Flotation Behavior of Different Colored Fluorites Using Sodium Oleate as a Collector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Micro-Flotation Tests
2.2.2. Measurement of Collector Adsorption Capacity
2.2.3. FT-IR Measurements
2.2.4. Surface Tension Measurement
2.2.5. Zeta Potential Measurements
2.2.6. Micro-Topography Studies
3. Results and Discussion
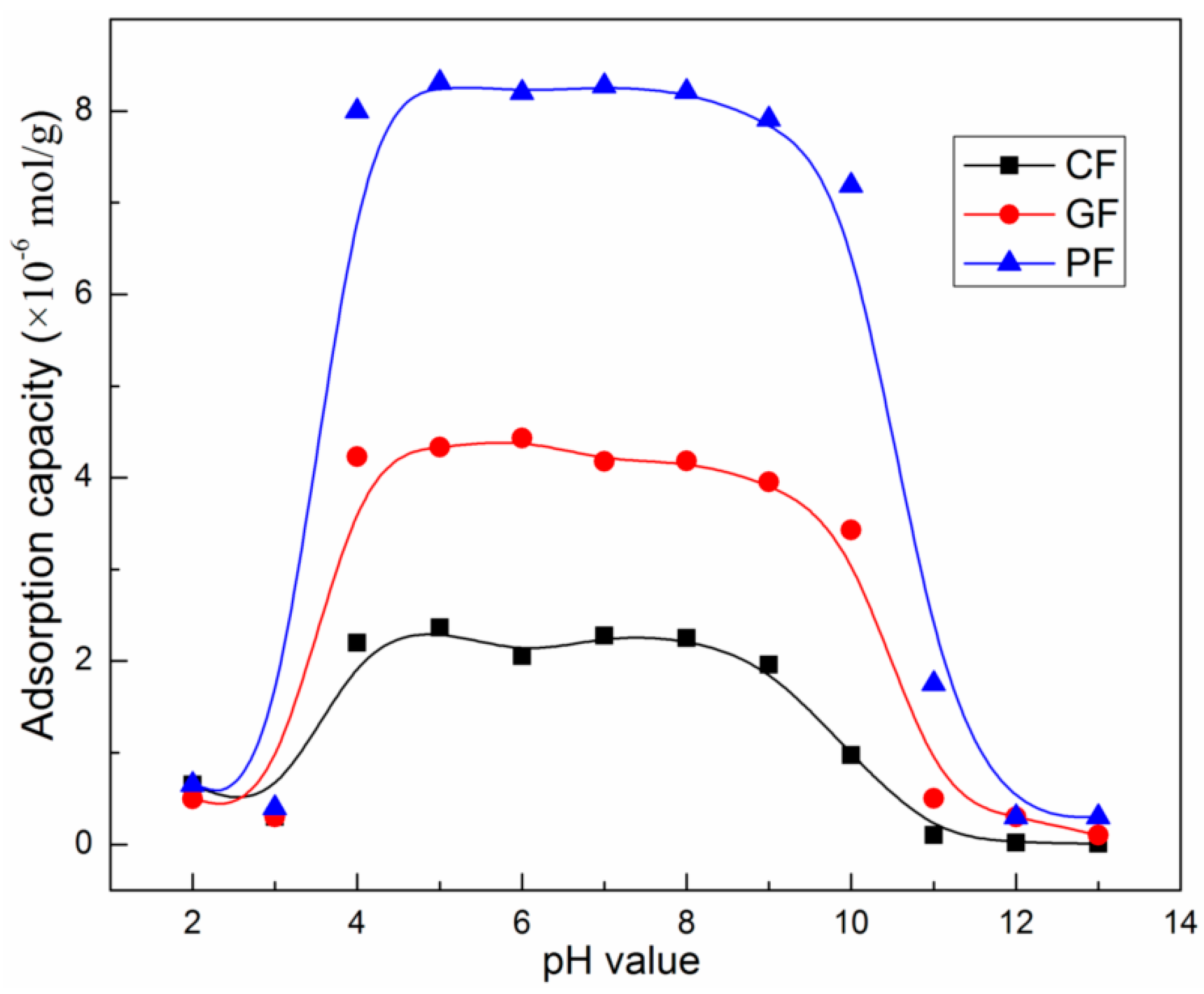
3.1. Micro-Flotation of Fluorites
3.2. Adsorption Behavior of NaOL on Fluorite
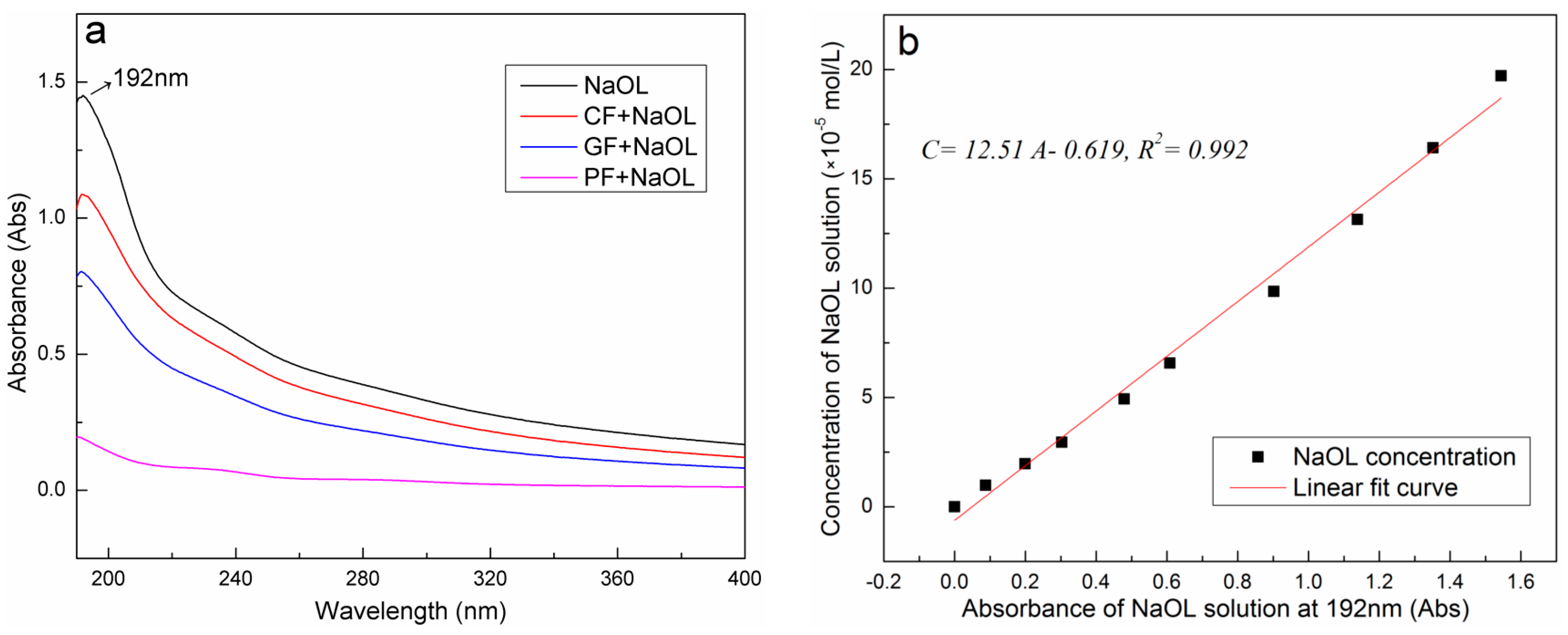
3.3. Measurement of NaOL Solution Surface Tension
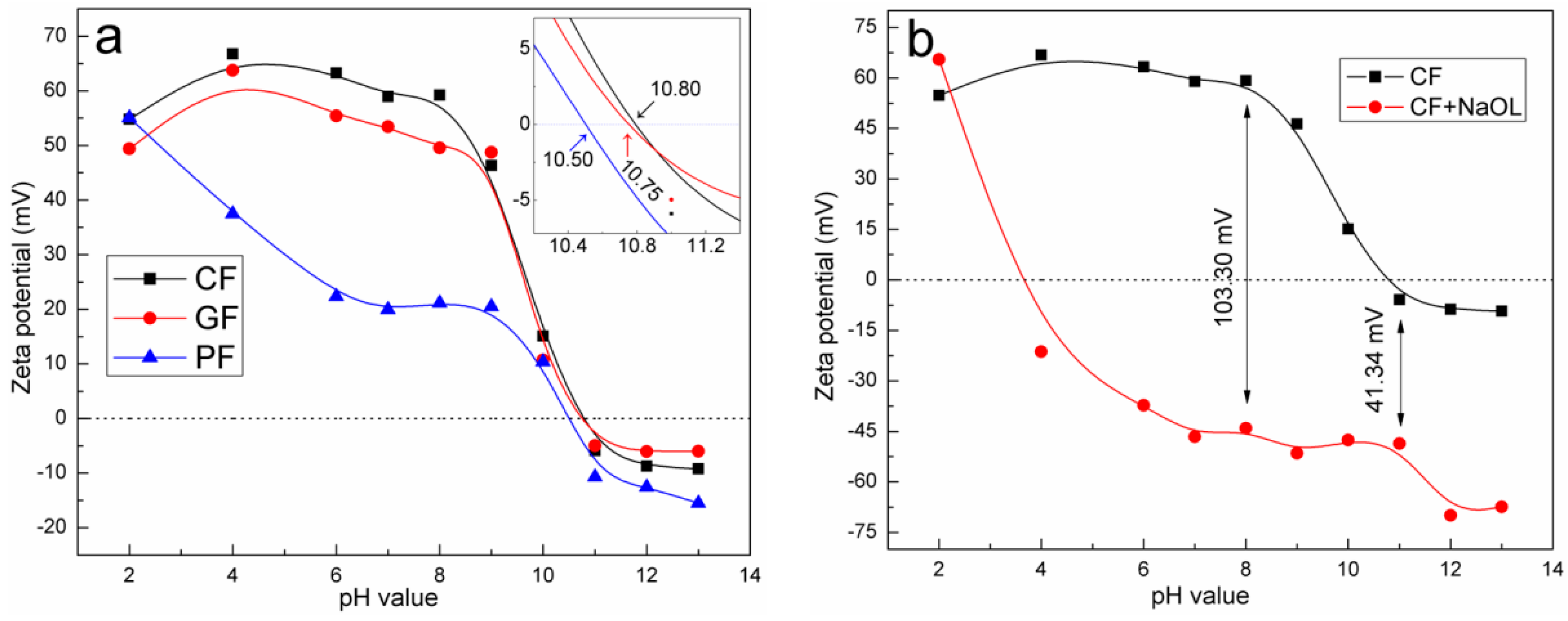
3.4. Zeta Potential Measurement Results
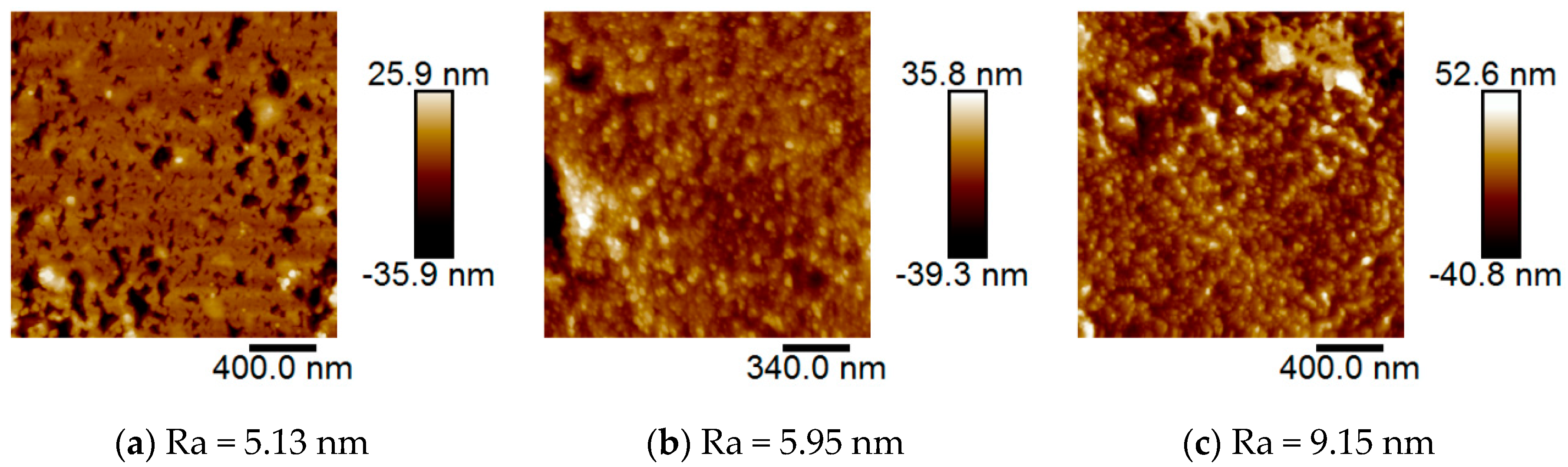
3.5. Surface Topographic Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garand, A.; Mucci, A. The solubility of fluorite as a function of ionic strength and solution composition at 25 °C and 1 atm total pressure. Mar. Chem. 2004, 91, 27–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S. Beneficiation of fluorite by flotation in a new chemical scheme. Miner. Eng. 2003, 16, 597–600. [Google Scholar] [CrossRef]
