Influence of Salinity and Pb on the Precipitation of Zn in a Model System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Experimental Series
- Purpose and Scope
- Nomenclature
- Influence of Salinity
- Influence of Pb
2.3. Experimental Setup
2.4. Methods
3. Results
3.1. Water Purification
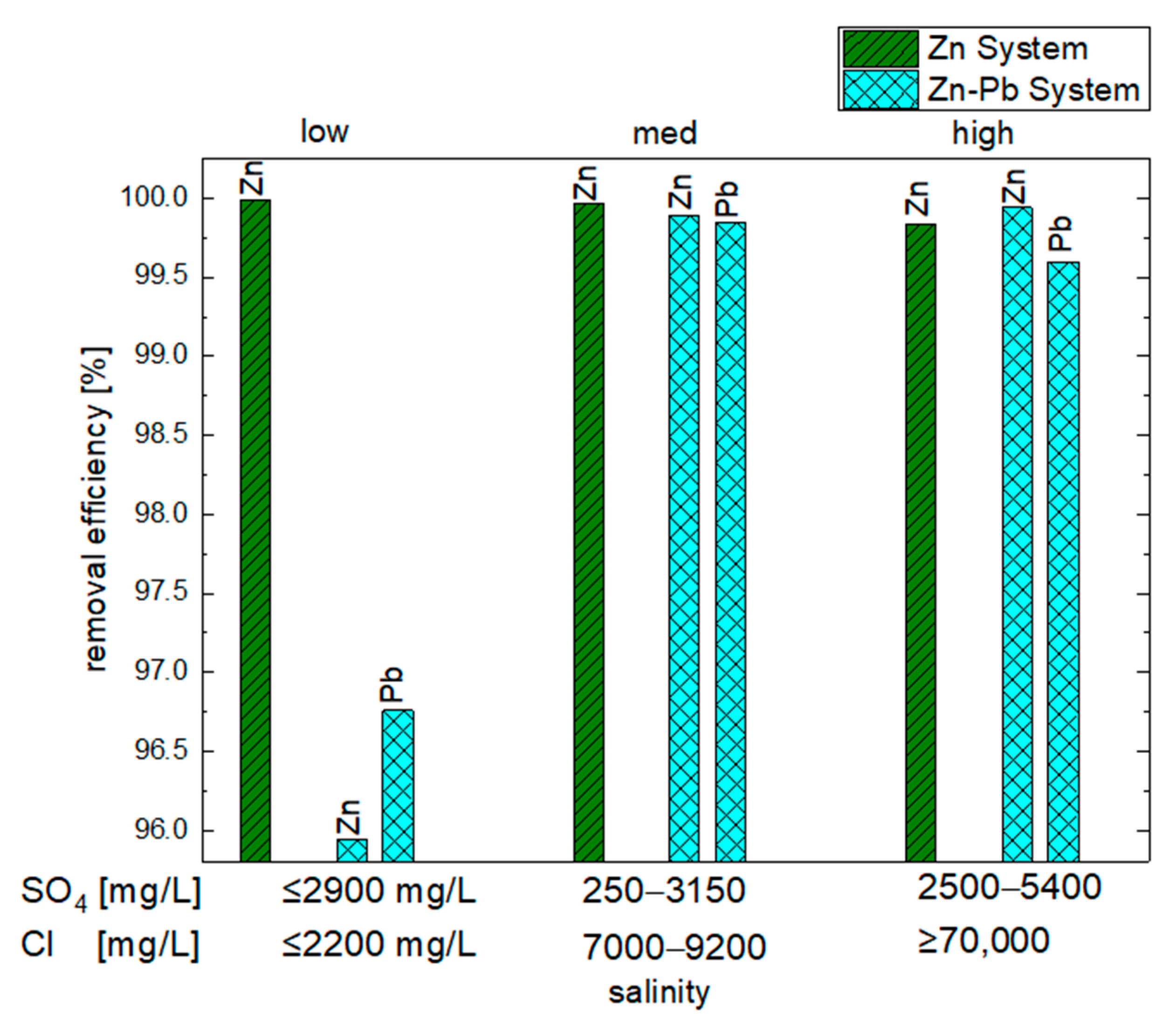
- Influence of Salinity on the Precipitation of Zn in the Zn System (Exp. Set 1) and the Zn-Pb System (Exp. Set 2)
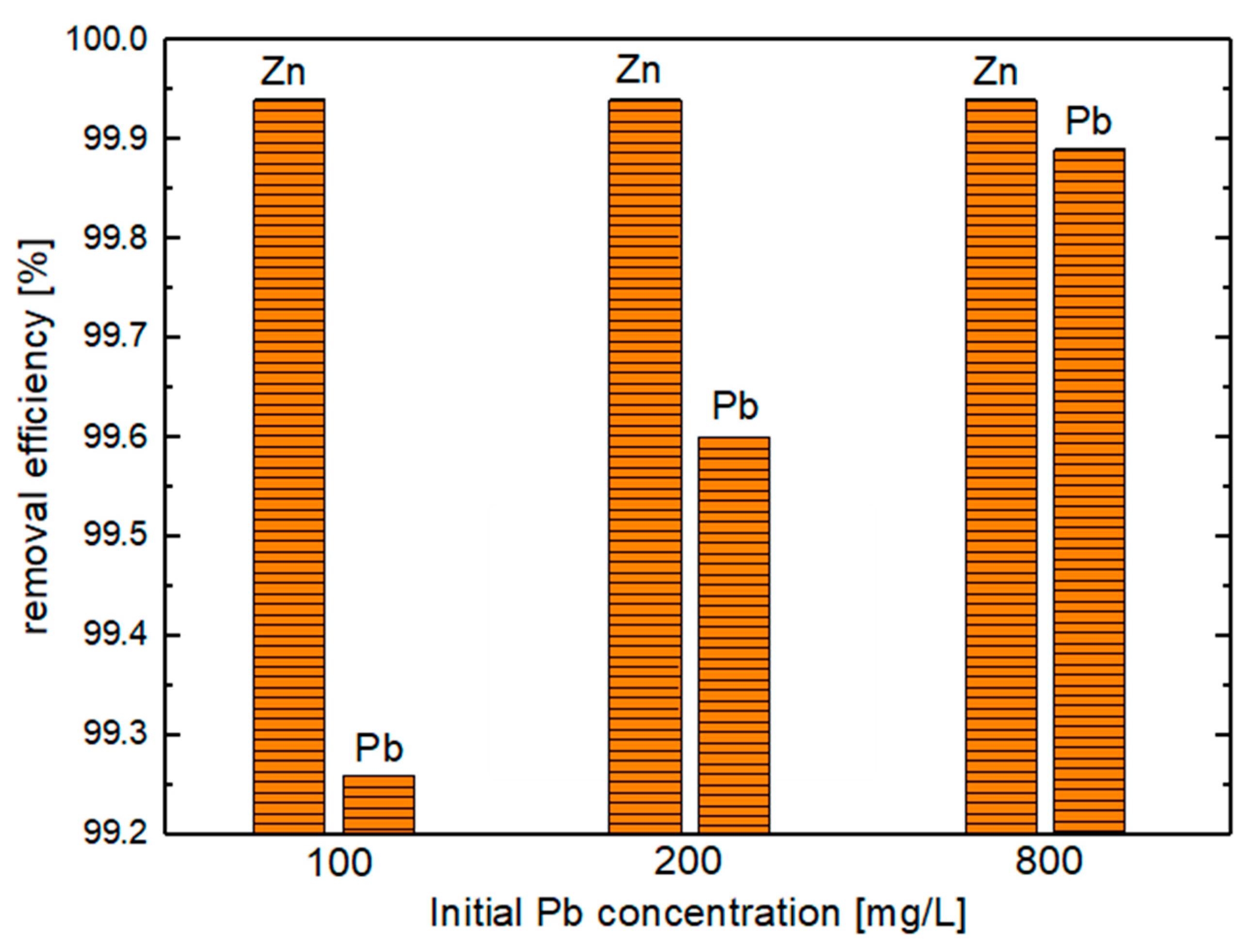
- Influence of Pb in the Zn–Pb–Salt System (Exp. Set 3)
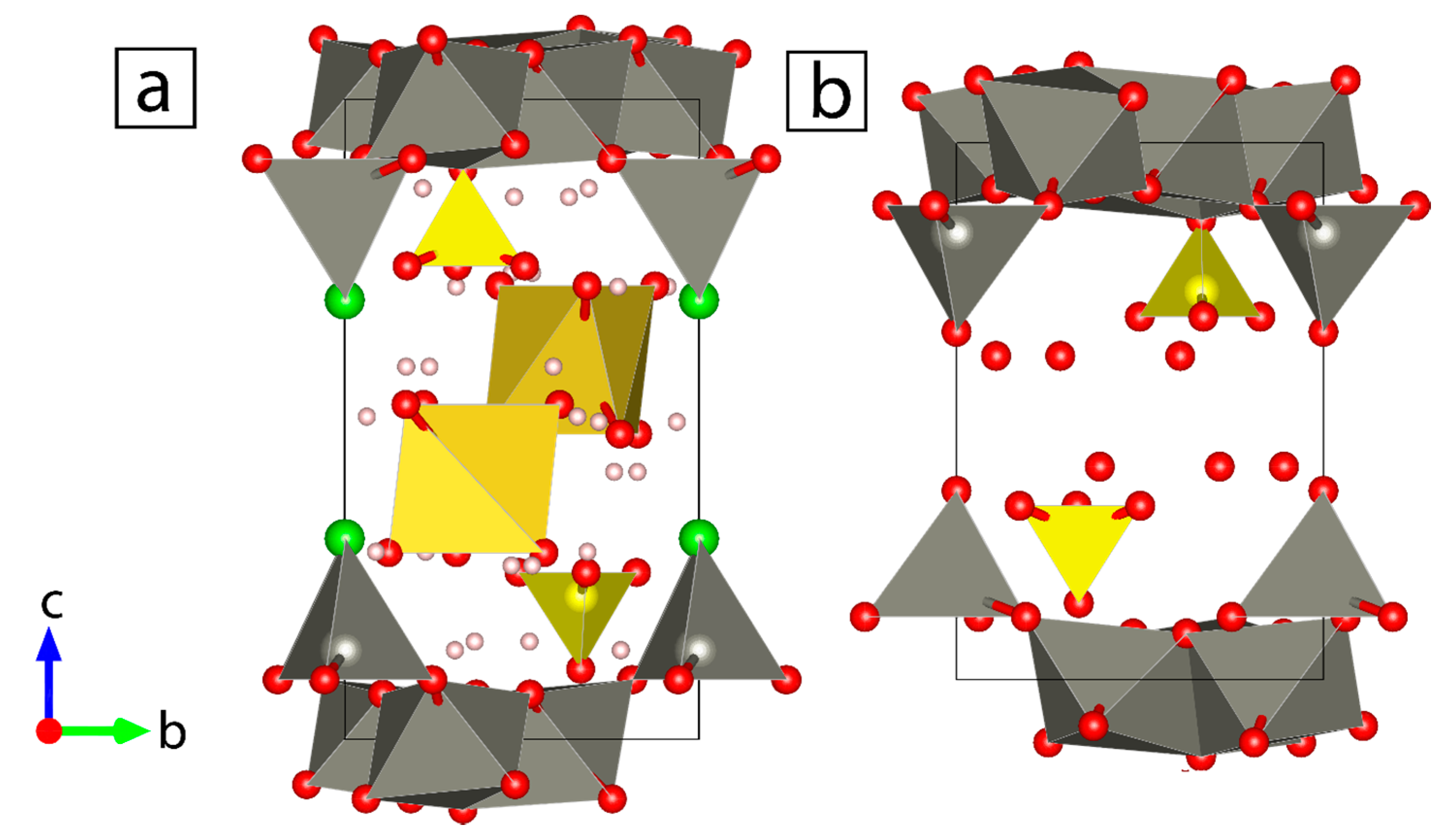
3.2. Mineralogical Characterization
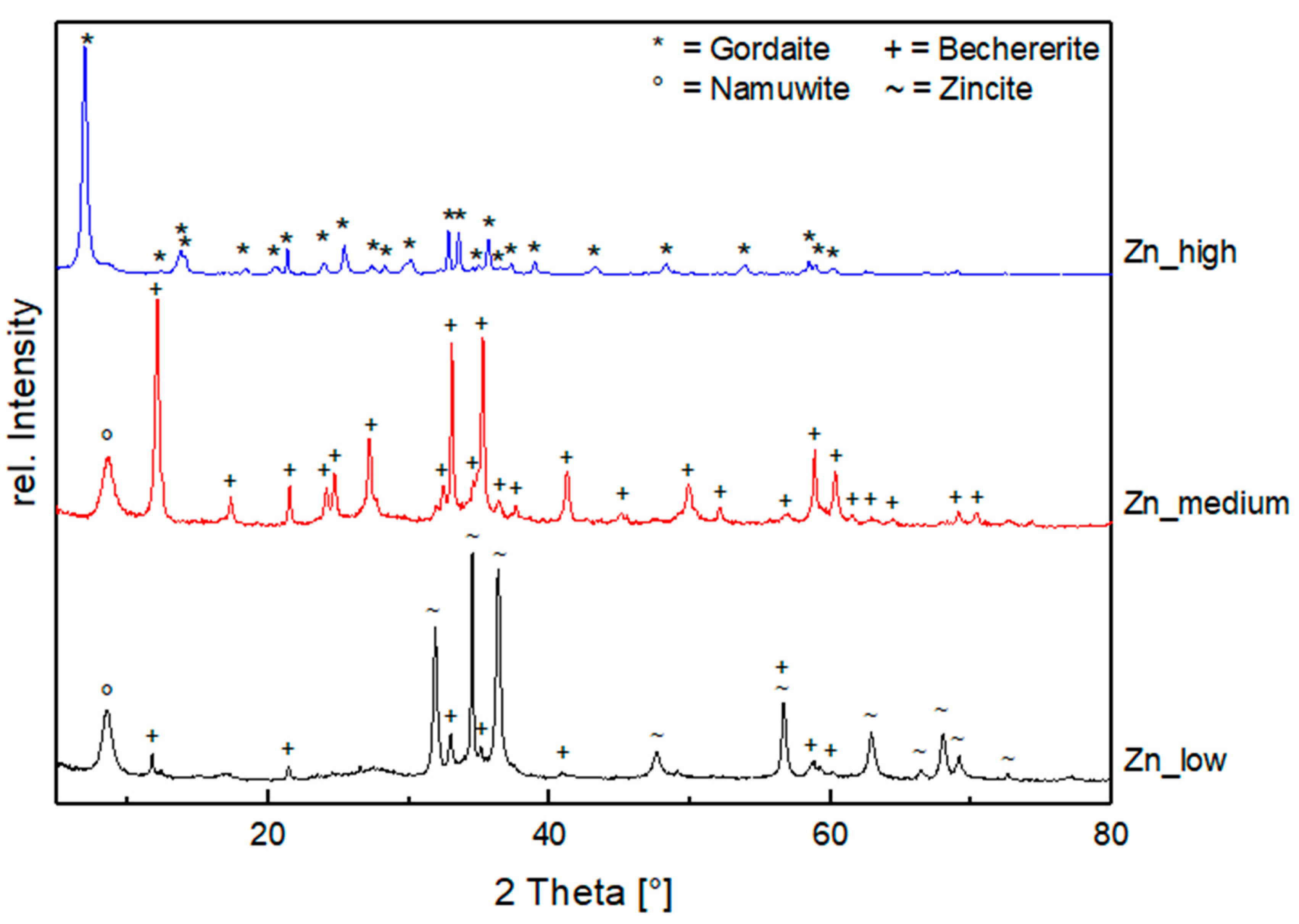
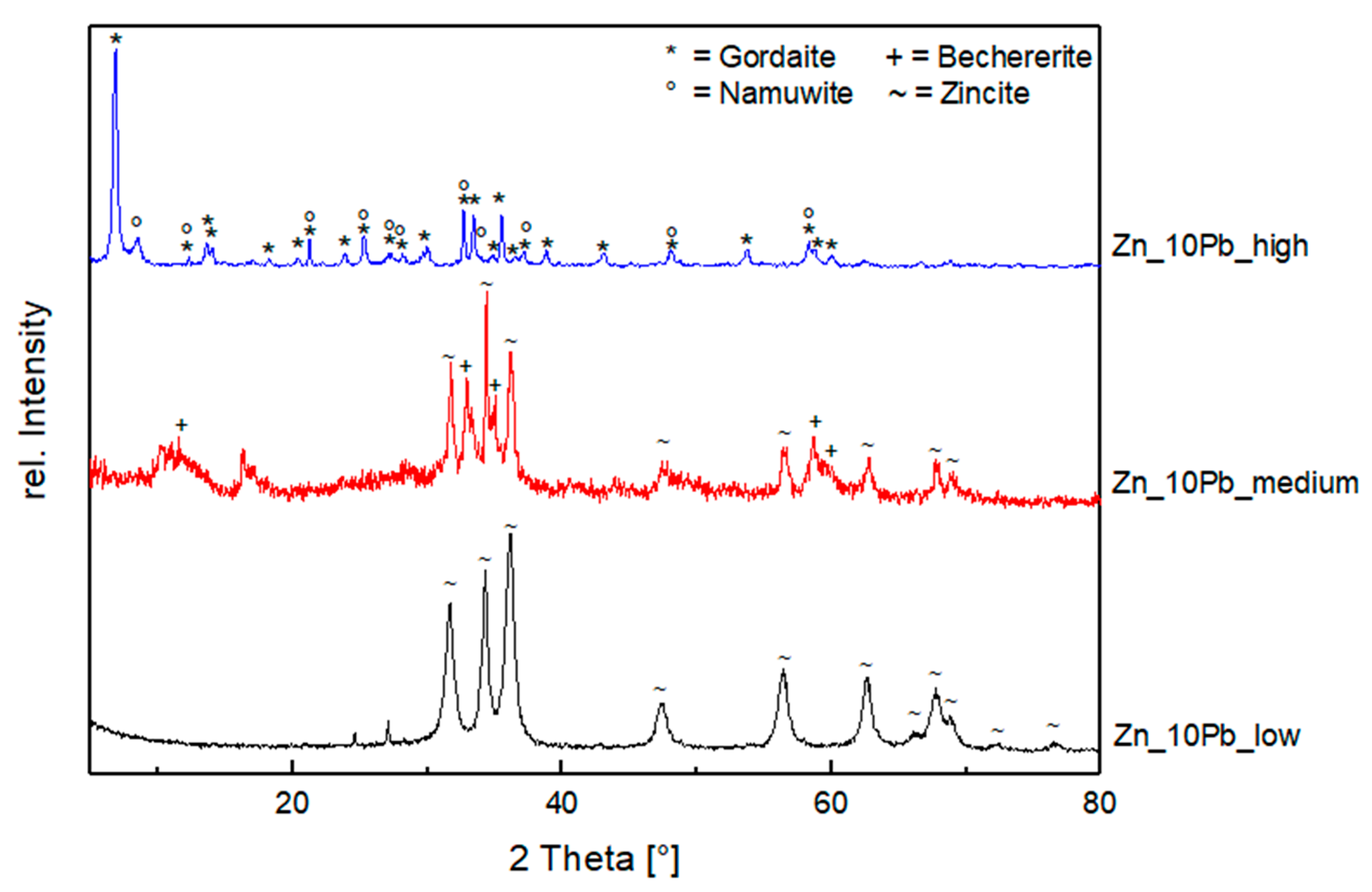
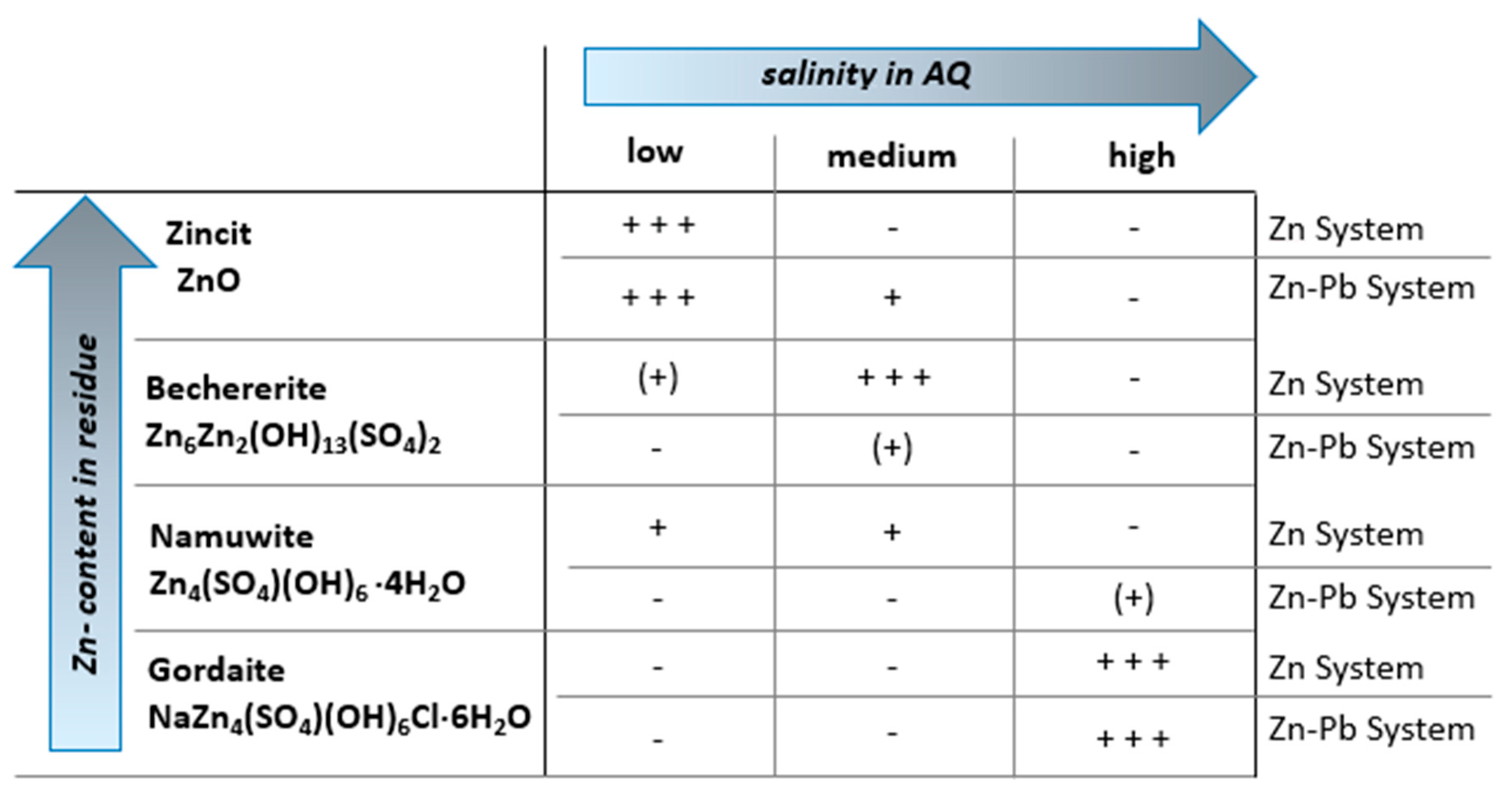
3.2.1. Influence of Salinity
- Zn System (Exp. Set 1)
- Zn–Pb System (Exp. Set 2)
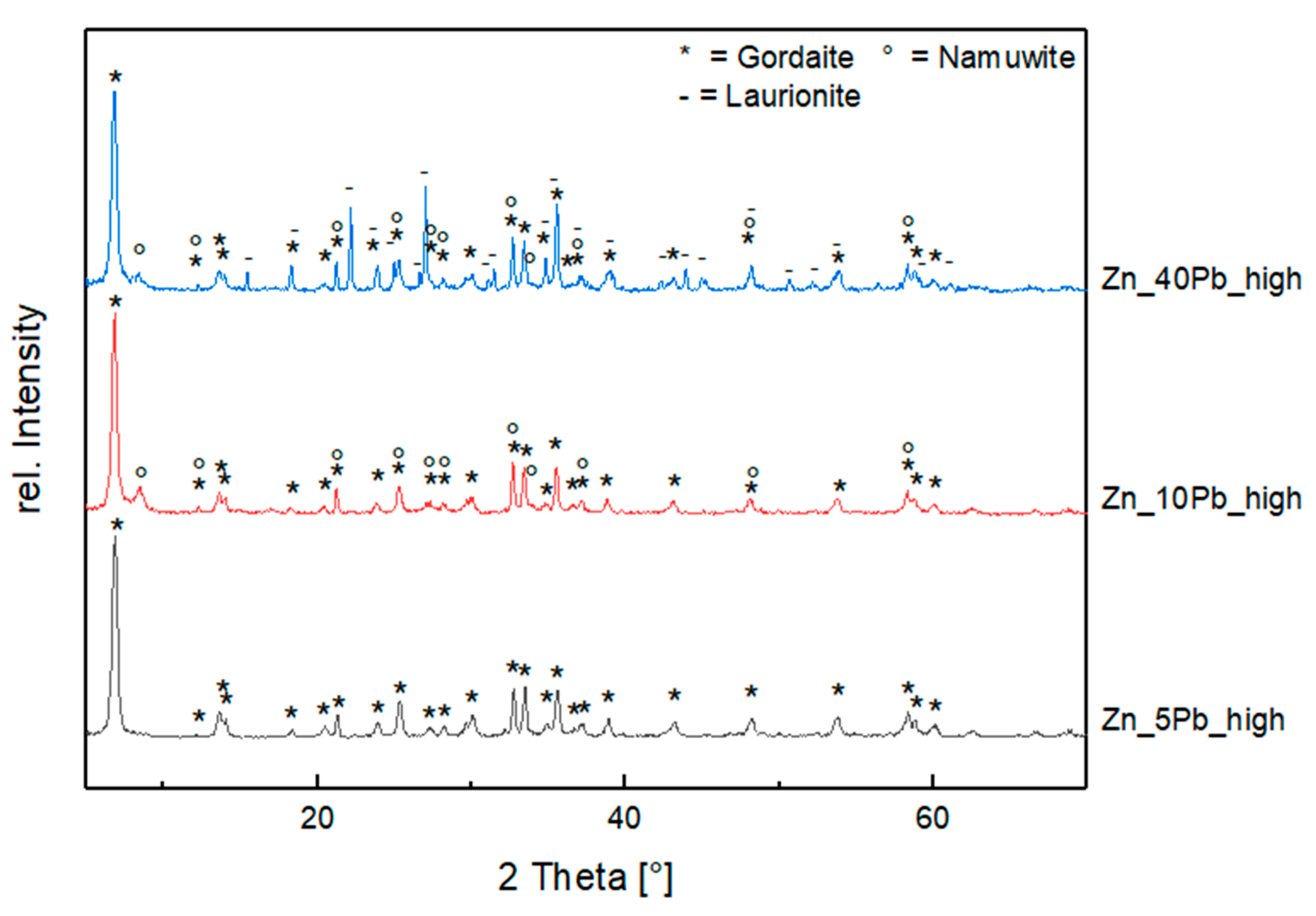
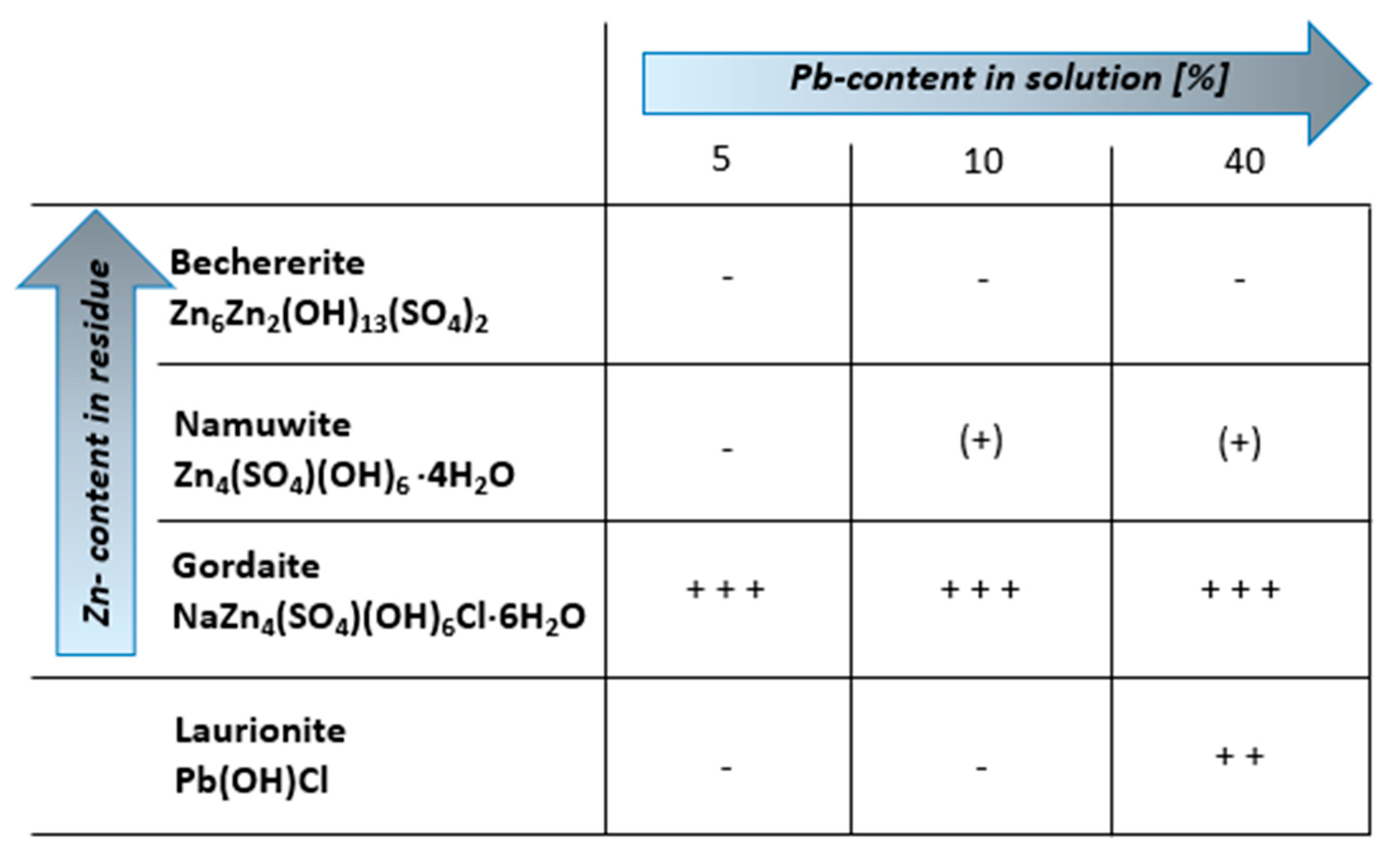
3.2.2. Influence of Pb in High Saline Solution (Exp. Set 3)
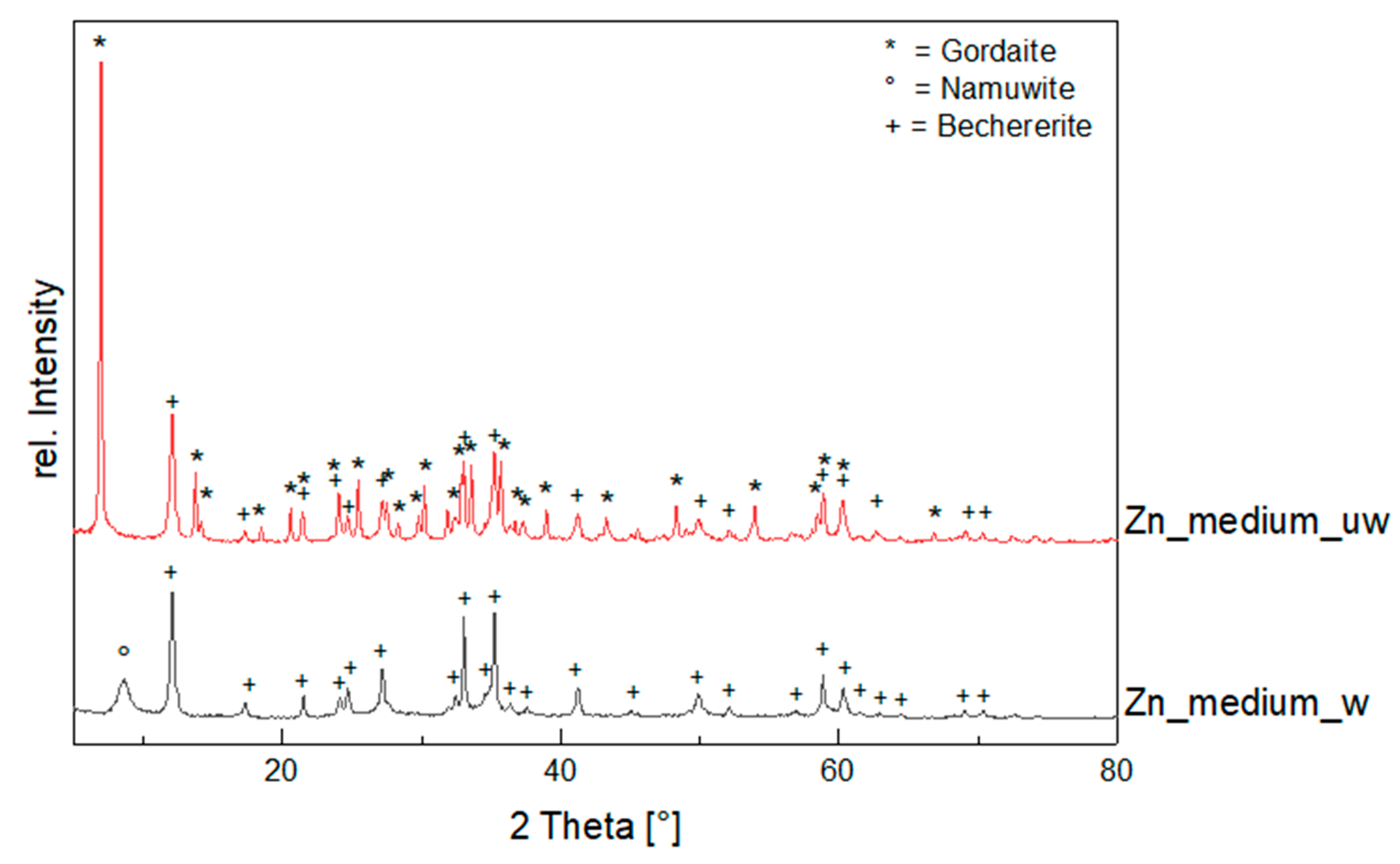
3.2.3. Washing of the Precipitates
- Zn System (Exp. Set 1)
- Zn–Pb System (Exp. Set 2)
- Zn–Pb–Salt System (Exp. Set 3)
4. Discussion
4.1. Influence of Salinity
4.2. Influence of Pb (Exp. Set 3)
4.3. Washing the Precipitates
5. Conclusions and Outlook
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Nordberg, G.F.; Fowler, B.A.; Nordberg, M. Handbook on the Toxicology of Metals, 4th ed.; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Zepf, V.; Simmons, J.; Reller, A.; Ashfield, M.; Rennie, C. Materials Critical to the Energy Industry: An Introduction; BP p.l.c.: London, UK, 2014. [Google Scholar]
