Efficient Extraction of Vanadium from Vanadium–Titanium Magnetite Concentrate by Potassium Salt Roasting Additives
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure and Methods
3. Results and Discussion
3.1. Roasting Process
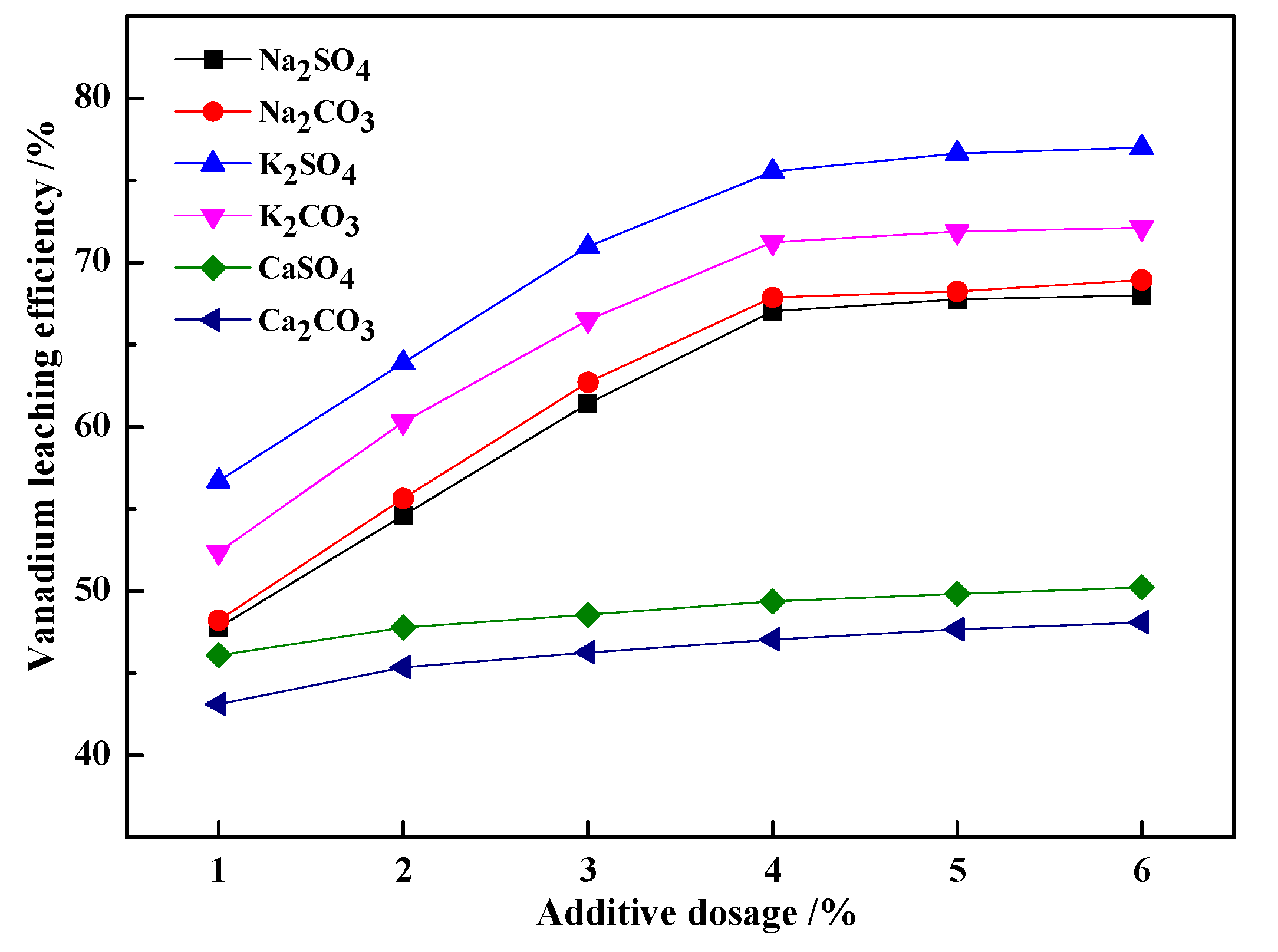
3.1.1. Effect of Different Additives and Their Dosage on the Vanadium Leaching Efficiency
