Density Functional Theory and Atomic Force Microscopy Study of Oleate Functioned on Siderite Surface
Abstract
:1. Introduction
2. Materials and Methods
2.1. Computational Details
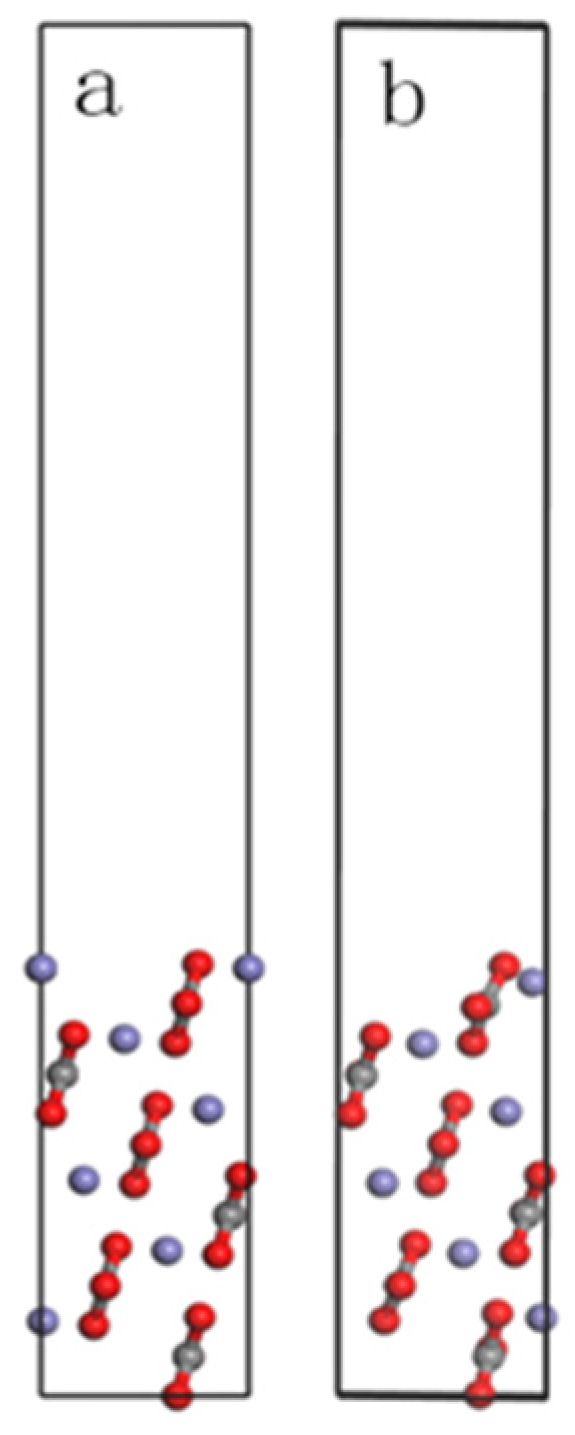
2.2. Models for Siderite Surface
2.3. Mineral-Reagent Complex
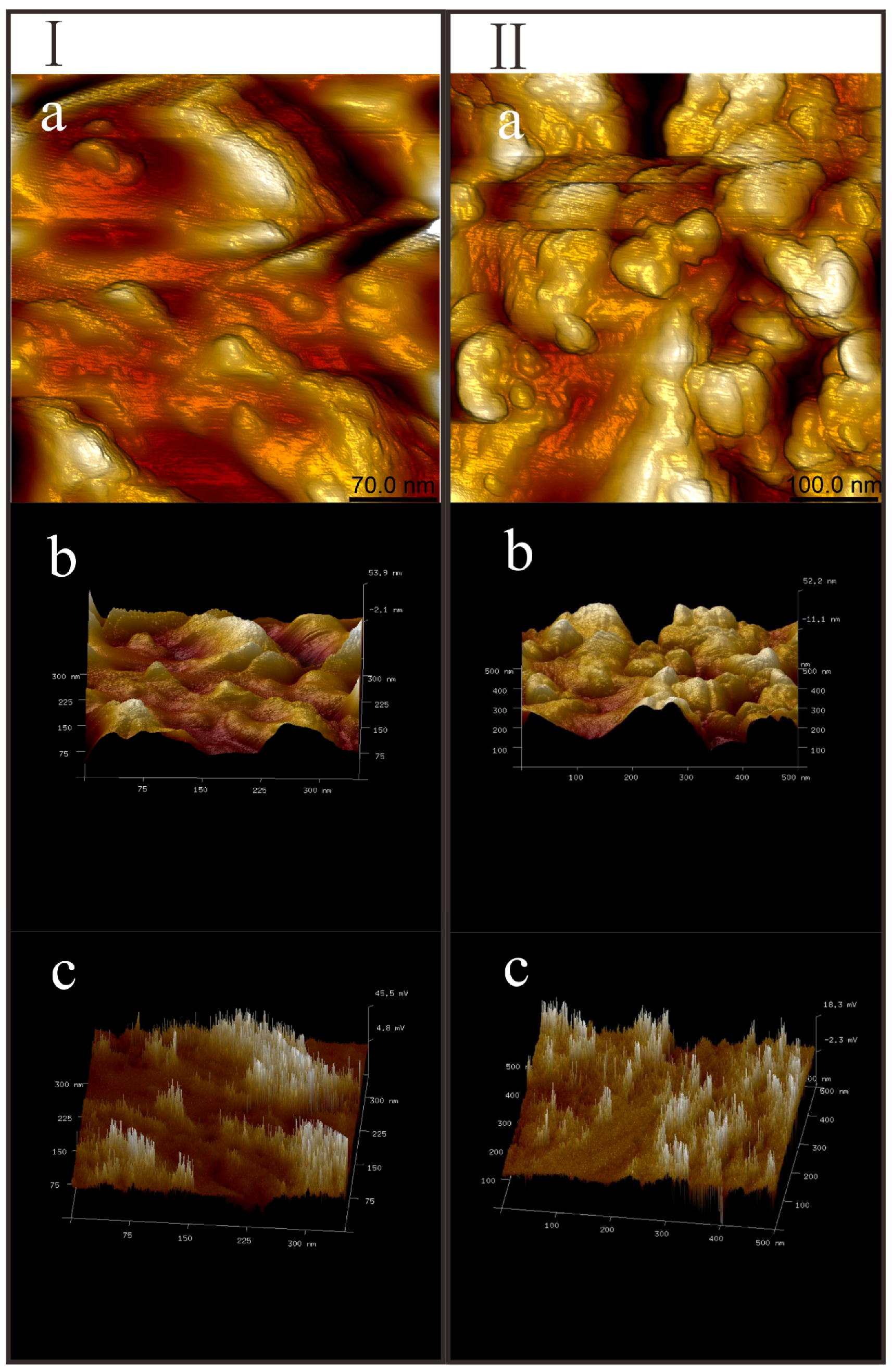
2.4. AFM Measurement
2.5. Materials and Sampling
3. Results and Discussion
3.1. Geometry Optimization of Siderite
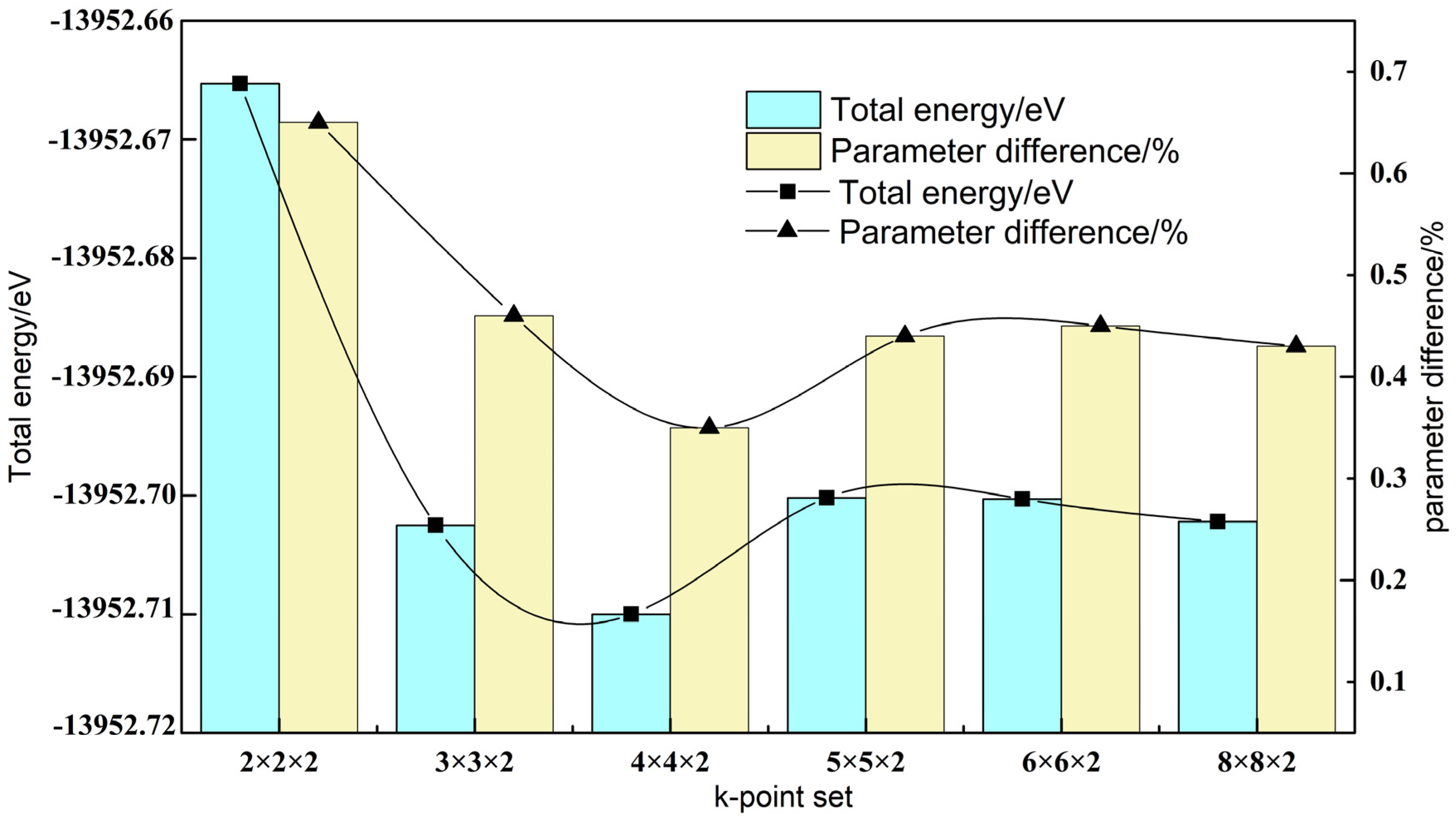
3.2. Siderite Surface Slab Optimization
3.3. Oleate Interaction with Siderite (101) Surface
3.4. PeakForce Tapping Imaging of Siderite
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pradip; Rai, B.A.; Rao, T.K.; Krishnamurthy, S.; Vetrivel, R.; Mielczarski, J.; Cases, J.M. Molecular modeling of Interactions of diphosphonic acid based surfactants with calcium minerals. Langmuir 2002, 18, 932–940. [Google Scholar] [CrossRef]
- Dzade, N.; Roldam, A.; Leeuw, N. A density functional theory study of the adsorption of benzene on hematite (α-Fe2O3) surfaces. Minerals 2014, 4, 89–115. [Google Scholar] [CrossRef]
- Filippov, L.O.; Severov, V.V.; Filippova, I.V. The use of collectors mixture in the reverse cationic flotation of magnetite ore: The role of Fe-bearing silicates. Miner. Eng. 2010, 23, 91–98. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, J. The flotation of quartz from iron minerals with a combined quaternary ammonium salt. Int. J. Miner. Process. 2005, 77, 116–122. [Google Scholar] [CrossRef]
- Turrer, H.D.G.; Peres, A.E.C. Investigation on alternative depressants for iron ore flotation. Miner. Eng. 2010, 23, 1066–1069. [Google Scholar] [CrossRef]
- Birinci, M.; Miller, J.D.; Sarıkaya, M.; Wang, X. The effect of an external magnetic field on cationic flotation of quartz from magnetite. Miner. Eng. 2010, 23, 813–818. [Google Scholar] [CrossRef]