- Akgün, A.; Teğin, İ.; Ziyadanoğullari, R. Enrichment of molybdenum and fluorite by flotation of fluorite ore containing molybdenum. J. Miner. Mater. Charact. Eng. 2006, 5, 103–117. [Google Scholar] [CrossRef]
- Hanna, H.S.; Somasundaran, P. Flotation of salt-type minerals. In Flotation: A. M. Gaudin Memorial Volume; Fuerstenau, M.C., Ed.; AIME: New York, NY, USA, 1976; pp. 197–272. [Google Scholar]
- Finkelstein, N.P. Review of interactions in flotation of sparingly soluble calcium minerals with anionic collectors. Trans. Inst. Min. Metall. 1989, 98, 157–177. [Google Scholar]
- Somasundaran, P.; Healy, T.W.; Fuerstenau, D.W. Surfactant adsorption at the solid-liquid interface-dependence of mechanism on chain length. J. Phys. Chem. 1964, 68, 3562–3566. [Google Scholar] [CrossRef]
- Chennakesavulu, K.; Raju, G.B.; Prabhakar, S.; Nair, C.M.; Murthy, K.V.G.K. Adsorption of oleate on fluorite surface as revealed by atomic force microscopy. Int. J. Miner. Process. 2009, 90, 101–104. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Ren, Z.; Yu, F.; Gao, H.; Chen, Z.; Peng, Y.; Liu, L. Selective separation of fluorite, barite and calcite with valonea extract and sodium fluosilicate as depressants. Minerals 2017, 7, 24. [Google Scholar] [CrossRef]