- Gidlow, D. Lead toxicity. Occup. Med. 2004, 54, 76–81. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewater: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Naja, G.M.; Volesky, B. Treatment of Metal-Bearing Effluents: Removal and Recovery. In Heavy Metals in the Environment; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; CRC Press: Boca Raton, FL, USA, 2009; p. 247. [Google Scholar]
- Youcai, Z. Pollution Control and Resource Recovery: Municipal Solid Wastes Incineration: Bottom Ash and Fly Ash; Butterworth-Heinemann: Oxford, UK, 2016. [Google Scholar]
- Tang, J.; Steenari, B.-M. Leaching optimization of municipal solid waste incineration ash for resource recovery: A case study of Cu, Zn, Pb and Cd. Waste Manag. 2016, 48, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zacco, A.; Borgese, L.; Gianoncelli, A.; Struis, R.P.; Depero, L.E.; Bontempi, E. Review of fly ash inertisation treatments and recycling. Environ. Chem. Lett. 2014, 12, 153–175. [Google Scholar] [CrossRef]
- Wang, K.-S.; Chiang, K.-Y.; Lin, S.-M.; Tsai, C.-C.; Sun, C.-J. Effects of chlorides on emissions of toxic compounds in waste incineration: Study on partitioning characteristics of heavy metal. Chemosphere 1999, 38, 1833–1849. [Google Scholar] [CrossRef]
- Li, J.-S.; Xue, Q.; Wang, P.; Wang, H.-Q.; Zhang, T.-T. Leaching characteristics of chlorine from municipal solid waste incineration fly ash by up-flow percolation column tests. Environ. Earth Sci. 2016, 75, 31. [Google Scholar] [CrossRef]
