Elimination of the Adverse Effect of Calcium Ion on the Flotation Separation of Magnesite from Dolomite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Minerals and Reagents
2.2. Experiments
2.2.1. Flotation Tests
2.2.2. Zeta Potential Measurements
2.2.3. XPS Measurements
3. Results and Discussion
3.1. Flotation Behaviors of Magnesite and Dolomite
3.2. Influence of Ca2+ on the Flotation of Magnesite and Its Elimination
3.3. Mechanism of Ca2+ Affecting the Flotation of Magnesite
3.3.1. XPS Analysis
3.3.2. Zeta Potential Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Karaoglu, H.; Yanmis, D.; Gurkok, S. Magnesite enrichment with pseudomonas oryzihabitans isolated from magnesite ore. Geomicrobiol. J. 2016, 33, 46–51. [Google Scholar] [CrossRef]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Proces. 2016, 149, 84–93. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Effect of solution chemistry on flotability of magnesite and dolomite. Int. J. Miner. Process. 2004, 74, 343–357. [Google Scholar] [CrossRef]
- Botero, A.E.C.; Torem, M.L.L.; De Mesquita, M.S. Fundamental studies of Rhodococcus opacus as a biocollector of calcite and magnesite. Miner. Eng. 2007, 20, 1026–1032. [Google Scholar] [CrossRef]