- Singh, R.K. EPR study of yellow and colourless fluorite from carbonatite rocks of Ambadongar, Gujarat. J. Geol. Soc. India 2011, 77, 381–384. [Google Scholar] [CrossRef]
- Bill, H.; Calas, G. Color centers, associated rare-earth ions and the origin of coloration in natural fluorites. Phys. Chem. Miner. 1978, 3, 117–131. [Google Scholar] [CrossRef]
- Gu, H.; Ma, D.; Chen, W.; Zhu, R.; Li, Y.; Li, Y. Electrolytic coloration and spectral properties of natural fluorite crystals containing oxygen impurities. Spectrochim. Acta. A 2011, 82, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, R.S.W. The cause of the colour of Blue John and other purple fluorites. Miner. Mag. 1973, 39, 401–411. [Google Scholar] [CrossRef]
- Engelhardt, J.B.; Dabringhaus, H.; Wandelt, K. Atomic force microscopy study of the CaF2(111) surface: From cleavage via island to evaporation topographies. Surf. Sci. 2000, 448, 187–200. [Google Scholar] [CrossRef]
- Ferreira, F.A.F., Jr.; Yoshimura, E.M.; Umisedo, N.K.; Nascimento, R.P.D. Correlation of optically and thermally stimulated luminescence of natural fluorite pellets. Radiat. Meas. 2014, 71, 254–257. [Google Scholar] [CrossRef]