- Montes-Sotomayor, S.; Houot, R.; Kongolo, M. Flotation of silicated gangue iron ores: mechanism and effect of starch. Miner. Eng. 1998, 11, 71–76. [Google Scholar] [CrossRef]
- Li, L.; Yin, W.; Wang, Y.; Tao, S.J. Effect of siderite on flotation separation of martite and quartz. J. Northeast. Univer. (Nat. Sci.) 2012, 33, 431–434. [Google Scholar]
- Rustad, J.R.; Felmy, A.R.; Bylaska, E.J. Molecular simulation of the magnetite-water interface. Geochim. Cosmochim. Acta 2003, 67, 1001–1016. [Google Scholar] [CrossRef]
- Kundu, T.K.; Rao, K.H.; Parker, S.C. Atomistic simulation studies of magnetite surface structures and adsorption behavior in the presence of molecular and dissociated water and formic acid. J. Colloid Interface Sci. 2006, 295, 364–373. [Google Scholar] [CrossRef] [PubMed]
- GMartin, J.; Cutting, R.S.; Vaughan, D.J.; Warren, M.C. Bulk and key surface structures of hematite, magnetite, and goethite: A density functional theory study. Am. Mineral. 2009, 94, 1341–1350. [Google Scholar] [CrossRef]
- Blengino, J.M.; Keddam, M.; Labbe, J.P.; Robbiola, L. Physico-chemical characterization of corrosion layers formed on iron in a sodium carbonate-bicarbonate containing environment. Corros. Sci. 1995, 37, 621–643. [Google Scholar] [CrossRef]
- Badaut, V.; Zeller, P.; Dorado, B.; Schlegel, M.L. Influence of Exchange Correlation on the Symmetry and Properties of Siderite According to Density-Functional Theory. Phys. Rev. B 2010, 82, 82. [Google Scholar] [CrossRef]
- Rath, S.S.; Sinhab, N.; Sahoo, H.; Das, B.; Mishra, B.K. Molecular modeling studies of oleate adsorption on iron oxides. Appl. Surf. Sci. 2014, 295, 115–122. [Google Scholar] [CrossRef]
- Shi, H.; Luo, W.; Johansson, B.; Ahuja, R. First-principles calculations of the electronic structure and pressure-induced magnetic transition in siderite FeCO3. Phys. Rev. B 2008, 78, 155119. [Google Scholar] [CrossRef]