- Schlumberger, S.; Schuster, M.; Ringmann, S.; Koralewska, R. Recovery of high purity zinc from filter ash produced during the thermal treatment of waste and inerting of residual materials. Waste Manag. Res. 2007, 25, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Weibel, G.; Eggenberger, U.; Schlumberger, S.; Mäder, U.K. Chemical associations and mobilization of heavy metals in fly ash from municipal solid waste incineration. Waste Manag. 2017, 62, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, S.; Bühler, J. Metallrückgewinnung aus Filterstäuben der thermischen Abfallbehandlung nach dem FLUREC-Verfahren. In Flaschen–Schlacken–Stäube–aus Abfallverbrennung und Metallurgie; TK Verlag Karl Thomé-Kozmiensky: Neuruppin, Germany, 2013; pp. 377–398. [Google Scholar]
- Heuss-Aßbichler, S.; John, M.; Klapper, D.; Bläß, U.W.; Kochetov, G. Recovery of copper as zero-valent phase and/or copper oxide nanoparticles from wastewater by ferritization. J. Environ. Manag. 2016, 181, 1–7. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Heuss-Aßbichler, S.; Park, S.-H.; Ullrich, A.; Benka, G.; Petersen, N.; Rettenwander, D.; Horn, S.R. Low-temperature synthesis of CuFeO2 (delafossite) at 70 °C: A new process solely by precipitation and ageing. J. Solid State Chem. 2016, 233, 390–396. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Ullrich, A. Conditions and mechanisms for the formation of nano-sized delafossite (CuFeO2) at temperatures ≤ 90° C in aqueous solution. J. Solid State Chem. 2016, 234, 55–62. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Ullrich, A.; Rettenwander, D. Purification of heavy metal loaded wastewater from electroplating industry under synthesis of delafossite (ABO2) by “lt-delafossite process”. Water Res. 2016, 100, 98–104. [Google Scholar] [CrossRef] [PubMed]
- John, M.; Heuss-Aßbichler, S.; Tandon, K.; Ullrich, A. Recovery of Ag and Au from synthetic and industrial wastewater by 2-step ferritization and lt-delafossite process via precipitation. J. Water Process Eng. 2017. [Google Scholar] [CrossRef]
- John, M.; Heuss-Aßbichler, S.; Ullrich, A. Recovery of Zn from wastewater of zinc plating industry by precipitation of doped ZnO nanoparticles. Int. J. Environ. Sci. Technol. 2016, 13, 2127–2134. [Google Scholar] [CrossRef]
- Water Quality—Determination of Dissolved Anions by Liquid Chromatography of Ions—Part 1: Determination of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate and Sulfate; ISO 10304-1:2007; German Version; ISO: Geneva, Switzerland, 2007.
- Bergerhoff, G.; Brown, I.; Allen, F. Crystallographic databases. Int. Union Crystallogr. Chester 1987, 360, 77–95. [Google Scholar]
- Downs, R.T.; Hall-Wallace, M. The American Mineralogist crystal structure database. Am. Mineral. 2003, 88, 247–250. [Google Scholar]
- Gražulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; Le Bail, A. Crystallography open database—An open-access collection of crystal structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quiros, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; Le Bail, A. Crystallography open database (COD): An open-access collection of crystal structures and platform for world-wide collaboration. Nucleic Acids Res. 2011, 40, D420–D427. [Google Scholar] [CrossRef] [PubMed]
- Gražulis, S.; Merkys, A.; Vaitkus, A.; Okulič-Kazarinas, M. Computing stoichiometric molecular composition from crystal structures. J. Appl. Crystallogr. 2015, 48, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Merkys, A.; Vaitkus, A.; Butkus, J.; Okulič-Kazarinas, M.; Kairys, V.; Gražulis, S. COD: CIF: Parser: An error-correcting CIF parser for the perl language. J. Appl. Crystallogr. 2016, 49, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, B.; Downs, R.T.; Yang, H.; Stone, N. The power of databases: The RRUFF project. In Highlights in Mineralogical Crystallography; Walter de Gruyter GmbH: Munich, Germany, 2016. [Google Scholar]
- Gelaude, P.; van Kalmthout, P.; Rewitzer, C. Laurion: The Minerals in the Ancient Slags; Janssen Print: Nijmegen, Netherlands, 1996. [Google Scholar]
- Hawthorne, F.C.; Sokolova, E. Simonkolleite, Zn5(OH)8Cl2(H2O), a decorated interrupted-sheet structure of the form [Mϕ2]4. Can. Mineral. 2002, 40, 939–946. [Google Scholar] [CrossRef]
- Adiwidjaja, G.; Friese, K.; Klaska, K.; Schlüter, J. The crystal structure of gordaite NaZn4(SO4)(OH)6Cl·6H2O. Z. Krist. 1997, 212, 704–707. [Google Scholar] [CrossRef]
