Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Procedure and Test Methods
- Chemical composition was detected by ICP-AES (inductively-coupled plasma atomic emission spectrometry) performed on an IRIS Advantage ER/S instrument (Thermo Elemental, Waltham, MA, USA).
- X-ray diffraction (XRD) analysis was conducted using a Rigaku D/MAX-RB X-ray diffraction (Rigaku, Akishima, Japan) using Cu Kα radiation. The phases were identified by comparison of the peak positions and d values with data published by the International Centre for Diffraction Data (ICDD).
- Optical microscopy (Leica, Wetzlar, Germany) and a scanning electron microscope (JSM-6610) (JEOL Co., Tokyo, Japan) with an energy dispersive spectrometer (BRUKER, Karlsruhe, Germany) were used to characterize the microstructure of the vanadium-titanium magnetite ore.
- The determination of the vanadium grade was measured in accordance with the test methods of vanadium in vanadium-bearing slag by ferrous ammonium sulfate titration using 2-(phenylamino)-benzoic acid as an indicator (GB/T 8704.5-2007) [17].
- Sizing analysis was conducted on the feed sub-samples by using laboratory wet and dry screening methods. Dry screening was carried out by a standard vibration sieve machine (HLSDB-Φ200, Wuhan Hengle Mineral Engineering Equipment Co., Wuhan, China).
3. Results and Discussion
3.1. Characterization Studies
3.1.1. Chemical Composition Analysis
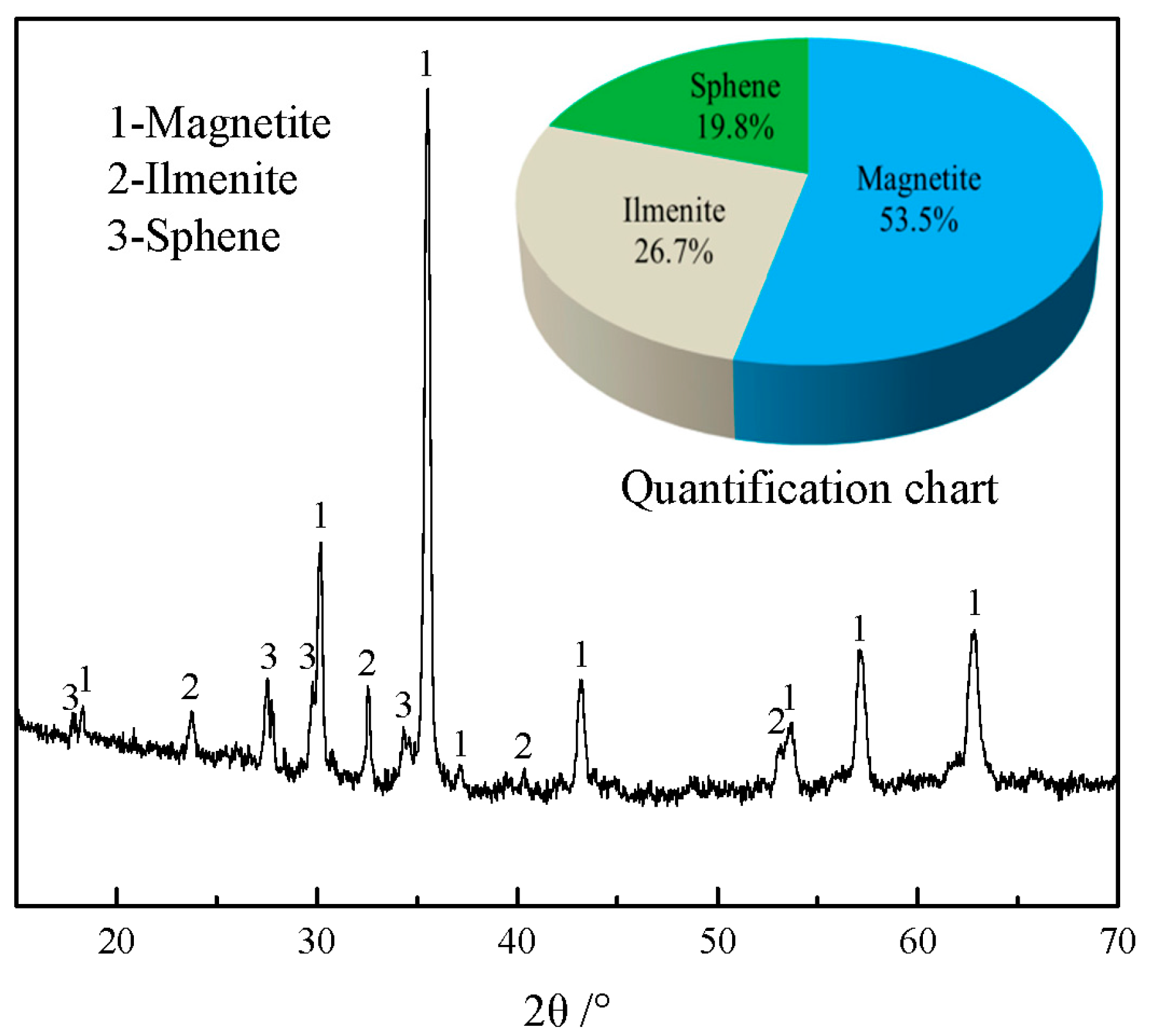
3.1.2. Mineral Composition Analysis
3.1.3. Occurrence of Valuable Elements