- Zawala, J.; Drzymala, J.; Malysa, K. Natural hydrophobicity and flotation of fluorite. Physicochem. Probl. Miner. 2007, 41, 5–11. [Google Scholar]
- Bakakin, V.V. Questions on relation of minerals structure and their flotation properties. J. Struct. Chem. 1960, 1, 89–97. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.; Xu, L. Adsorption mechanism of mixed anionic/cationic collectors in muscovite-quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Mielczarski, E.; Donato, P.D.; Mielczarski, J.A.; Cases, J.M.; Barres, O.; Bouquet, E. Solution chemistry in adsorption layer formation of oleate on fluorite. J. Colloid. Interf. Sci. 2000, 226, 269–276. [Google Scholar] [CrossRef]
- Mielczarski, J.A.; Mielczarski, A.E.; Cases, J.M. Dynamics of fluorite-oleate interactions. Langmuir 1999, 15, 500–508. [Google Scholar] [CrossRef]
- Mielczarski, E.; Mielczarski, J.A.; Cases, J.M.; Bai, B.; Pradip. Influence of solution conditions and mineral surface structure on the formation of oleate adsorption layers on fluorite. Colloid. Surf. A 2002, 205, 73–84. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Zhang, L.; Zhu, G. Role of oleic acid ionic-molecular complexes in the flotation of spodumene. Miner. Eng. 2015, 71, 7–12. [Google Scholar] [CrossRef]