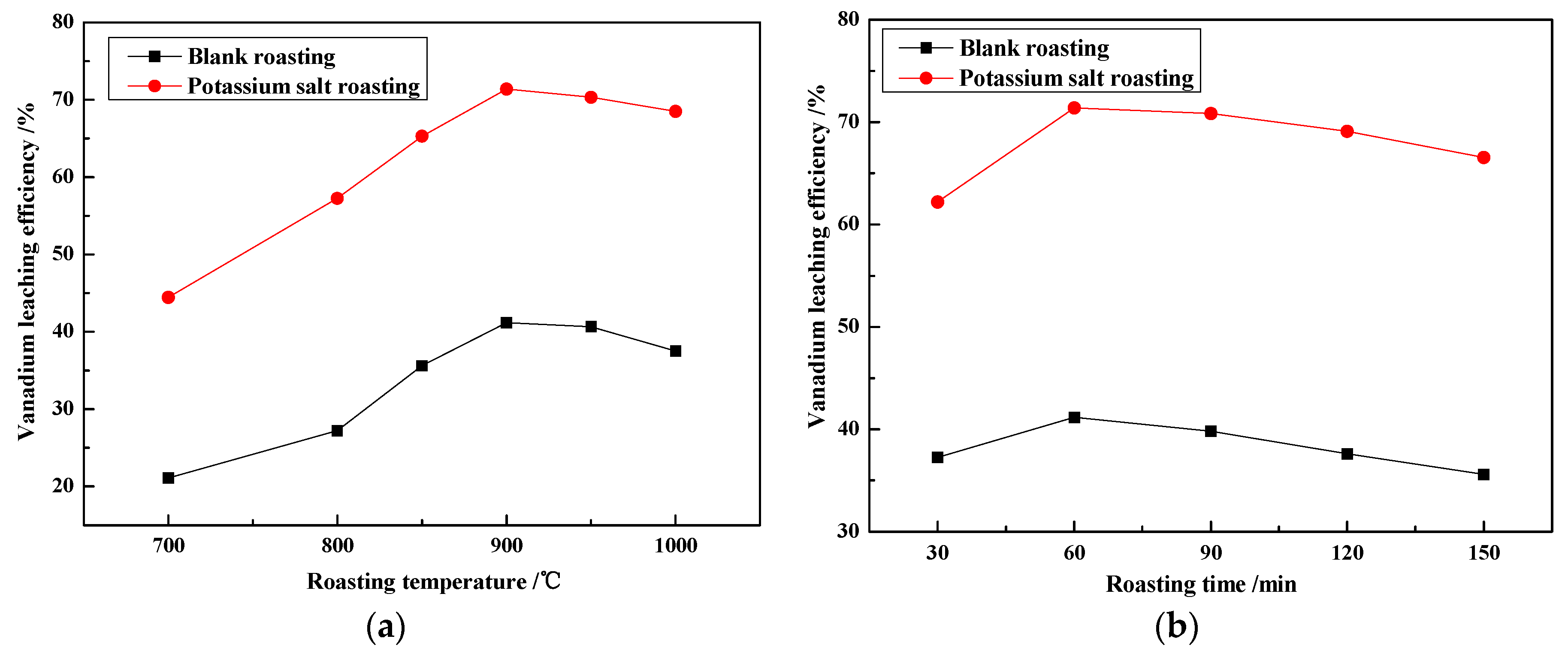
3.1.2. Effect of Roasting Temperature and Roasting Time on Vanadium Leaching Efficiency
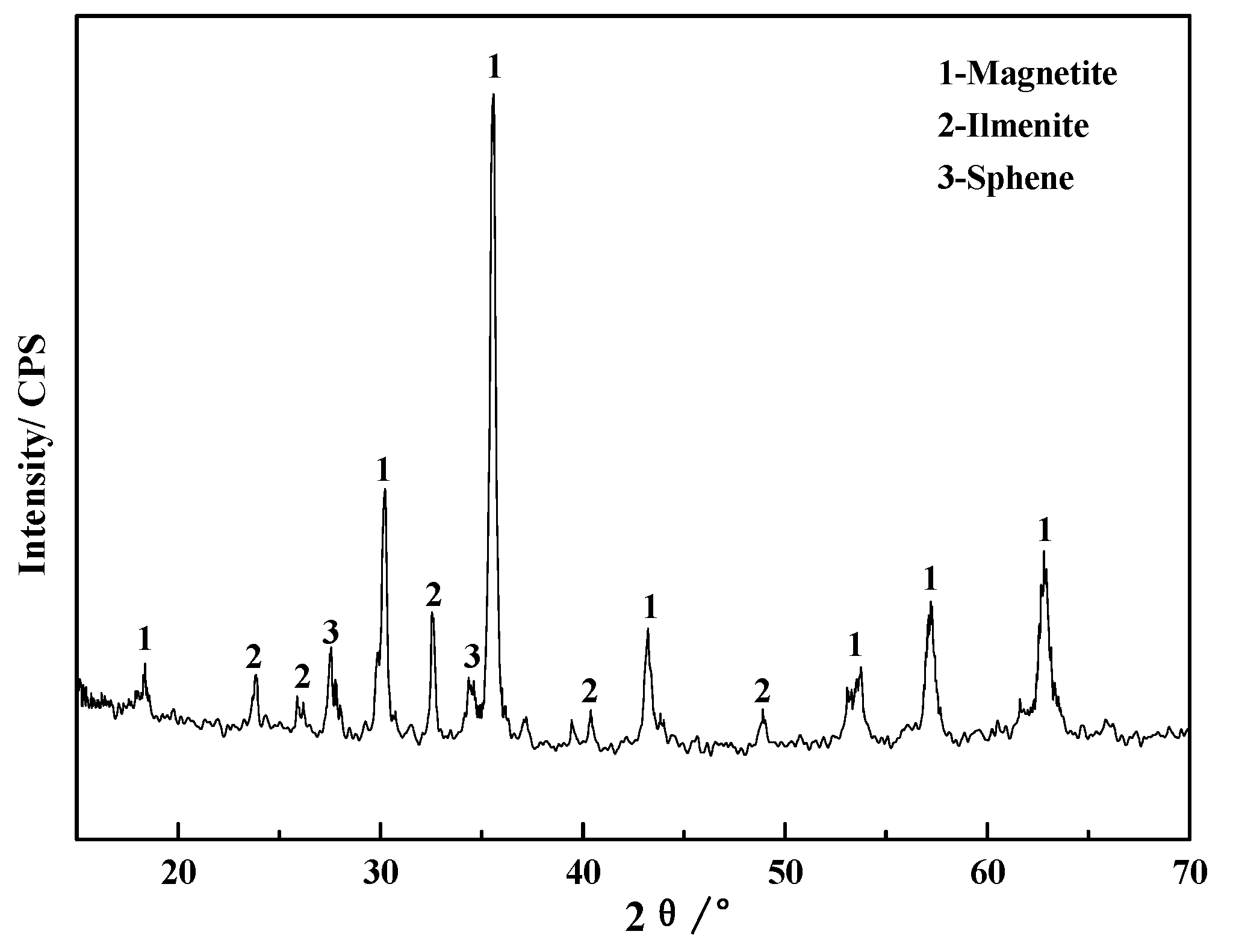
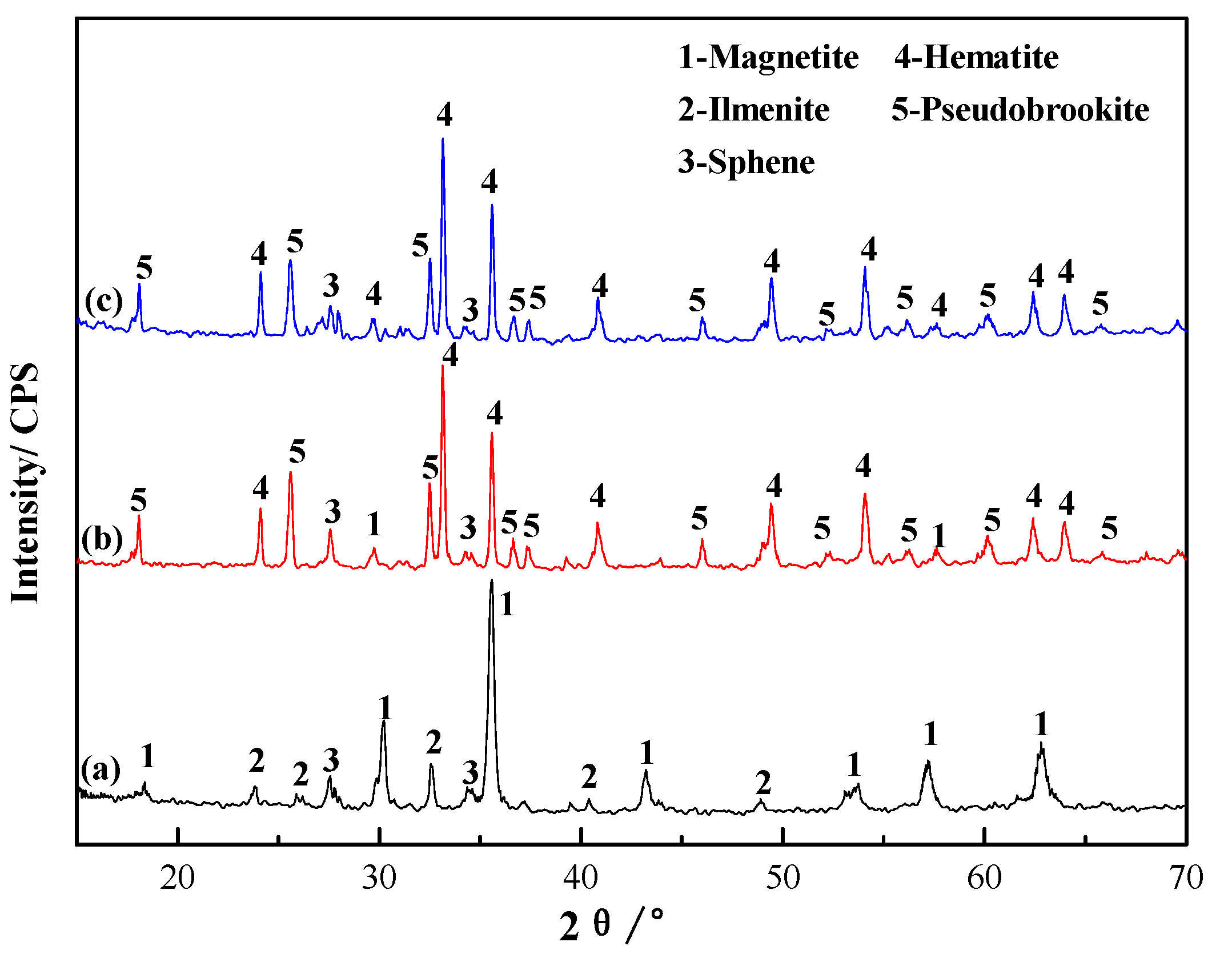
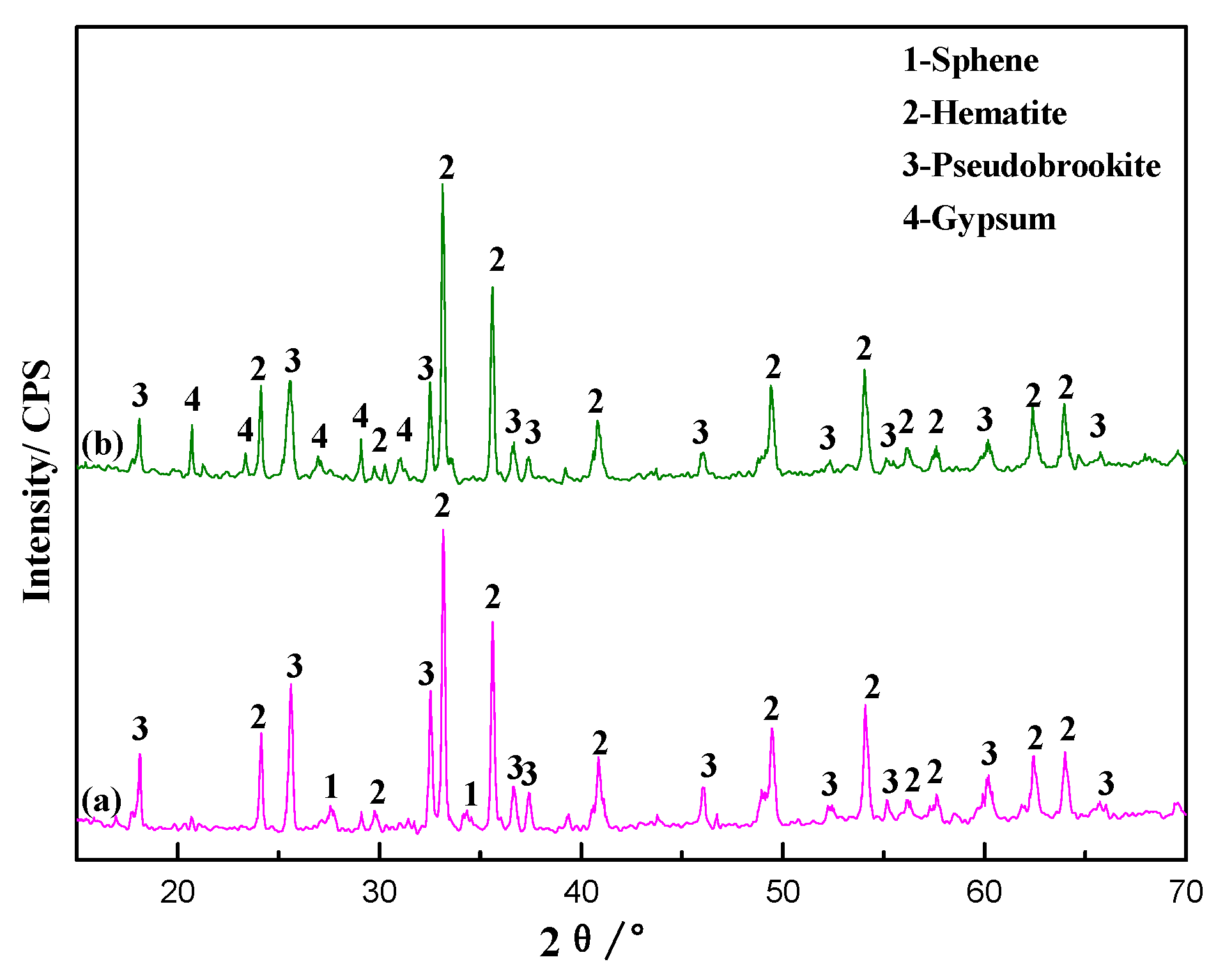
3.1.3. Analyses of Phase Transformation in the Roasting Process
3.2. Leaching Process