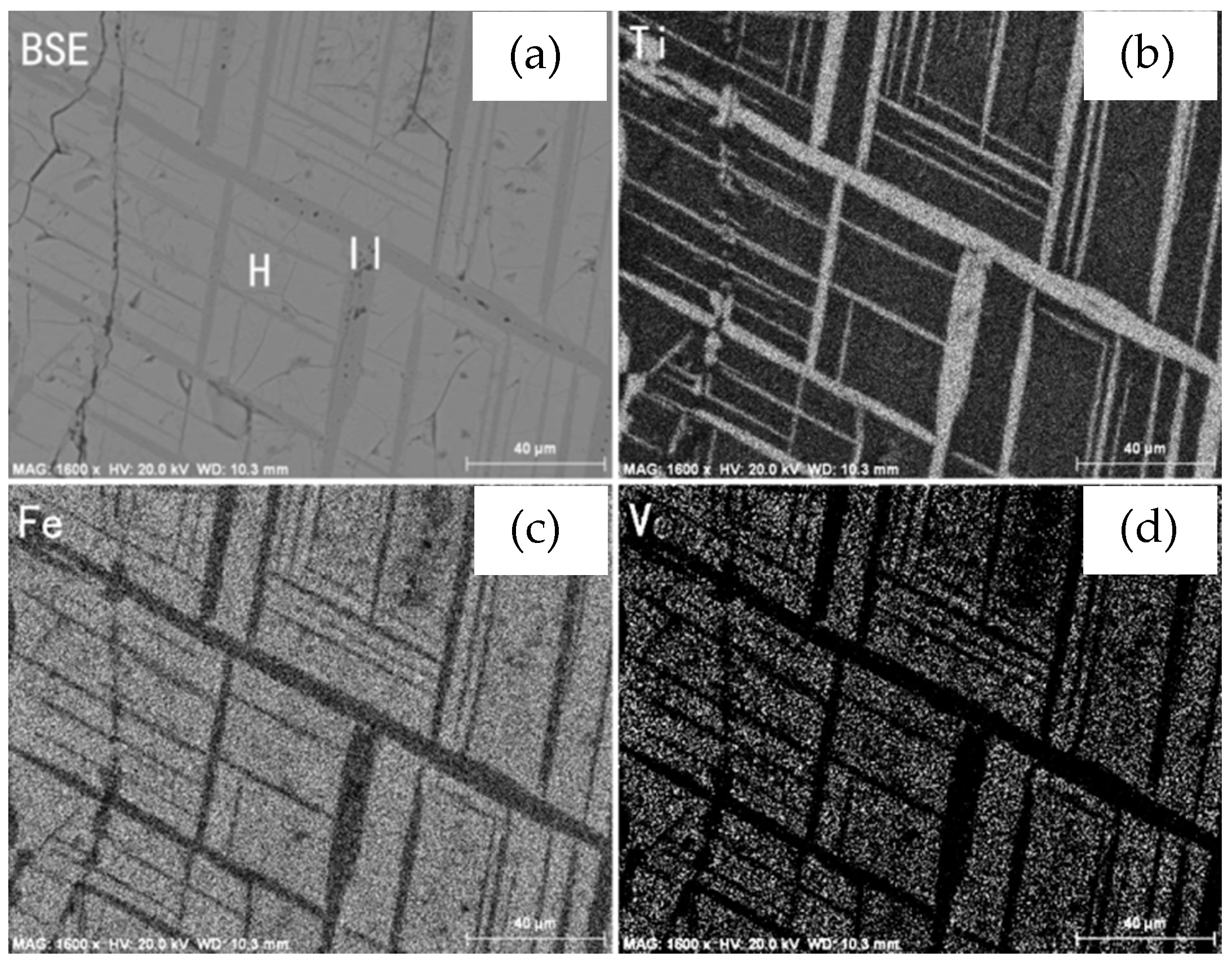
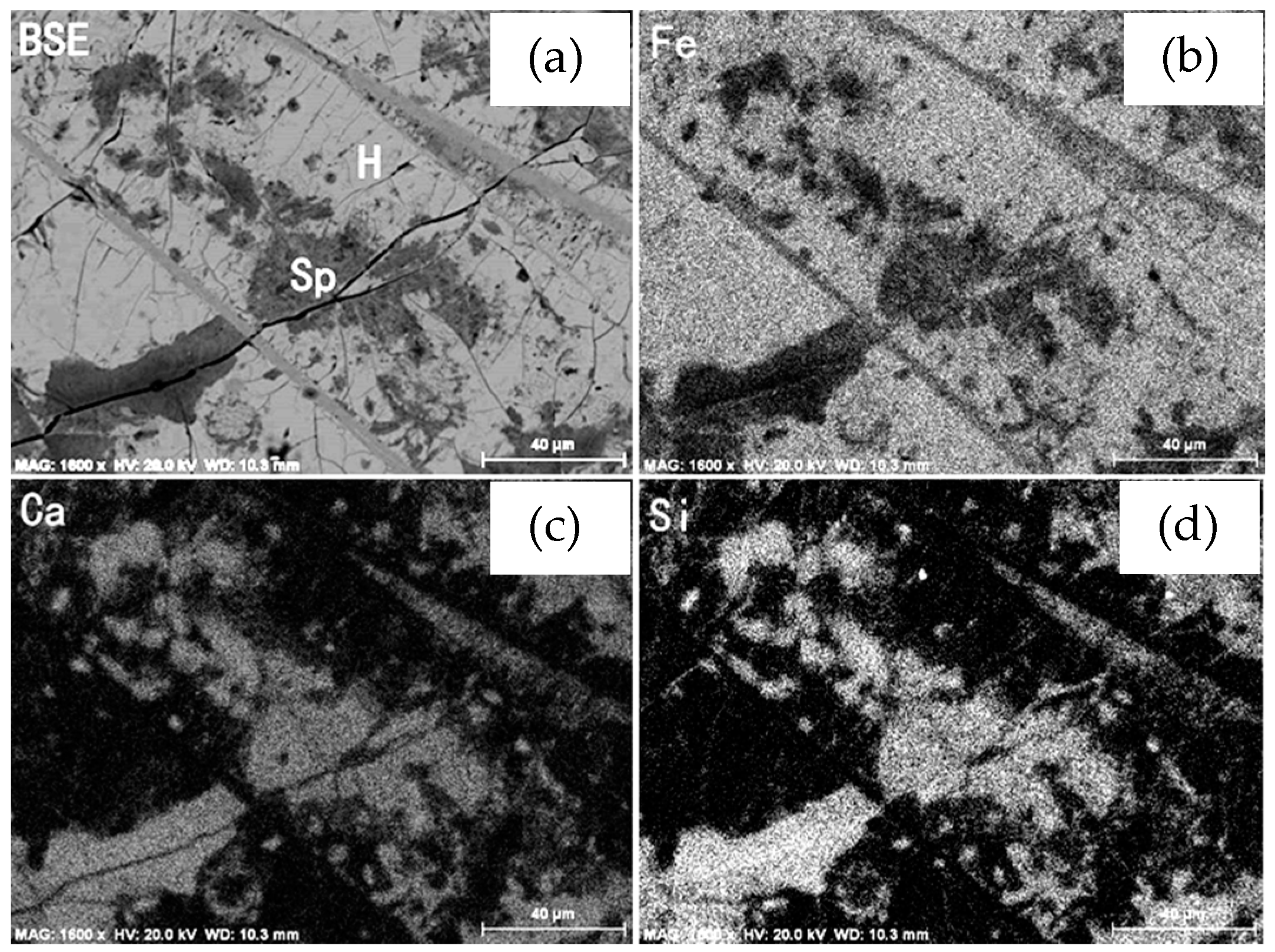
3.1.4. Alteration of Vanadium-titanium Magnetite
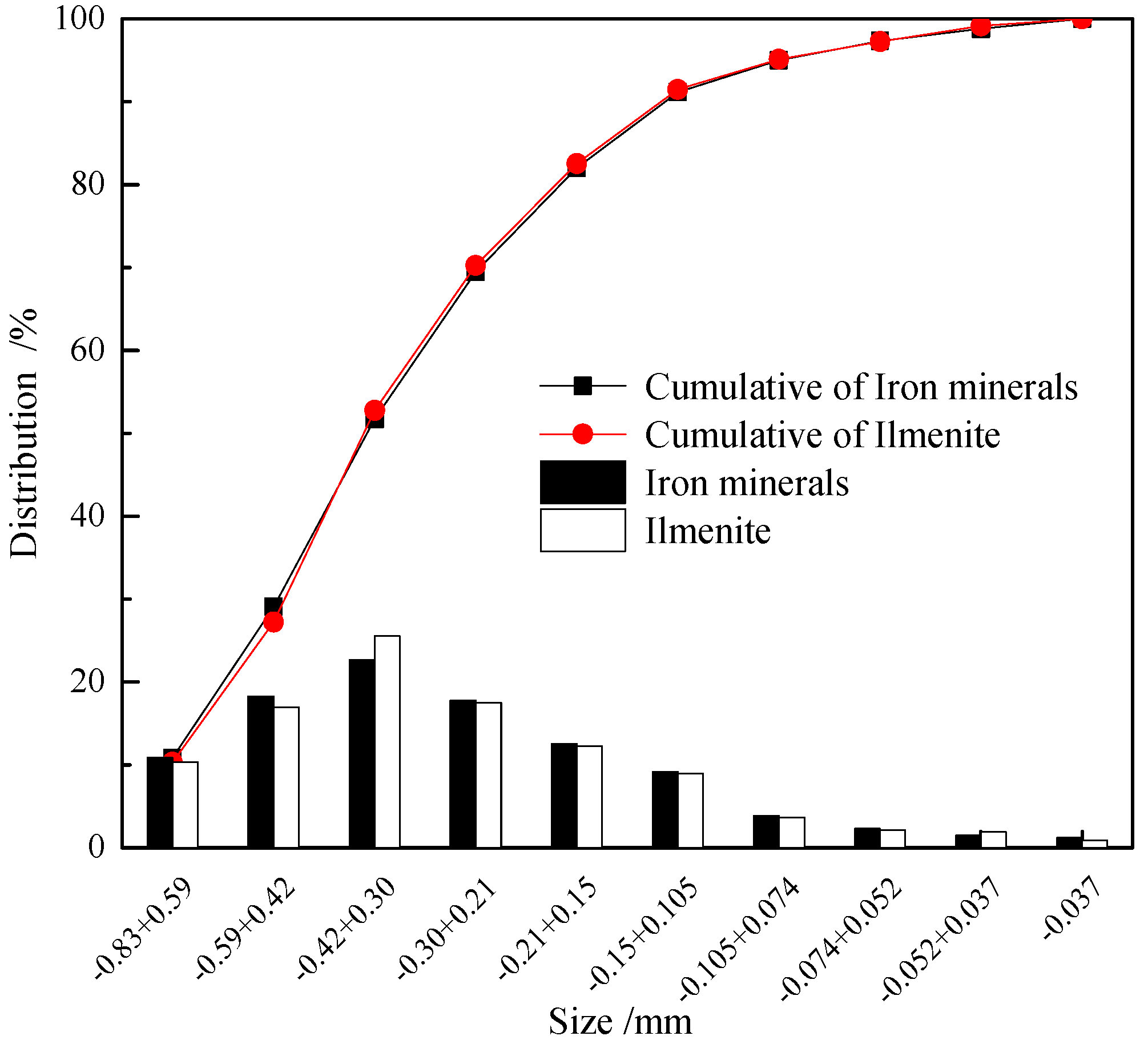
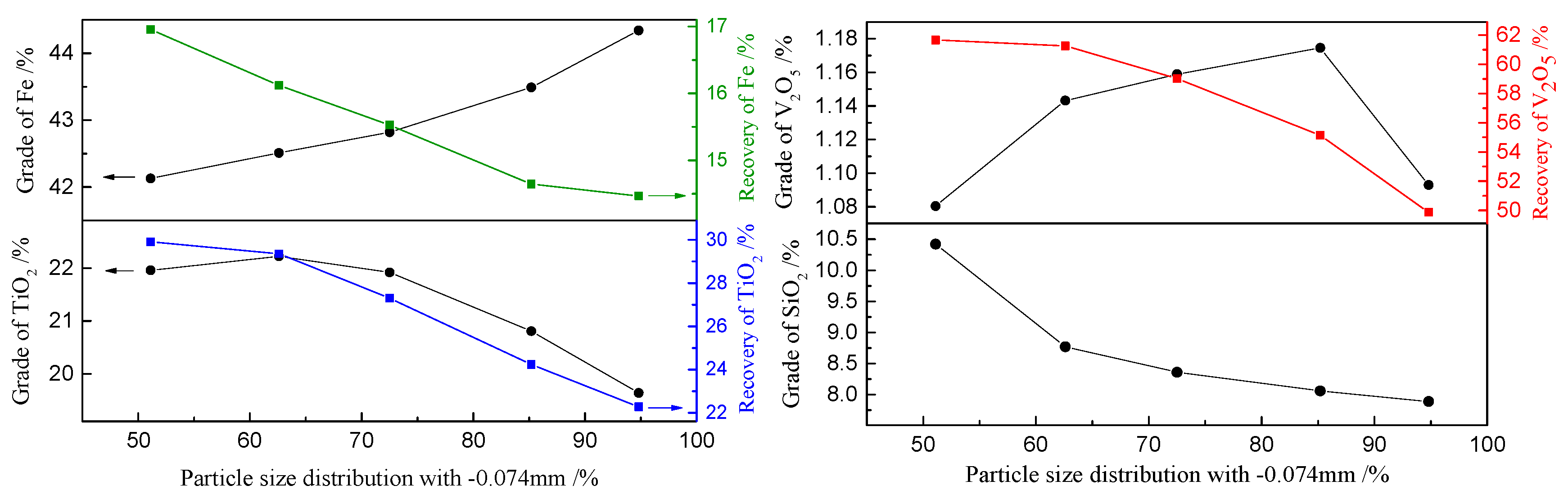
3.1.5. Grain-Size Distribution of Metallic Minerals in Raw Sample
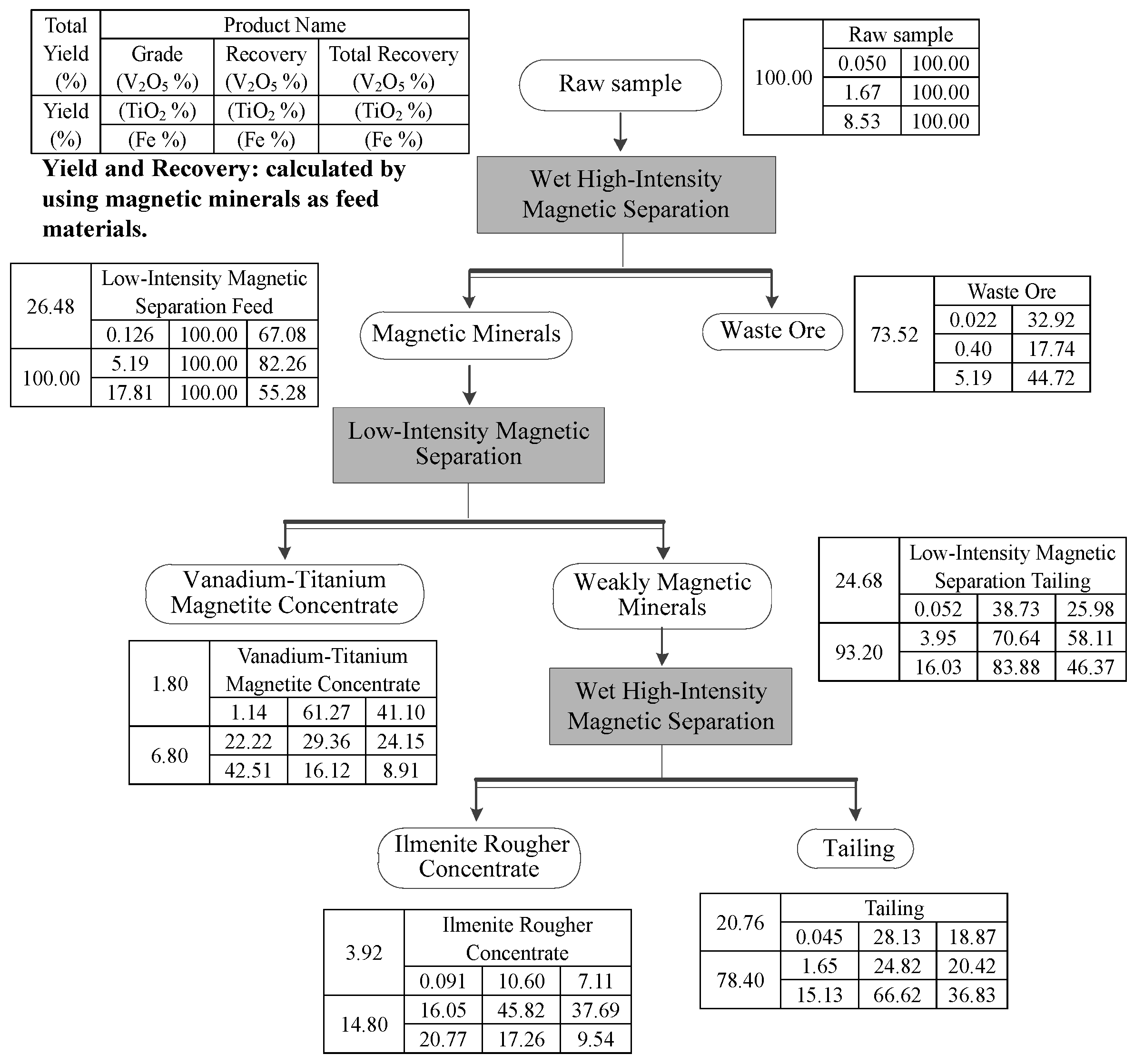