- Marouf, R.; Marouf-Khelifa, K.; Schott, J.; Khelifa, A. Zeta potential study of thermally treated dolomite samples in electrolyte solutions. Microporous Mesoporous Mater. 2009, 122, 99–104. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, I: Flotation of single minerals and mineral mixtures. Miner. Eng. 2004, 17, 855–863. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Q. Flotation separation of carbonate from sulfide minerals, II: Mechanisms of flotation depression of sulfide minerals by thioglycollic acid and citric acid. Miner. Eng. 2004, 17, 865–878. [Google Scholar] [CrossRef]
- Luo, X.; Yin, W.; Wang, Y.; Sun, C.; Ma, Y.; Liu, J. Effect and mechanism of dolomite with different size fractions on hematite flotation using sodium oleate as collector. J. Cent. South Univ. 2016, 23, 529–534. [Google Scholar] [CrossRef]
- Śláczka, A.S.; Paprotny, J. Flocculation of smithsonite and dolomite using polymers containing nitrogen atoms. Int. J. Miner. Process. 1985, 14, 319–325. [Google Scholar] [CrossRef]
- Santillán, J.; Williams, Q. A high-pressure infrared and X-ray study of FeCO3 and MnCO3: Comparison with CaMg(CO3)2-dolomite. Phys. Earth Planet. Inter. 2004, 143–144, 291–304. [Google Scholar] [CrossRef]
- Gence, N.; Ozbay, N. pH dependence of electrokinetic behavior of dolomite and magnesite in aqueous electrolyte solutions. Appl. Surf. Sci. 2006, 252, 8057–8061. [Google Scholar] [CrossRef]