- Groat, L.A. The crystal structure of namuwite, a mineral with Zn in tetrahedral and octahedral coordination, and its relationship to the synthetic basic zinc sulfates. Am. Mineral. 1996, 81, 238–243. [Google Scholar] [CrossRef]
- Hoffmann, C.; Armbruster, T.; Giester, G. Acentric structure (P3) of bechererite, Zn7Cu(OH)13[SiO(OH)3SO4]. Am. Mineral. 1997, 82, 1014–1018. [Google Scholar] [CrossRef]
- Santana, J.; Fernández-Pérez, B.; Morales, J.; Vasconcelos, H.; Souto, R.; González, S. Characterization of the corrosion products formed on zinc in archipelagic subtropical environments. Int. J. Electrochem. Sci. 2012, 7, 12730–12741. [Google Scholar]
- Le Bail, A. The profile of a Bragg reflection for extracting intensities. In Powder Diffraction: Theory and Practice; Dinnebier, R.E., Billinge, S.J., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 134–165. [Google Scholar]
- Wu, C.; Qiao, X.; Chen, J.; Wang, H. Controllable ZnO morphology via simple template-free solution route. Mater. Chem. Phys. 2007, 102, 7–12. [Google Scholar] [CrossRef]
- Abdelmohsen, A.H.; El Rouby, W.M.; Ismail, N.; Farghali, A.A. Morphology transition engineering of ZnO nanorods to nanoplatelets grafted Mo8O23–MoO2 by polyoxometalates: Mechanism and possible applicability to other oxides. Sci. Rep. 2017, 7, 5946. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Billinge, S.J. Principles of powder diffraction. In Powder Diffraction: Theory and Practice; Dinnebier, R.E., Billinge, S.J., Eds.; RSC Publishing: Cambridge, UK, 2008; pp. 1–19. [Google Scholar]