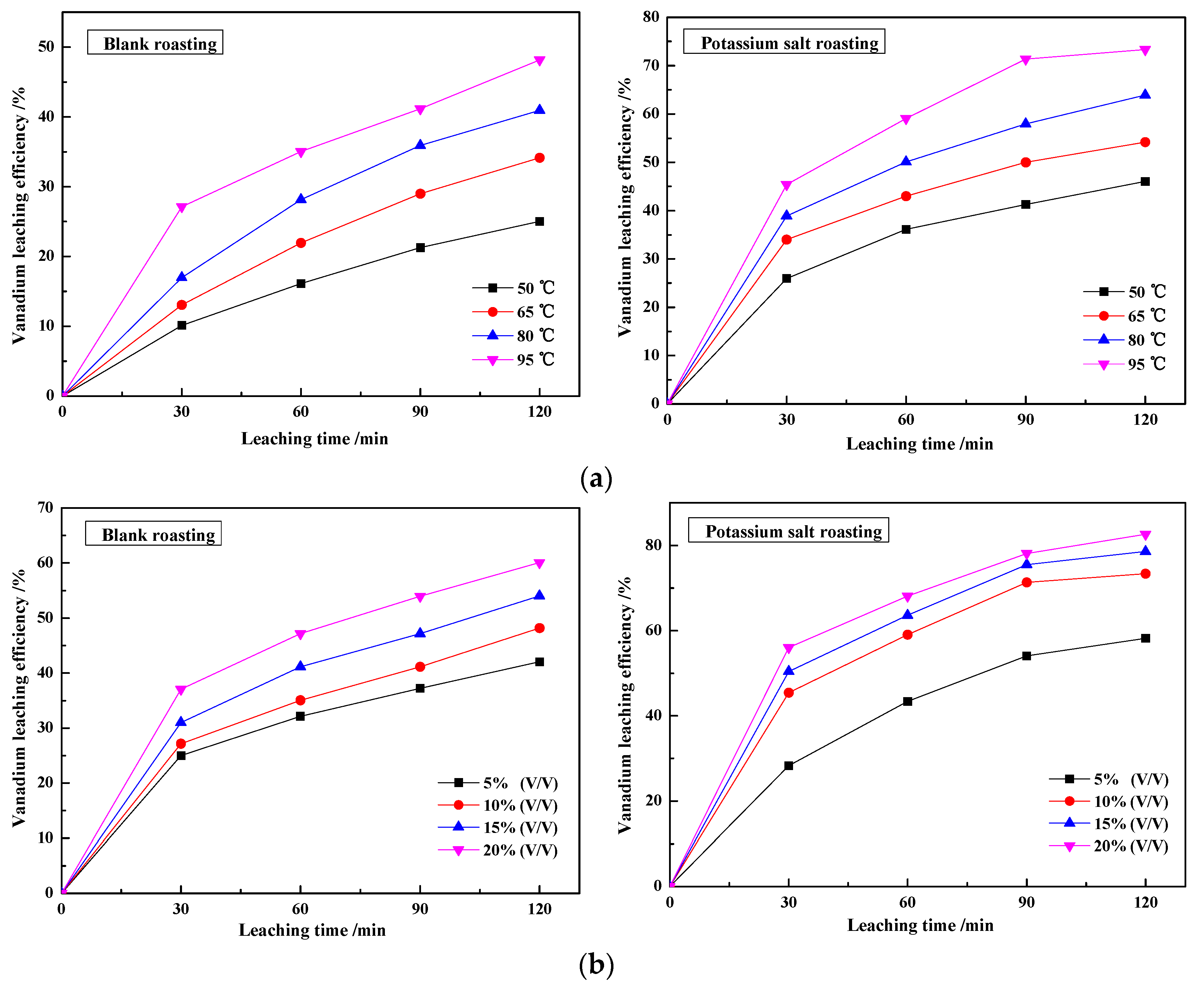
3.2.1. Effect of Leaching Temperature and Sulfuric Acid Concentration on the Vanadium Leaching Efficiency
3.2.2. Analyses of Phase Transformation in the Leaching Process
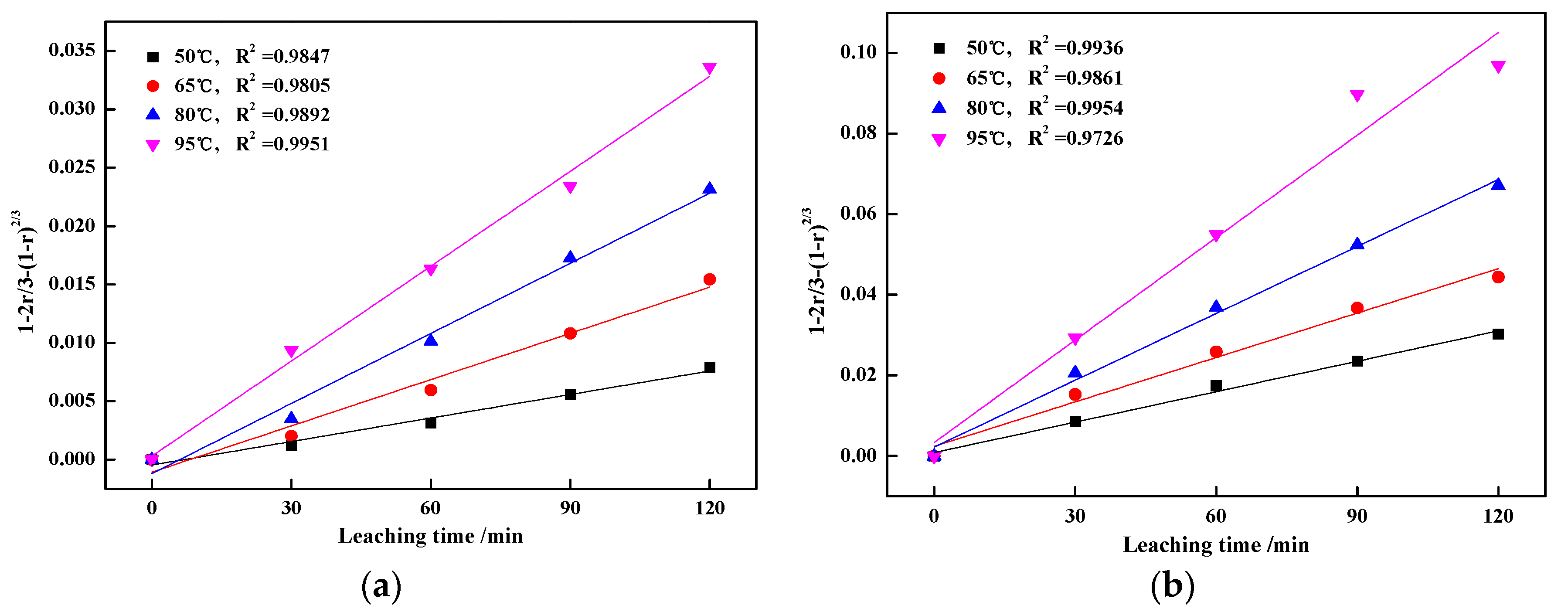
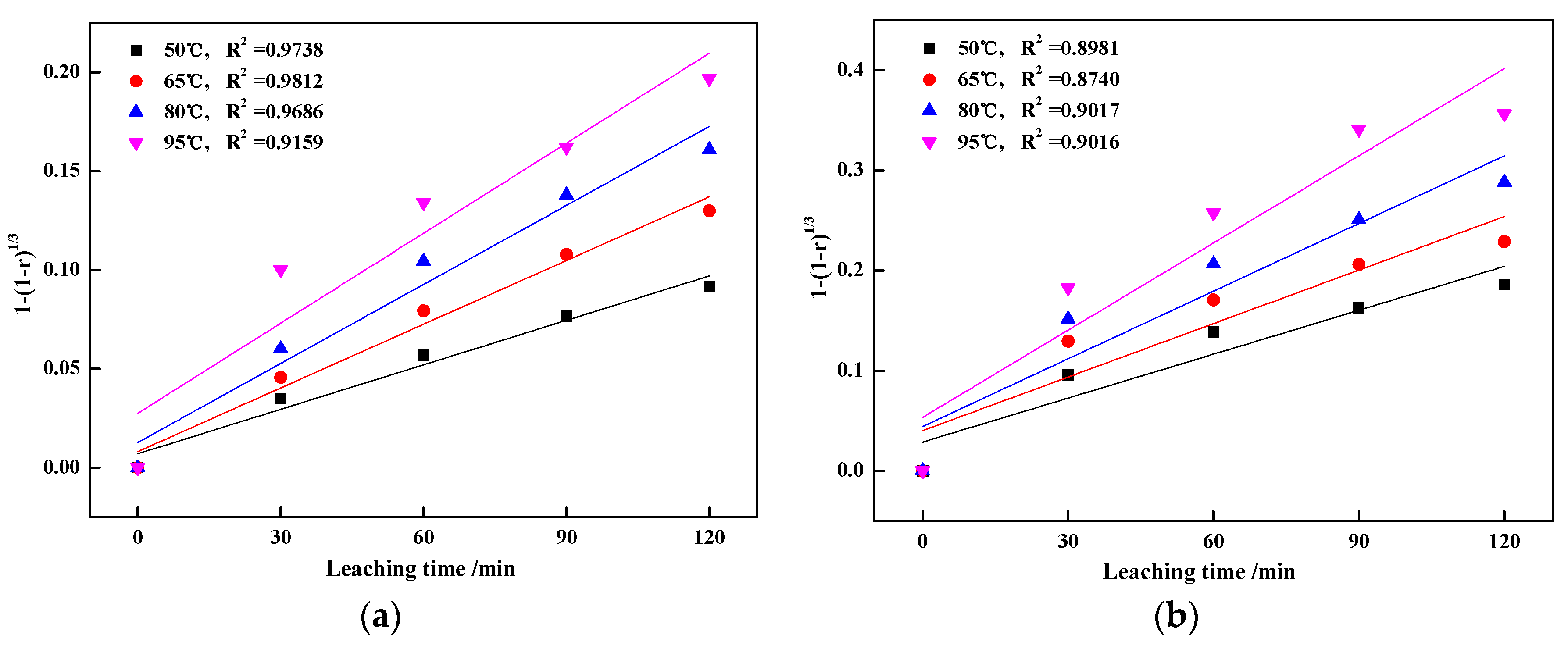
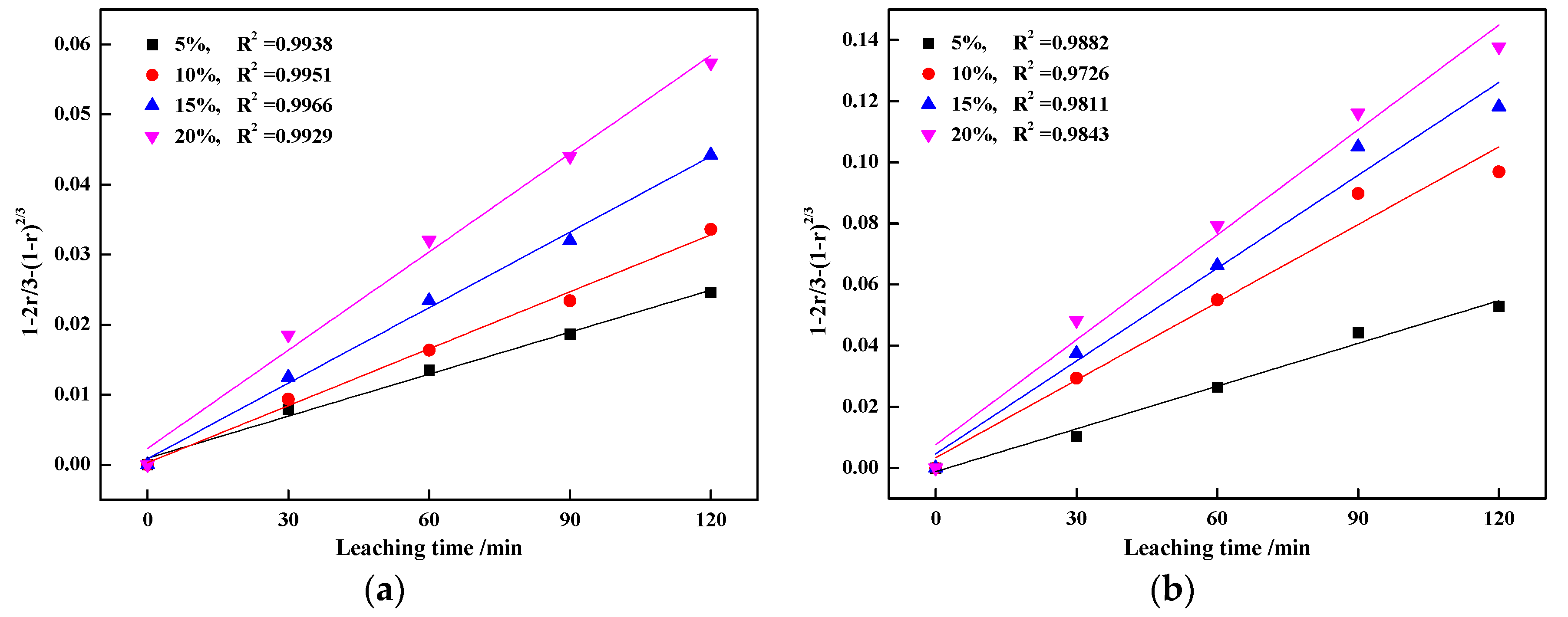