- Gence, N. Wetting behavior of magnesite and dolomite surfaces. Appl. Surf. Sci. 2006, 252, 3744–3750. [Google Scholar] [CrossRef]
- Hu, Y.; Chi, R.; Xu, Z. Solution chemistry study of salt-type mineral flotation systems: Role of inorganic dispersants. Ind. Eng. Chem. Res. 2003, 42, 1641–1647. [Google Scholar] [CrossRef]
- Pokrovsky, O.S.; Golubev, S.V.; Schott, J.; Castillo, A. Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumnetral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constrains on CO2 sequestration in sedimentary basins. Chem. Geol. 2009, 265, 20–32. [Google Scholar] [CrossRef]
- Van Cappellen, P.; Charlet, L.; Stumm, W.; Wersin, P.A. Surface complexation model of the carbonate mineral-aqueous solution interface. Geochim. Cosmochim. Acta 1993, 57, 3505–3518. [Google Scholar] [CrossRef]
- Vučinić, D.R.; Radulović, D.S.; Deušić, S.Đ. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.L.; Tao, D. Reverse flotation of magnesite by dodecyl phosphate from dolomite in the presence of sodium silicate. Sep. Sci. Technol. 2004, 34, 377–390. [Google Scholar] [CrossRef]
- Luo, X.M.; Wang, Y.F.; Wen, S.M.; Ma, M.Z.; Sun, C.Y. Effect of carbonate minerals on quartz flotation behavior under conditions of reverse anionic flotation of iron ores. Int. J. Miner. Process. 2016, 152, 1–6. [Google Scholar] [CrossRef]