- Regenspurg, S.; Feldbusch, E.; Byrne, J.; Deon, F.; Driba, D.L.; Henninges, J.; Kappler, A.; Naumann, R.; Reinsch, T.; Schubert, C. Mineral precipitation during production of geothermal fluid from a Permian Rotliegend reservoir. Geothermics 2015, 54, 122–135. [Google Scholar] [CrossRef]
- Maruyama, S.A.; Krause, F.; Tavares Filho, S.R.; Leitão, A.A.; Wypych, F. Synthesis, cation exchange and dehydration/rehydration of sodium gordaite: NaZn4(OH)6(SO4)Cl·6H2O. Appl. Clay Sci. 2017, 146, 100–105. [Google Scholar] [CrossRef]
- Hawthorne, F.C.; Schindler, M. Topological enumeration of decorated [Cu2+ϕ2]N sheets in hydroxy-hydrated copper-oxysalt minerals. Can. Mineral. 2000, 38, 751–761. [Google Scholar] [CrossRef]

| Element | WW1 (mg/L) | WW2 (mg/L) | WW3 (mg/L) | WW4 (mg/L) |
|---|---|---|---|---|
| Zn | 3570 | 1650 | 711 | 2600 |
| Pb | 1780 | 204 | 70 | 970 |
| Na | 32,200 | 22,500 | n.d. | 27,900 |
| K | 6730 | 7780 | n.d. | 4580 |
| Ca | 4340 | 3450 | n.d. | 7880 |
| SO4 | 2400 | 3000 | 1400 | 1900 |
| Cl | 71,000 | 60,000 | 69,000 | 71,000 |
| Set | System | Experiment | Zn (mg/L) | Pb (mg/L) | Na (mg/L) | K (mg/L) | Ca (mg/L) | SO4 (mg/L) | Cl (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Zn | Zn_low | 2000 | 0 | 0 | 2900 | 0 | ||
| Zn_med | 3000 | 700 | 400 | 3150 | 7000 | ||||
| Zn_high | 30,000 | 7000 | 4000 | 5400 | 70,000 | ||||
| 2 | Zn–Pb | Zn_10Pb_low | 2000 | 200 | 0 | 2200 | |||
| Zn_10Pb_med | 3000 | 700 | 400 | 250 | 9200 | ||||
| Zn_10Pb_high | 30,000 | 7000 | 4000 | 2500 | 72,200 | ||||