3.3. Kinetics Analyses of Vanadic Acid Leaching Process
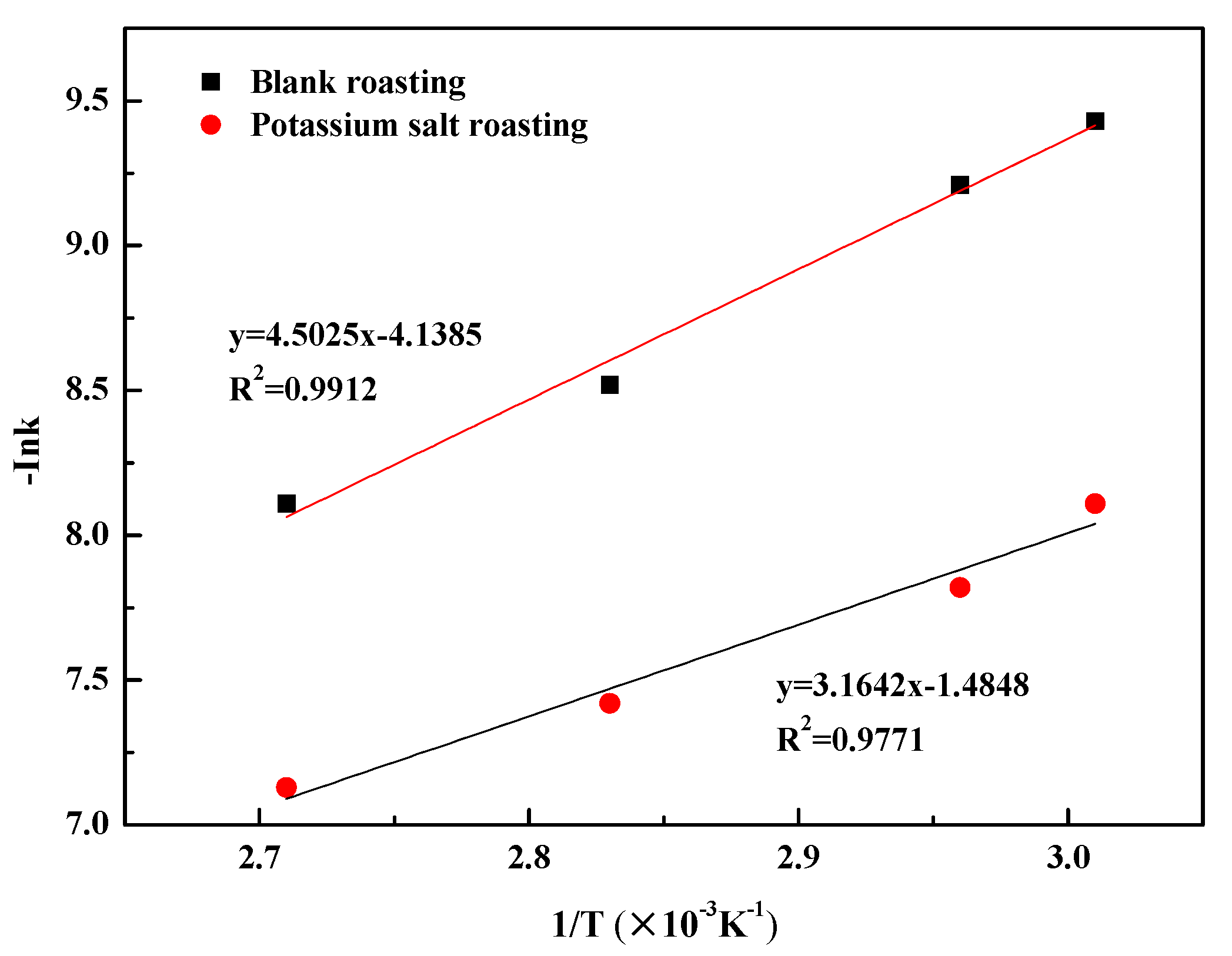
3.3.1. Calculation of Apparent Activation Energy
3.3.2. Calculation of Reaction Orders
4. Conclusions
- The effects of potassium salt roasting additives were more efficient than traditional sodium and calcium salt. Particularly, K2SO4 was preferred as the roasting additive. Under certain conditions (the dosage of K2SO4 was 4 wt %, the roasting temperature was 900 °C, the roasting time was 1 h, the leaching temperature was 95 °C, the sulfuric acid concentration was 10% (v/v), and the leaching time was 1.5 h, with a liquid to solid ratio of 3 mL/g) the vanadium leaching efficiency increased from 41.17% with blank roasting to 71.37%.