- Blanchard, M.; Poitrasson, F.; Méheut, M.; Lazzeri, M.; Mauri, F.; Balan, E. Iron isotope fractionation between pyrite (FeS2), hematite (Fe2O3) and siderite (FeCO3): A first-principles density functional theory study. Geochim. Cosmochim. Acta 2009, 73, 6565–6578. [Google Scholar] [CrossRef]
- Li, L.; Hao, H.; Yuan, Z.; Liu, J. Molecular dynamics simulation of siderite-hematite-quartz flotation with sodium oleate. Appl. Surf. Sci. 2017, 419, 557–563. [Google Scholar] [CrossRef]
- Banerjee, S.; Gayathri, N.; Dash, S.; Tyagi, A.K.; Raj, B. Imaging distribution of local stiffness over surfaces using atomic force acoustic microscopy. J. Phys. D Appl. Phys. 2007, 40, 2539–2547. [Google Scholar] [CrossRef]
- Kajiyama, K.; Tanaka, K.; Ge, S.R.; Takahara, A. Morphology and mechanical properties of polymer surfaces via scanning force microscopy. Prog. Surf. Sci. 1996, 52, 1–52. [Google Scholar] [CrossRef]
- Zhong, J.; Yan, J. Seeing is believing: Atomic force microscopy imaging for nanomaterial research. RSC Adv. 2015, 6, 1103–1121. [Google Scholar] [CrossRef]
- Renard, F.; Putnis, C.V.; Montes-Hernandez, G.; King, H.E. Interactions of arsenic with calcite surfaces revealed by in situ nanoscale imaging. Geochim. Cosmochim. Acta 2015, 159, 61–79. [Google Scholar] [CrossRef]
- Zhong, Q.; Inniss, D.; Kjoller, K.; Elings, V.B. Fractured polymer/silica fiber surface studied by tapping mode atomic force microscopy. Surf. Sci. Lett. 1993, 290, L688–L692. [Google Scholar] [CrossRef]
- Megevand, B.; Pruvost, S.; Lins, L.C.; Livi, S.; Gerard, J.; Duchet-Rumeau, J. Probing nanomechanical properties with AFM to understand the structure and behavior of polymer blends compatibilized with ionic liquids. RSC Adv. 2016, 6, 96421–96430. [Google Scholar] [CrossRef]