| Set | System | Experiment | Zn (mg/L) | Pb (mg/L) | Na (mg/L) | K (mg/L) | Ca (mg/L) | SO4 (mg/L) | Cl (mg/L) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | Zn–Pb–salt | Zn_5Pb_high | 2000 | 100 | 30,000 | 7000 | 4000 | 2500 | 72,200 |
| Zn_10Pb_high | 200 | 72,240 | |||||||
| Zn_40Pb_high | 800 | 72,440 |
| Exp. Set | Experiment | Conc. Zn (mg/L) | Removal Efficiency of Zn (%) | Conc. Pb (mg/L) | Removal Efficiency of Pb (%) |
|---|---|---|---|---|---|
| 1 | Zn_low | ≤0.05 | >99.99 | - | - |
| Zn_med | 0.60 | 99.97 | - | - | |
| Zn_high | 3.20 | 99.84 | - | - | |
| 2 | Zn_10Pb_low | 81.24 | 95.94 | 6.48 | 96.76 |
| Zn_10Pb_med | 0.30 | 99.98 | 0.29 | 99.85 | |
| Zn_10Pb_high | 1.22 | 99.94 | 0.8 | 99.60 | |
| 3 | Zn_5Pb_high | 1.17 | 99.94 | 0.74 | 99.26 |
| Zn_10Pb_high | 1.22 | 99.94 | 0.80 | 99.6 | |
| Zn_40Pb_high | 1.23 | 99.94 | 0.86 | 99.89 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandon, K.; John, M.; Heuss-Aßbichler, S.; Schaller, V. Influence of Salinity and Pb on the Precipitation of Zn in a Model System. Minerals 2018, 8, 43. https://doi.org/10.3390/min8020043
Tandon K, John M, Heuss-Aßbichler S, Schaller V. Influence of Salinity and Pb on the Precipitation of Zn in a Model System. Minerals. 2018; 8(2):43. https://doi.org/10.3390/min8020043
Chicago/Turabian StyleTandon, Kai, Melanie John, Soraya Heuss-Aßbichler, and Valentin Schaller. 2018. "Influence of Salinity and Pb on the Precipitation of Zn in a Model System" Minerals 8, no. 2: 43. https://doi.org/10.3390/min8020043