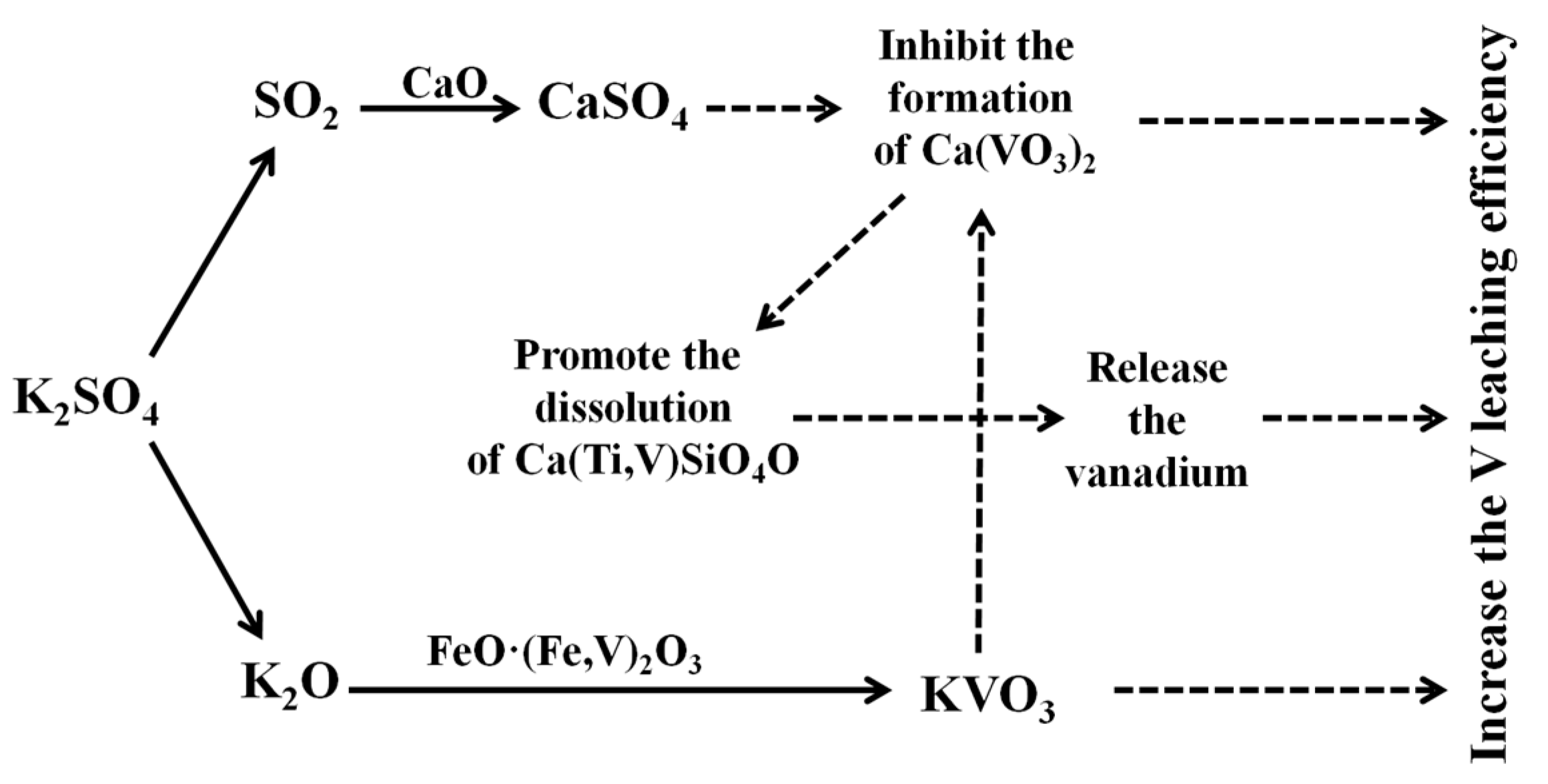
- K2SO4 could fully destroy the structure of vanadium-bearing minerals such as magnetite, and could promote the formation of KVO3 to inhibit the formation of Ca(VO3)2 in the roasting process. Moreover, promoting the dissolution of sphene to release its vanadium in the leaching process significantly increases the vanadium leaching efficiency.
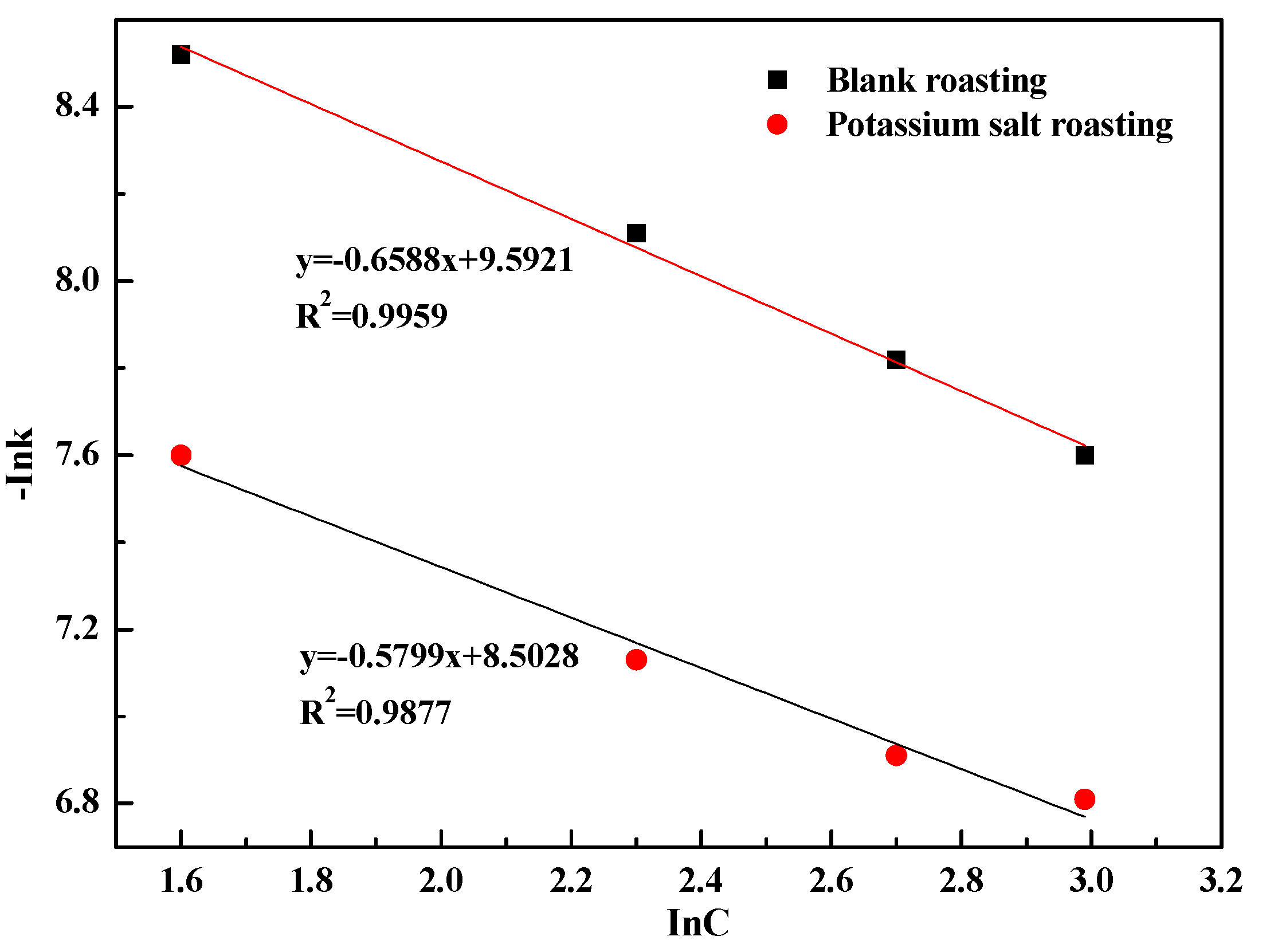
- The leaching process was controlled by internal diffusion; the apparent activation energy decreased from 37.43 kJ/mol of blank roasting to 26.31 kJ/mol of potassium salt roasting. At the same time, the reaction order with regard to sulfuric acid concentration decreased from 0.6588 to 0.5799. Therefore, potassium salt roasting could accelerate the leaching process and reduce the dependence on high temperature and high acidity to improve mineral activity.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy. 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, W.; Guan, X.M. Vanadium extraction from titano-magnetite by hydrofluoric acid. Int. J. Miner. Process. 2016, 157, 55–59. [Google Scholar] [CrossRef]
- Qiu, H.D.; Zhang, H.; Zhao, B.; Zhu, J.F.; Liu, D.R. Dynamics study on vanadium extraction technology from chloride leaching steel slag. Rare Metal. Mater. Eng. 2013, 42, 696–699. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Huang, Q.Y.; Lv, X.W.; Bai, C.G. Multistage utilization process for the gradient-recovery of V, Fe, and Ti from vanadium-bearing converter slag. J. Hazard. Mate 2017, 336, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.G.; Lin, X.; Wang, X.Y.; Gao, H.B. High-efficient extraction of vanadium and its application in the utilization of the chromium-bearing vanadium slag. Chem. Eng. J. 2016, 301, 132–138. [Google Scholar] [CrossRef]
- Aarabi-Karasgani, M.; Rashchi, F.; Mostoufi, N.; Vahidi, E. Leaching of vanadium from LD converter slag using sulfuric acid. Hydrometallurgy 2010, 102, 14–21. [Google Scholar] [CrossRef]
- Li, M.; Liu, B.; Zheng, S.L.; Wang, S.N.; Du, H.; Dreisinger, D.B.; Zhang, Y. A cleaner vanadium extraction method featuring non-salt roasting and ammonium bicarbonate leaching. J. Clean. Prod. 2017, 149, 206–217. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Zhang, T.A.; Zhang, Y.; Lv, G.Z.; Liu, Y.; Liu, Z.L. Pressure leaching of converter vanadium slag with waste titanium dioxide. Rare Met. 2016, 35, 576–580. [Google Scholar] [CrossRef]
- Xu, C.B.; Zhang, Y.M.; Liu, T.; Huang, J. Characterization and pre-concentration of low-grade vanadium-titanium magnetite ore. Minerals 2017, 7, 137. [Google Scholar] [CrossRef]
- Zhao, L.S.; Wang, L.N.; Qi, T.; Chen, D.S.; Zhao, H.X.; Liu, Y.H. A novel method to extract iron, titanium, vanadium, and chromium from high-chromium vanadium-bearing titanomagnetite concentrates. Hydrometallurgy 2014, 149, 106–109. [Google Scholar] [CrossRef]