- Mou, J.; Czajkowsky, D.M.; Sheng, S.J.; Ho, R.; Shao, Z. High resolution surface structure of E. coli GroES oligomer by atomic force microscopy. FEBS Lett. 1996, 381, 161–164. [Google Scholar] [CrossRef]
- Li, Q.; Qian, H.; Guo, D.; Liu, Y. Complexation of p-sulfonatocalixarenes with local anaesthetics guests: binding structures, stabilities, and thermodynamic origins. Eur. J. Org. Chemi. 2012, 2012, 3962–3971. [Google Scholar] [CrossRef]
- Bag, M.; Renna, L.A.; Adhikari, R.Y.; Karak, S.; Liu, F.; Lahti, P.M.; Russell, T.P.; Tuominen, M.T.; Venkataraman, D. Kinetics of ion transport in perovskite active layers and its implications for active layer stability. J. Am. Chem. Soc. 2015, 137, 13130–13137. [Google Scholar] [CrossRef] [PubMed]
- Kemnade, N.; Shearer, C.J.; Dieterle, D.J.; Cherevan, A.S.; Gebhardt, P.; Wilde, G.; Eder, D. Non-destructive functionalisation for atomic layer deposition of metal oxides on carbon nanotubes: Effect of linking agents and defects. Nanoscale 2015, 7, 3028–3034. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Li, W.; Yu, A.; Wu, P. Carbon-coated mesoporous tio2 nanocrystals grown on graphene for lithium-ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 10395. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Martin, S.T. Siderite dissolution in the presence of chromate. Geochim. Cosmochim. Acta 2011, 75, 4951–4962. [Google Scholar] [CrossRef]
- Weaver, R.M.; Grandstaff, D.E.; Myer, G.H. Surface stability of siderite under acidic atmosphere: An atomic force microscopy study. Mater. Res. Soc. 1996, 453, 727–732. [Google Scholar] [CrossRef]
- Lavina, B.; Dera, P.; Downs, R.T.; Yang, W.; Sinogeikin, S.; Meng, Y.; Shen, G.; Schiferl, D. Siderite at lower mantle conditions and the effects of the pressure-induced spin-pairing transition. Geophys. Res. Lett. 2009, 36, L23306. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Chen, Z.; Yang, K. A homochiral metal-organic framework membrane for enantioselective separation. Chem. Commun. 