- Zhu, Y.; Luo, B.; Sun, C.; Li, Y.; Han, Y. Influence of bromine modification on collecting property of lauric acid. Miner. Eng. 2015, 79, 24–30. [Google Scholar] [CrossRef]
- Rao, K.H.; Antti, B.M.; Forssberg, E. Mechanism of oleate interaction on salt-type minerals, part II. Adsorption and electrokinetic studies of apatite in the presence of sodium oleate and sodium metasilicate. Int. J. Miner. Process. 1990, 28, 59–79. [Google Scholar] [CrossRef]
- Gautelier, M.; Schott, J.; Oelkers, E.H. An experimental study of dolomite dissolution rates at 80 °C as a function of chemical affinity and solution composition. Chem. Geol. 2007, 242, 509–517. [Google Scholar] [CrossRef]
- Liu, A.; Ni, W.; Wu, W. Mechanism of separating pyrite and dolomite by flotation. J. Univ. Sci. Technol. B. 2007, 14, 291–296. [Google Scholar] [CrossRef]
- Gence, N.; Özdaǧ, H. Surface properties of magnesite and adsorption mechanism. Int. J. Miner. Process. 1995, 43, 37–47. [Google Scholar] [CrossRef]
- Chen, Z.; Ren, Z.; Gao, H.; Liu, J.; Jin, J.; Min, F. The effects of calcium ions on the flotation of sillimanite using dodecylammonium chloride. Minerals 2017, 7, 28. [Google Scholar] [CrossRef]
- Shi, Q.; Feng, Q.; Zhang, G.; Deng, H. A novel method to improve depressants actions on calcite flotation. Miner. Eng. 2013, 55, 186–189. [Google Scholar] [CrossRef]