- Miller, J.D.; Hiskey, J.B. Electrokinetic behavior of fluorite as influenced by surface carbonation. J. Colloid. Interf. Sci. 1972, 41, 567–573. [Google Scholar] [CrossRef]
- Honig, E.P.; Hengst, J.H.T. Points of zero charge of inorganic precipitates. J. Colloid. Interf. Sci. 1969, 29, 510–520. [Google Scholar] [CrossRef]
- Miller, J.D.; Fa, K.; Calara, J.V.; Paruchuri, V.K. The surface charge of fluorite in the absence of surface carbonation. Colloid. Surf. A 2004, 238, 91–97. [Google Scholar] [CrossRef]
- Chernyshova, I.V.; Ponnurangam, S.; Somasundaran, P. Adsorption of fatty acids on iron (hydr) oxides from aqueous solutions. Langmuir 2011, 27, 10007–10018. [Google Scholar] [CrossRef] [PubMed]
- Khalfaoui, N.; Görlich, M.; Müller, C.; Schleberger, M.; Lebius, H. Latent tracks in CaF2 studied with atomic force microscopy in air and in vacuum. Nucl. Instrum. Methods B 2006, 245, 246–249. [Google Scholar] [CrossRef]
- Xia, W.; Ni, C.; Xie, G. The influence of surface roughness on wettability of natural/gold-coated ultra-low ash coal particles. Powder Technol. 2016, 288, 286–290. [Google Scholar] [CrossRef]