- Li, M.; Du, H.; Zheng, S.L.; Wang, S.N.; Zhang, Y.; Liu, B.; Dreisinger, D.B.; Zhang, Y. Extraction of vanadium from vanadium slag via non-salt roasting and ammonium oxalate leaching. JOM 2017, 69, 1970–1975. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, K.; Hua, H.W.; Yang, Z.; Zhou, W.; Xie, B. Selective leaching of vanadium in calcification-roasted vanadium slag by ammonium carbonate. Hydrometallurgy 2016, 160, 18–25. [Google Scholar] [CrossRef]
- Shi, Q.H.; Zhang, Y.M.; Huang, J.; Liu, T.; Liu, H.; Wang, L.Y. Synergistic solvent extraction of vanadium from leaching solution of stone coal using D2EHPA and PC88A. Sep. Purif. Technol. 2017, 181, 1–7. [Google Scholar] [CrossRef]
- Zhang, X.F.; Liu, F.G.; Xue, X.X.; Jiang, T. Effects of microwave and conventional blank roasting on oxidation behavior, microstructure and surface morphology of vanadium slag with high chromium content. J. Alloys Compd. 2016, 686, 356–365. [Google Scholar] [CrossRef]
- Li, L.J.; Zhang, L.; Zheng, S.L.; Lou, T.P.; Zhang, Y.; Chen, D.H.; Zhang, Y. Acid leaching of calcined vanadium titano magnetite with calcium compounds for extraction of vanadium. Chin. J. Process. Eng. 2011, 11, 573–578. [Google Scholar]
- Li, X.S.; Xie, B.; Wang, G.E.; Li, X.J. Oxidation process of low-grade vanadium slag in presence of Na2CO3. Trans. Nonferrous Met. Soc. 2011, 21, 1860–1867. [Google Scholar] [CrossRef]
- Zhao, Y.; Hong, Y.L.; Yin, X.C.; Yan, Z.M.; Yan, X.M. Leaching kinetics of calcification roasted vanadium slag with high CaO content by sulfuric acid. Int. J. Miner. Process. 2014, 133, 105–111. [Google Scholar]
- Ippolito, N.M.; Innocenzi, V.; De Michelis, I.; Medici, F.; Vegliò, F. Rare earth elements recovery from fluorescent lamps: A new thermal pretreatment to improve the efficiency of the hydrometallurgical process. J. Clean. Prod. 2017, 153, 287–298. [Google Scholar] [CrossRef]
- Innocenzi, V.; Ippolito, N.M.; De Michelis, I.; Medici, F.; Vegliò, F. A hydrometallurgical process for the recovery of rare earths from fluorescent lamps: Experimental design, optimization of terbium acid leaching process and process analysis. J. Environ. Manag. 2016, 184, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, S.; Wang, S.; Qin, Y.; Du, H.; Zhang, Y. Electrochemical decomposition of vanadium slag in concentrated NaOH solution. Hydrometallurgy 2015, 151, 51–55. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Y.M.; Liu, T. Experiment and mechanism study on vanadium extraction from stone coal by activation roasting. Chin. J. Rare Met. 2013, 37, 284–288. [Google Scholar]
- Li, H.Y.; Fang, H.X.; Wang, K.; Zhou, W.; Yang, Z.; Yan, X.M.; Ge, W.S.; Li, Q.W.; Xie, B. Asynchronous extraction of vanadium and chromium from vanadium slag by stepwise sodium roasting-water leaching. Hydrometallurgy 2015, 156, 124–135. [Google Scholar] [CrossRef]
- Beijing General Research Institute of Mining & Metallurgy. Chemical Phase Analyses; Metallurgical Industry Press: Beijing, China, 1979. [Google Scholar]