- Feng, B.; Lu, Y.; Feng, Q.; Zhang, M.; Gu, Y. Talc–serpentine interactions and implications for talc depression. Miner. Eng. 2012, 32, 68–73. [Google Scholar] [CrossRef]
- Nia, X.; Liu, Q. Adsorption behaviour of sodium hexametaphosphate on pyrochlore and calcite. Can. Metall. Quart. 2013, 52, 473–478. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Feng, Q.; Long, T.; Ou, L.; Zhang, G. Effect of sodium hexametaphosphate on separation of serpentine from pyrite. Trans. Nonferrous Met. Soc. China 2011, 21, 208–213. [Google Scholar] [CrossRef]
- Ding, H.; Lin, H.; Deng, Y. Depressing effect of sodium hexametaphosphate on apatite in flotation of rutile. J. Univ. Sci. Technol. B. 2007, 14, 200–203. [Google Scholar] [CrossRef]
- Larson, C.E. Use of sodium hexametaphosphate as an anticoagulant. Exp. Biol. Med. 1940, 44, 554–555. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, J.; Luo, C.; Yuan, C. Solution chemistry studies on dodecylamine flotation of smithsonite/calcite. J. Cent. South Univ. 1995, 26, 589–594. (In Chinese) [Google Scholar]
- Kangal, O.; Sirkeci, A.A.; Güney, A. Flotation behaviour of huntite (Mg3Ca(CO3)4) with anionic collectors. Int. J. Miner. Process. 2005, 75, 31–39. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; De Armando, A.C.; Valadão, G.E.S. Electrokinetic properties of wavellite and its floatability with cationicand anionic collector. J. Colloid Interface Sci. 2011, 361, 632–638. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Álvarez-Ayuso, E. Sorption of Zn, Cd and Cr on calcite. Application to purification of industrial wastewaters. Miner. Eng. 2002, 15, 539–547. [Google Scholar] [CrossRef]
- Demri, B.; Muster, D. XPS study of some calcium compounds. J. Mater. Process. Technol. 1995, 55, 311–314. [Google Scholar] [CrossRef]
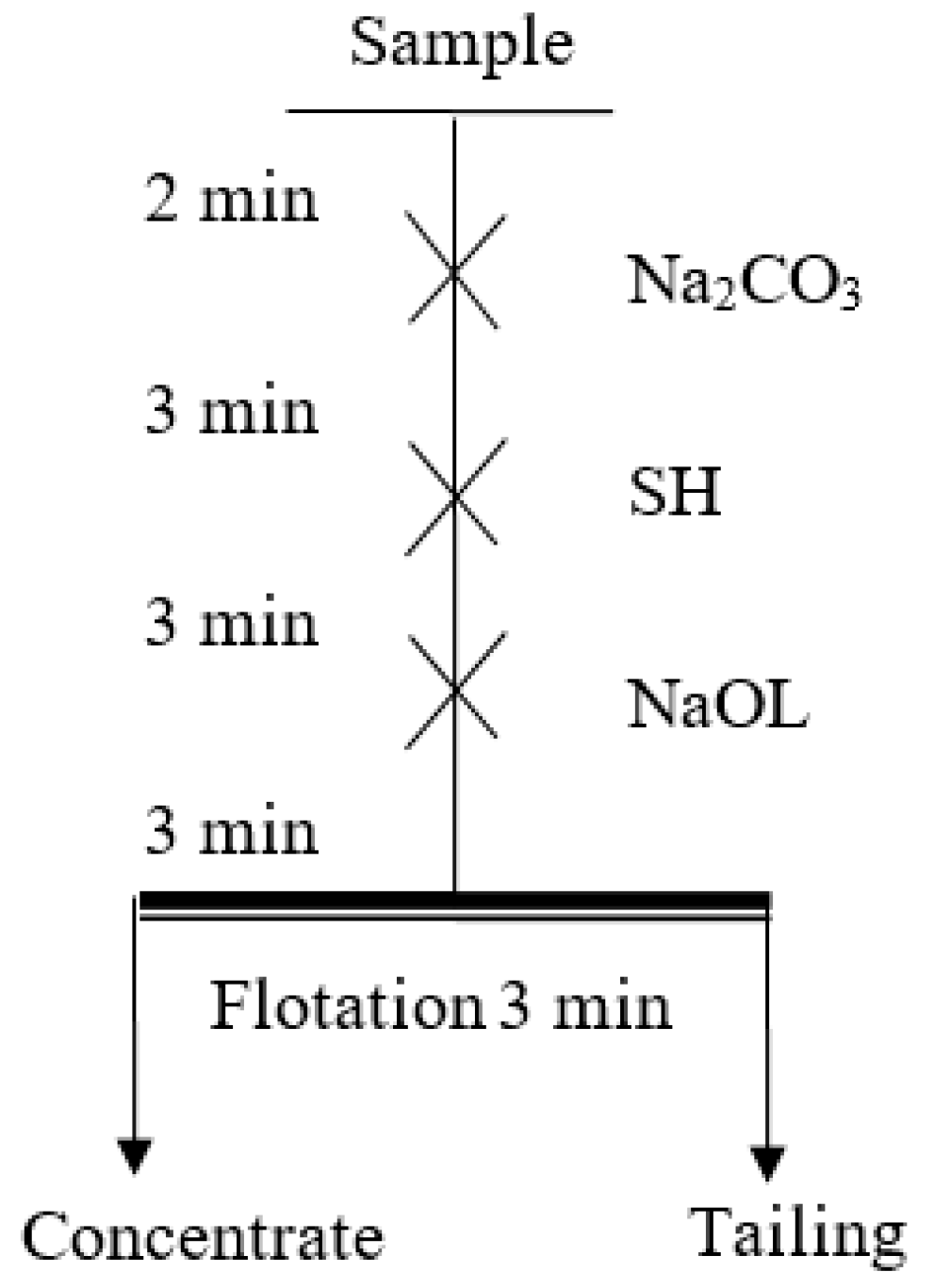
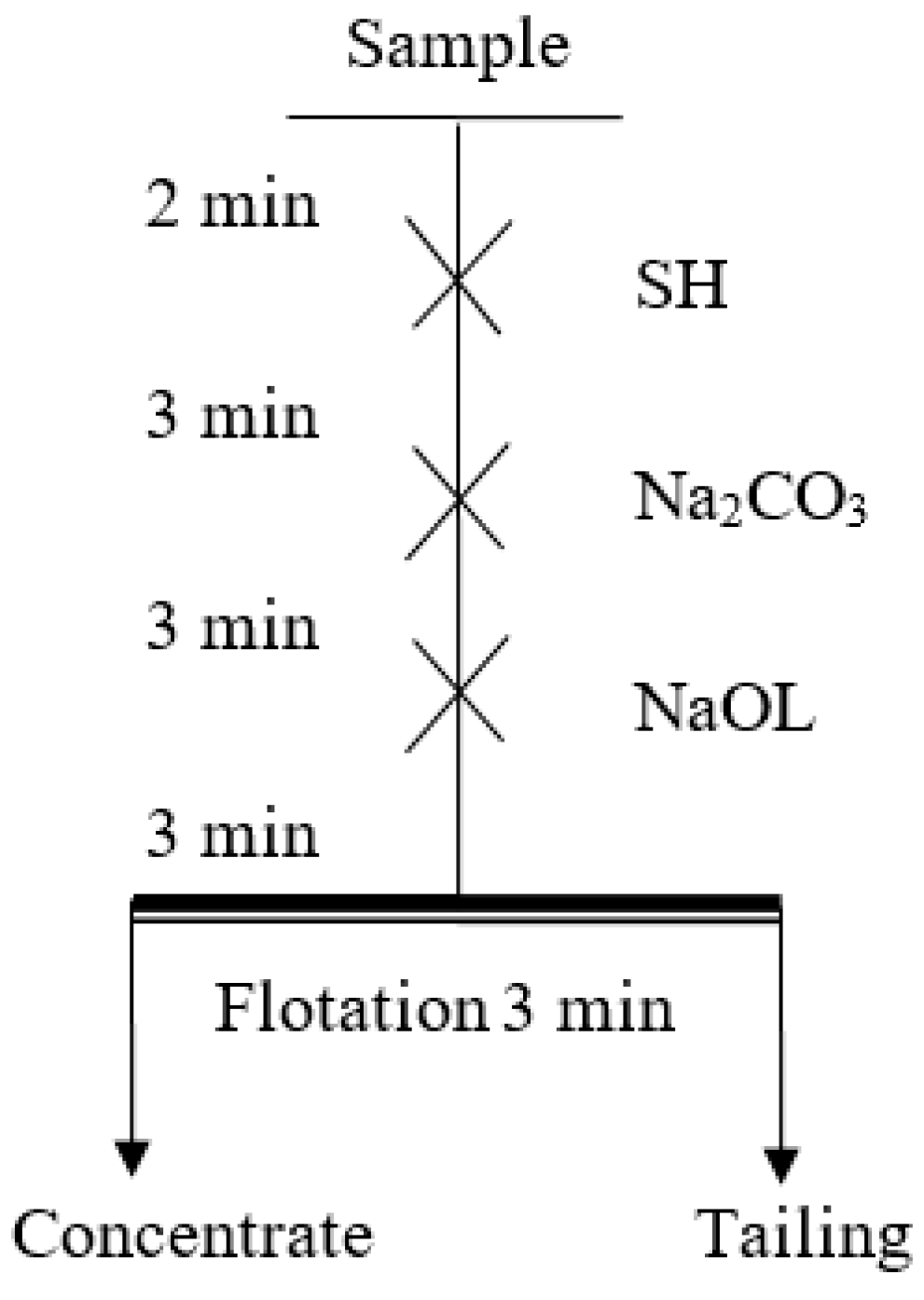


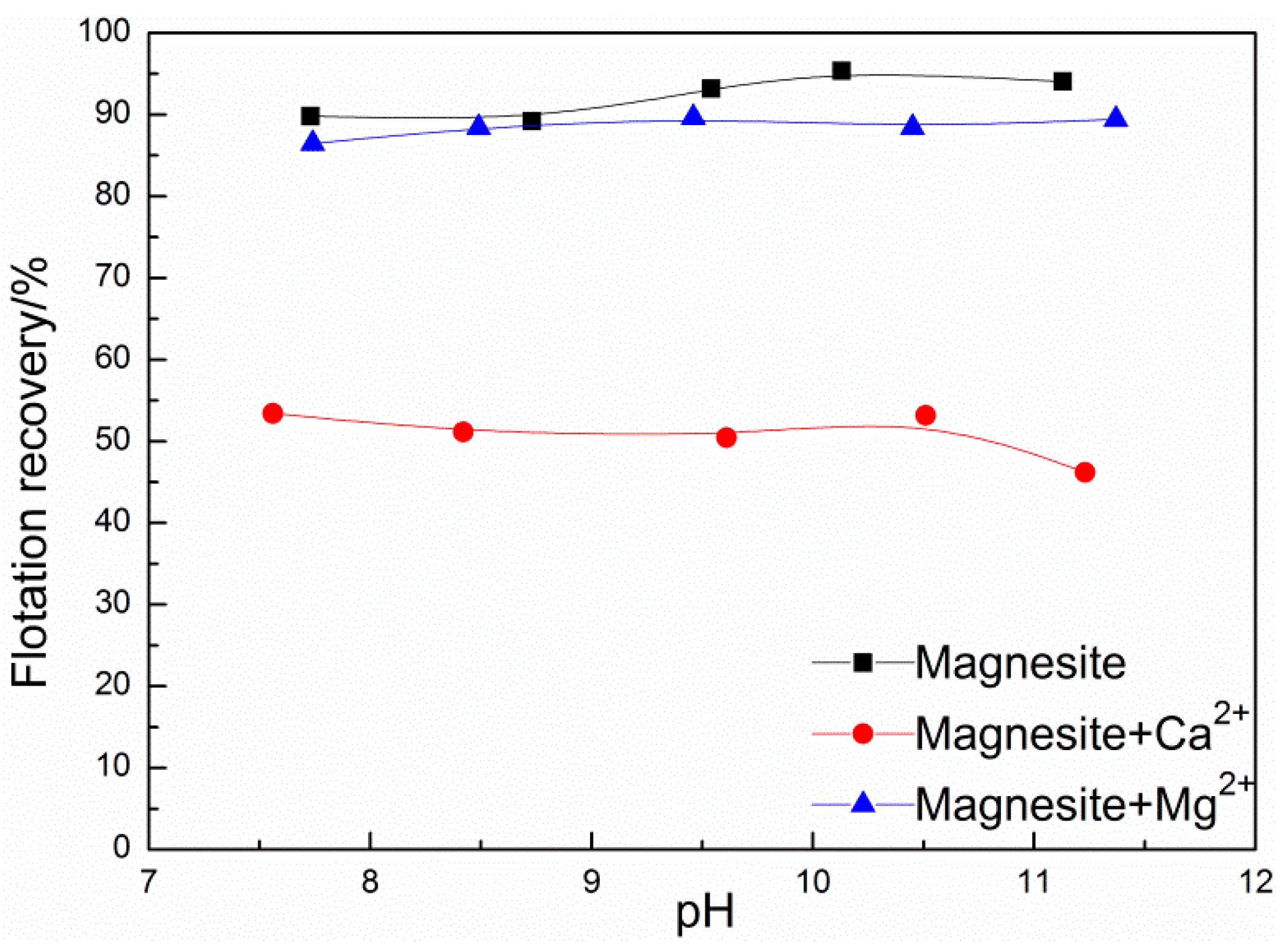



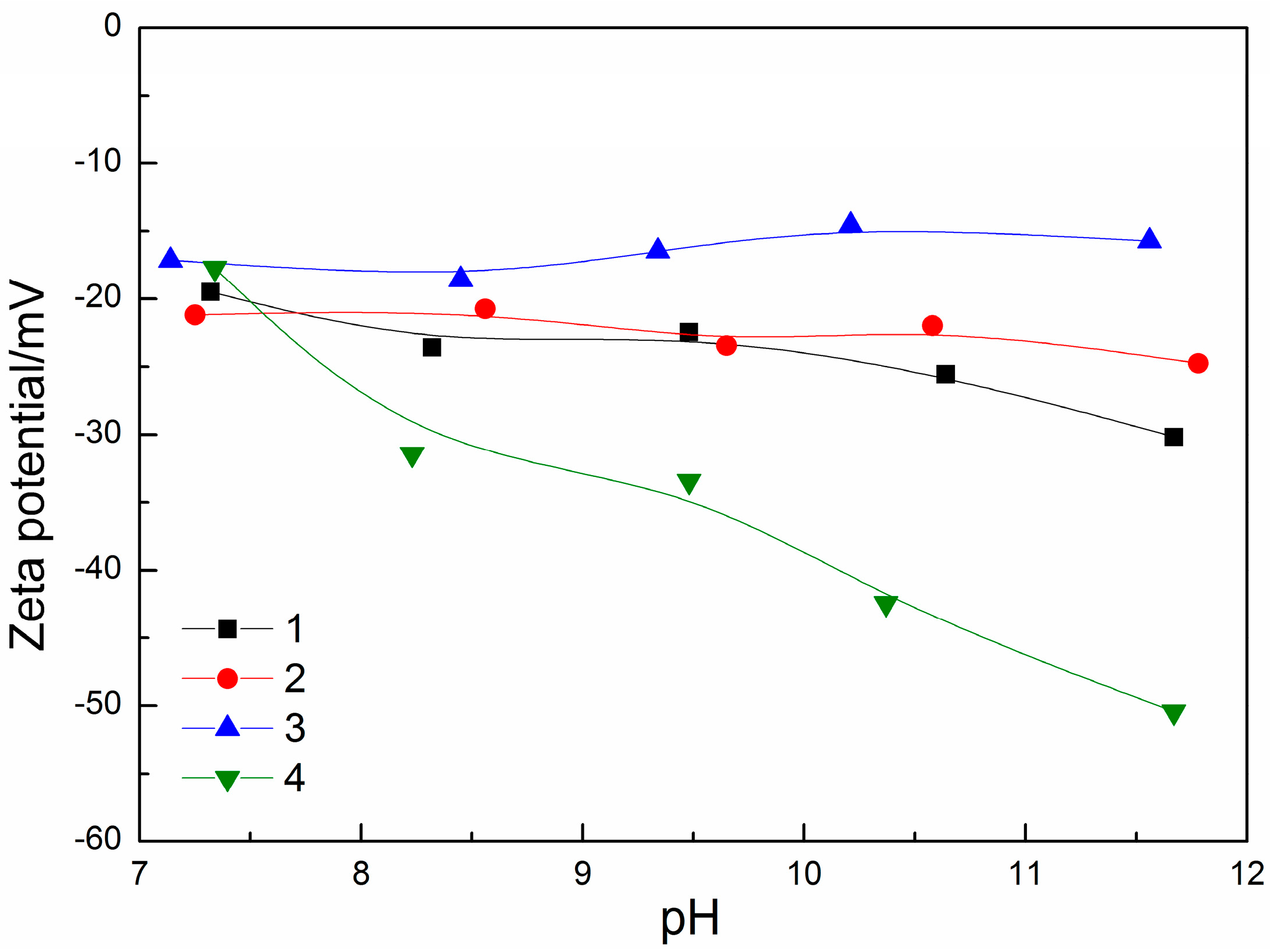
| Sample | MgO/% | CaO/% | SiO2/% | Al2O3/% | Purity/% |
|---|---|---|---|---|---|
| Magnesite | 47.24 | 0.17 | 0.19 | / | 99.20 |
| Dolomite | 21.52 | 30.13 | 0.21 | 0.10 | 98.99 |
| SH Dosage (mg/L) | Product | Weight Recovery (%) | MgCO3 Grade (%) | MgCO3 Recovery (%) | CaMg(CO3)2 Grade (%) | CaMg(CO3)2 Recovery (%) |
|---|---|---|---|---|---|---|
| 0 | concentrate | 97.45 | 49.85 | 97.39 | 50.15 | 97.46 |
| tailing | 2.55 | 50.19 | 2.61 | 49.81 | 2.54 | |
| raw ore | 100 | 49.88 | 100 | 50.14 | 100 | |
| 20 | concentrate | 79.14 | 48.89 | 75.97 | 51.11 | 80.79 |
| tailing | 20.86 | 53.89 | 24.03 | 46.11 | 19.21 | |
| raw ore | 100 | 50.93 | 100 | 50.06 | 100 | |