- Zawala, J.; Drzymala, J.; Malysa, K. An investigation into the mechanism of the three-phase contact formation at fluorite surface by colliding bubble. Int. J. Miner. Process. 2008, 88, 72–79. [Google Scholar] [CrossRef]
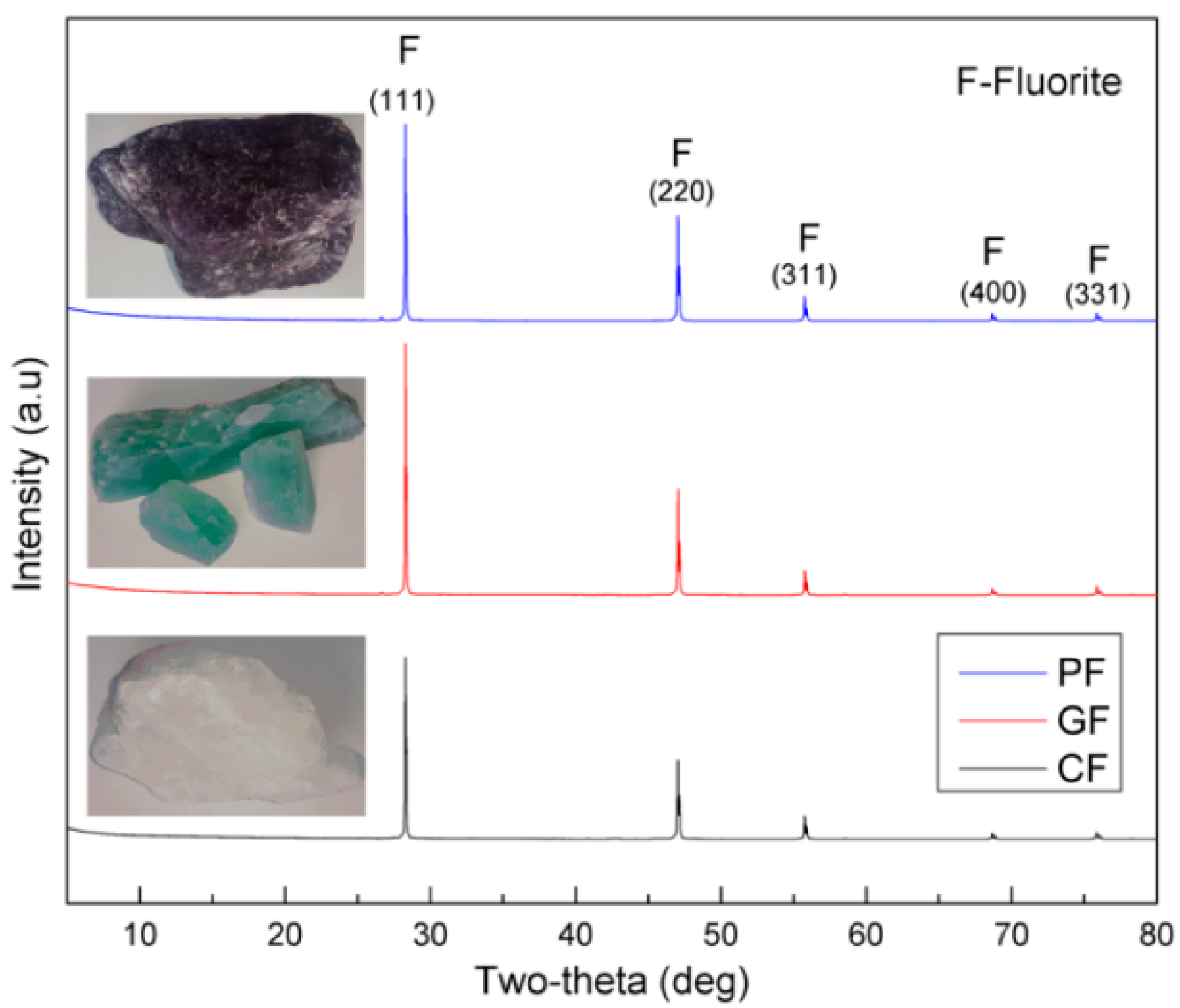






| Size Distribution and SSA | Fluorite Sample | ||
|---|---|---|---|
| CF | GF | PF | |
| D50 (μm) | 23.95 | 24.40 | 21.86 |
| D90 (μm) | 58.77 | 59.33 | 58.95 |
| SSA (m2/g) | 0.3908 | 0.3953 | 0.4149 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, R.; Ren, Z.; Gao, H.; Qian, Y. Flotation Behavior of Different Colored Fluorites Using Sodium Oleate as a Collector. Minerals 2017, 7, 159. https://doi.org/10.3390/min7090159
Zheng R, Ren Z, Gao H, Qian Y. Flotation Behavior of Different Colored Fluorites Using Sodium Oleate as a Collector. Minerals. 2017; 7(9):159. https://doi.org/10.3390/min7090159
Chicago/Turabian StyleZheng, Renji, Zijie Ren, Huimin Gao, and Yupeng Qian. 2017. "Flotation Behavior of Different Colored Fluorites Using Sodium Oleate as a Collector" Minerals 7, no. 9: 159. https://doi.org/10.3390/min7090159