- Chen, F.; Zhang, Y.M.; Huang, J.; Liu, T.; Xue, N.N. Mechanism of enhancing extraction of vanadium from stone coal by roasting with MgO. Minerals 2017, 7. [Google Scholar] [CrossRef]
- Higher Education Press. Inorganic Chemistry; Higher Education Press: Beijing, China, 2010. [Google Scholar]
- Wang, B.; Liu, T.; Zhang, Y.M.; Huang, J. Effect of CaF2/CaO composite additive on roasting of vanadium-bearing stone coal and acid leaching kinetics. Minerals 2017, 7, 43. [Google Scholar] [CrossRef]
- Hu, P.C.; Zhang, Y.M.; Liu, T.; Huang, J.; Yuan, Y.Z.; Zheng, Q.S. Highly selective separation of vanadium over iron from stone coal by oxalic acid leaching. J. Ind. Eng. Chem. 2017, 45, 241–247. [Google Scholar] [CrossRef]
- Yuan, Y.Z.; Zhang, Y.M.; Liu, T.; Chen, T.J.; Huang, J. Source separation of V and Fe by two-stage selective leaching during V extraction from stone coal. RSC Adv. 2017, 7, 18438–18446. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M. V and Ni recovery from a vanadium-rich power plant residual ash using acid producing fungi: Aspergillus Niger and Penicillium simplicissimum. RSC Adv. 2016, 6, 9139–9151. [Google Scholar] [CrossRef]
- Zhu, X.B.; Zhang, Y.M.; Huang, J.; Liu, T.; Wang, Y. A kinetics study of multi-stage counter-current circulation acid leaching of vanadium from stone coal. Int. J. Miner. Process. 2012, 114–117, 1–6. [Google Scholar] [CrossRef]
- Cai, Z.L.; Zhang, Y.M.; Liu, T.; Huang, J. Mechanisms of vanadium recovery from stone coal by novel BaCO3/CaO composite additive roasting and acid leaching technology. Minerals 2016, 6, 26. [Google Scholar] [CrossRef]
- Espiari, S.; Rashchi, F.; Sadrnezhaad, S.K. Hydrometallurgical treatment of tailings with high zinc content. Hydrometallurgy 2006, 82, 54–62. [Google Scholar] [CrossRef]
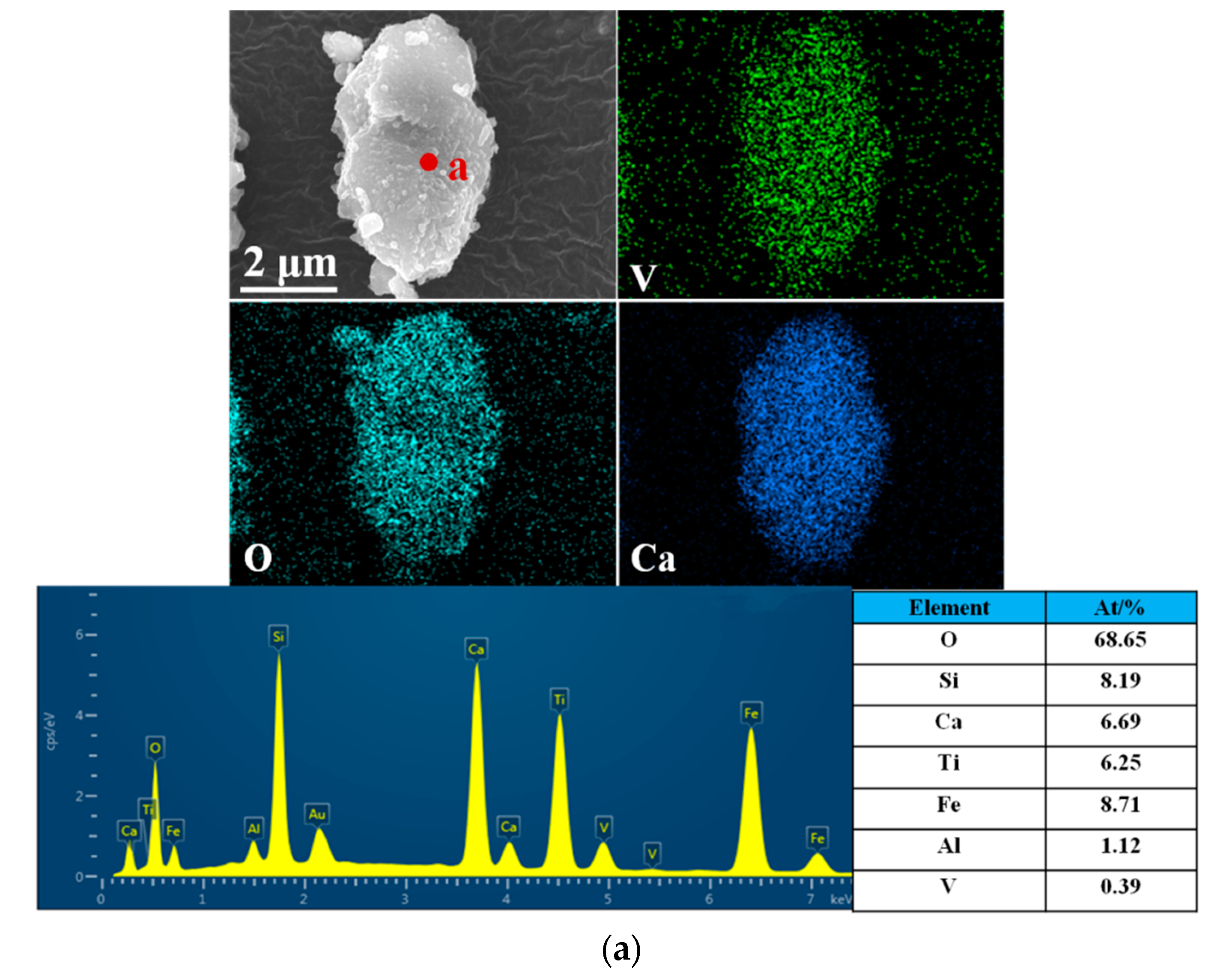


| Element | V2O5 | TiO2 | TFe | SiO2 | Al2O3 | CaO | MgO | S | Cu |
|---|---|---|---|---|---|---|---|---|---|
| Content | 1.10 | 19.72 | 44.2 | 9.85 | 3.14 | 4.71 | 0.78 | 0.026 | 0.002 |
| Vanadium Phase | Magnetite | Ilmenite | Sphene |
|---|---|---|---|
| Content | 63.54 | 5.47 | 30.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Liu, T.; Zhang, Y.; Huang, J.; Xu, C. Efficient Extraction of Vanadium from Vanadium–Titanium Magnetite Concentrate by Potassium Salt Roasting Additives. Minerals 2018, 8, 25. https://doi.org/10.3390/min8010025
Li R, Liu T, Zhang Y, Huang J, Xu C. Efficient Extraction of Vanadium from Vanadium–Titanium Magnetite Concentrate by Potassium Salt Roasting Additives. Minerals. 2018; 8(1):25. https://doi.org/10.3390/min8010025
Chicago/Turabian StyleLi, Renmin, Tao Liu, Yimin Zhang, Jing Huang, and Chengbao Xu. 2018. "Efficient Extraction of Vanadium from Vanadium–Titanium Magnetite Concentrate by Potassium Salt Roasting Additives" Minerals 8, no. 1: 25. https://doi.org/10.3390/min8010025