| 40 | concentrate | 59.13 | 52.54 | 61.35 | 47.46 | 56.85 |
| tailing | 40.87 | 47.89 | 38.65 | 52.11 | 43.15 | |
| raw ore | 100 | 50.64 | 100 | 49.36 | 100 |
| Sample | Binding Energy/eV | ||||
|---|---|---|---|---|---|
| Atomic orbital | Mg1s | O1s | C1s | Ca2s | Ca2p |
| Magnesite | 1304.9 | 530.5 | 283.1 | / | / |
| Magnesite + Ca2+ + Na2CO3 + SH | 1305.39 | 530.88 | 283.62 | 438.0 | 346.8 |
| Offset/eV | 0.49 | 0.38 | 0.52 | / | / |
| Magnesite | 1304.9 | 530.5 | 283.1 | / | / |
| Magnesite + Ca2+ + SH + Na2CO3 | 1304.9 | 530.37 | 283.1 | / | / |
| Offset/eV | 0 | −0.13 | 0 | / | / |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, N.; Wei, D.; Shen, Y.; Han, C.; Zhang, C. Elimination of the Adverse Effect of Calcium Ion on the Flotation Separation of Magnesite from Dolomite. Minerals 2017, 7, 150. https://doi.org/10.3390/min7080150
Luo N, Wei D, Shen Y, Han C, Zhang C. Elimination of the Adverse Effect of Calcium Ion on the Flotation Separation of Magnesite from Dolomite. Minerals. 2017; 7(8):150. https://doi.org/10.3390/min7080150
Chicago/Turabian StyleLuo, Na, Dezhou Wei, Yanbai Shen, Cong Han, and Caie Zhang. 2017. "Elimination of the Adverse Effect of Calcium Ion on the Flotation Separation of Magnesite from Dolomite" Minerals 7, no. 8: 150. https://doi.org/10.3390/min7080150