2012, 48, 7022. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Sun, W.; Liu, X. Anisotropic surface energies and adsorption behaviors of scheelite crystal. Colloid Surf. Physicochem. Eng. Asp. 2012, 415, 439–448. [Google Scholar] [CrossRef]
- Xua, L.; Hu, Y.; Dong, F.; Gao, Z.; Wu, H.; Wang, Z. Anisotropic adsorption of oleate on diaspore and kaolinite crystals: Implications for their flotation separation. Appl. Surf. Sci. 2014, 321, 331–338. [Google Scholar] [CrossRef]
- Pavel, T.; Josef, K.; Udo, V. On the use of peak-force tapping atomic force microscopy for quantification of the local elastic modulus in hardened cement paste. Cem. Concr. Res. 2012, 42, 215–221. [Google Scholar] [CrossRef]
- Prywer, J.; Cryst, J. Effect of crystal geometry on disappearance of slow-growing faces. J. Cryst. Growth 2001, 224, 134–144. [Google Scholar] [CrossRef]
- Yin, W.; Li, D.; Luo, X.; Yao, J.; Sun, Q. Effect and mechanism of siderite on reverse flotation of hematite. Int. J. Miner. Metall. Mater. 2016, 23, 373–379. [Google Scholar] [CrossRef]
- Fakhrullina, G.; Akhatova, F.; Kibardina, M.; Fokin, D.; Fakhrullin, R. Nanoscale imaging and characterisation of Caenorhabditis elegans epicuticle using atomic force microscopy. Nanomed. Nanotechnol. Biol. Med. 2016, 13, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Seiedi, O.; Rahbar, M.; Nabipour, M.; Emadi, M.A.; Ghatee, M.H.; Ayatollahi, S. Atomic force microscopy (afm) investigation on the surfactant wettability alteration mechanism of aged mica mineral surfaces. Energy Fuels 2011, 25, 183–188. [Google Scholar] [CrossRef]



| Category | Correlation-Exchange Potential | a/Å | V/(Å3) | α/° | Difference 1/% |
|---|---|---|---|---|---|
| Experimental | 5.797 | 98.14 | 47.73 | - | |
| Simulation | GGA-PBE | 5.962 | 102.60 | 46.72 | 2.8 |
| GGA-RPBE | 6.157 | 109.07 | 45.71 | 6.2 | |
