581.3 | 602 | 542 | 493 |
| NNP | 494.9 | 594.2 | 537.9 | 472.7 |
| Mineral | Chemical Formula | Phase (wt %) |
|---|---|---|
| Ankerite | Ca(Fe,Mg,Mn)(CO3)2 | 34.5 |
| Siderite | FeCO3 | 16.0 |
| Monazite | (La,Ce,Nd)PO4 | 3.4 |
| Biotite | K(Fe,Mg)3(AlSi3)O10(F,OH)2 | 6.6 |
| Burbankite | (Na,Ca)3(Sr,Ba,Ce)3(CO3)5 | 1.3 |
| Strontianite | SrCO3 | 0.2 |
| Kukharenkoite | Ba2Ce(CO3)3F | 1.5 |
| Calcite | CaCO3 | 11.4 |
| Pyrrhotite | Fe(1−x)S | 3.2 |
| Pyrite | FeS2 | 2.1 |
| Quartz | SiO2 | 3.2 |
| Synchysite-Ce | CaCe(CO3)2F | 0.3 |
| Ilmenite | FeTiO3 | 5.2 |
| Bassanite | 2CaSO4·(H2O) | 9.6 |
| Hematite | Fe2O3 | 1.1 |
| Fraction | LREE | Ba | Sr | |||
|---|---|---|---|---|---|---|
| (ppm) | 2σ | (ppm) | 2σ | (ppm) | 2σ | |
| −106 µm | 26,612 | 1030 | 39,487 | 732 | 18,158 | 267 |
| 106–315 µm | 22,091 | 873 | 31,438 | 542 | 14,606 | 200 |
| 315–630 µm | 19,411 | 782 | 26,239 | 441 | 13,140 | 173 |
| 630 µm–2 mm | 20,748 | 845 | 28,999 | 506 | 13,474 | 184 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edahbi, M.; Plante, B.; Benzaazoua, M.; Kormos, L.; Pelletier, M. Rare Earth Elements (La, Ce, Pr, Nd, and Sm) from a Carbonatite Deposit: Mineralogical Characterization and Geochemical Behavior. Minerals 2018, 8, 55. https://doi.org/10.3390/min8020055
Edahbi M, Plante B, Benzaazoua M, Kormos L, Pelletier M. Rare Earth Elements (La, Ce, Pr, Nd, and Sm) from a Carbonatite Deposit: Mineralogical Characterization and Geochemical Behavior. Minerals. 2018; 8(2):55. https://doi.org/10.3390/min8020055
Chicago/Turabian StyleEdahbi, Mohamed, Benoît Plante, Mostafa Benzaazoua, Lori Kormos, and Mia Pelletier. 2018. "Rare Earth Elements (La, Ce, Pr, Nd, and Sm) from a Carbonatite Deposit: Mineralogical Characterization and Geochemical Behavior" Minerals 8, no. 2: 55. https://doi.org/10.3390/min8020055