| GGA-PW91 | 5.907 | 101.29 | 47.13 | 1.8 | |
| GGA-WC | 5.783 | 96.71 | 47.64 | 0.2 | |
| GGA-PBESOL | 5.734 | 95.90 | 48.15 | 1.1 |
| Parameter | Experiment | Simulation | Difference/% |
|---|---|---|---|
| V/Å3 | 98.14 | 96.36 | 1.8 |
| a/Å | 75.797 | 75.758 | 0.6 |
| α/° | 47.73 | 47.73 | 0.1 |
| Conditions | Slab Depth/Å | Vacuum Thickness/Å | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 19.132 | 23.039 | 26.946 | 30.852 | 34.758 | 20 | 22 | 24 | 26 | 28 | |
| Surface energy/J·m−2 | 0.212 | 0.326 | 0.328 | 0.294 | 0.284 | 0.303 | 0.309 | 0.357 | 0.327 | 0.330 |
| Atom | Bond | Population | Length/Å |
|---|---|---|---|
| Fe1 | Fe1-O1 | 0.50 | 1.746 |
| Fe1-O4 | 0.27 | 1.901 | |
| Fe1-O5 | 0.04 | 2.937 | |
| Fe1-C2 | −0.32 | 2.794 | |
| O1 | O1-C1 | 0.66 | 1.364 |
| O1-H1 | −0.01 | 2.496 | |
| O1-O3 | −0.01 | 2.931 |
| Atom | Status | s | d | p | Total/e | Charge/e |
|---|---|---|---|---|---|---|
| Fe1 | Before adsorption | 0.31 | 6.21 | 0.31 | 6.83 | 1.17 |
| After adsorption | 0.37 | 6.51 | 0.13 | 7.01 | 0.99 | |
| O1 | Before adsorption | 1.83 | 0 | 4.88 | 6.71 | −0.71 |
| After adsorption | 1.81 | 0 | 4.73 | 6.54 | −0.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, C.; Yuan, Z.; Hao, H.; Zhao, C. Density Functional Theory and Atomic Force Microscopy Study of Oleate Functioned on Siderite Surface. Minerals 2018, 8, 33. https://doi.org/10.3390/min8010033
Li L, Zhang C, Yuan Z, Hao H, Zhao C. Density Functional Theory and Atomic Force Microscopy Study of Oleate Functioned on Siderite Surface. Minerals. 2018; 8(1):33. https://doi.org/10.3390/min8010033
Chicago/Turabian StyleLi, Lixia, Chen Zhang, Zhitao Yuan, Haiqing Hao, and Chenyang Zhao. 2018. "Density Functional Theory and Atomic Force Microscopy Study of Oleate Functioned on Siderite Surface" Minerals 8, no. 1: 33. https://doi.org/10.3390/min8010033