3.2. Pre-Discarding Tailing
3.3. Low-Intensity Magnetic Separation
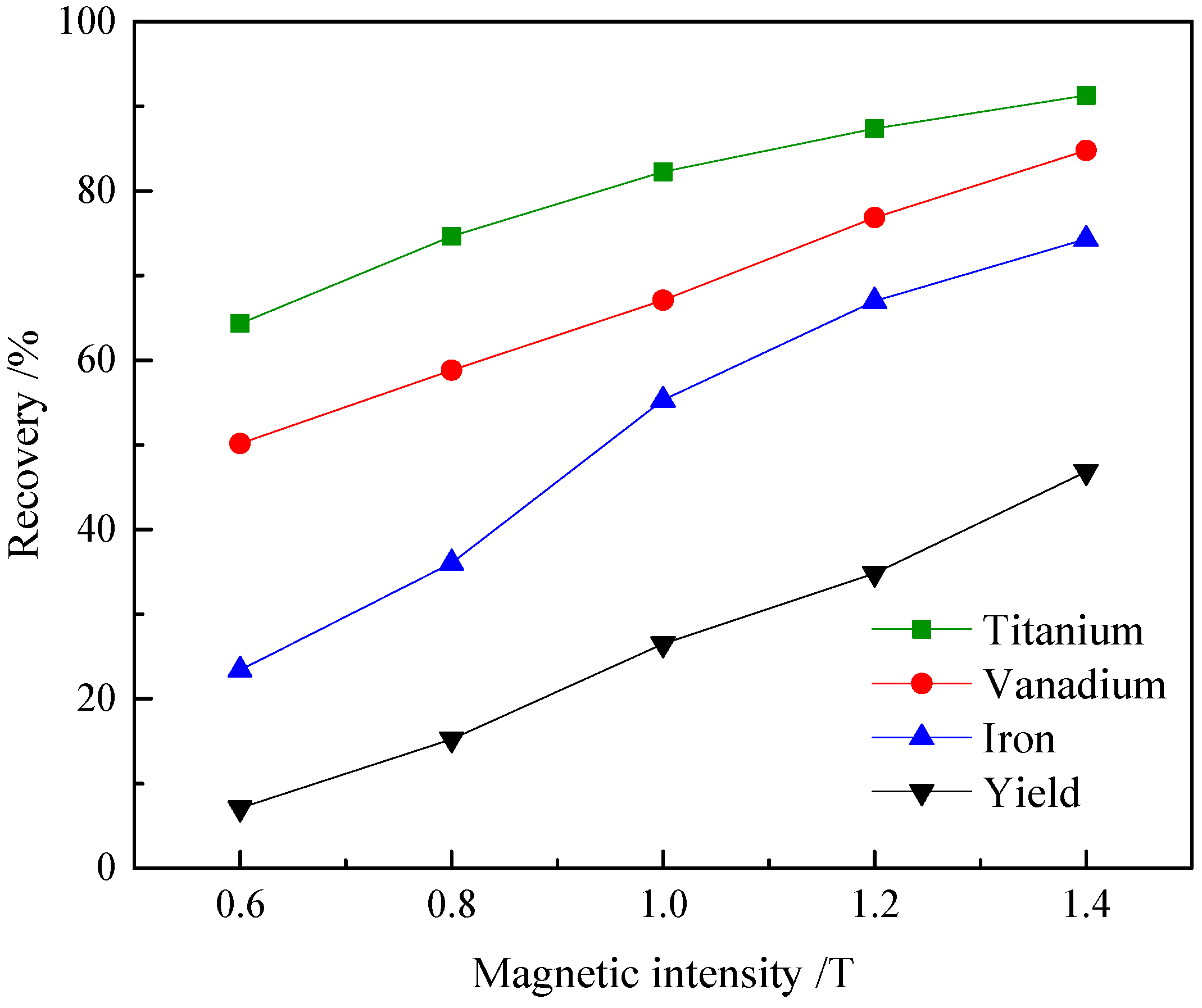
3.4. High Intensity Magnetic Separation
3.5. Flow-Sheet Test
4. Conclusions
- The mineralogical study of the raw sample indicates that it is a typical, low-grade vanadium-titanium magnetite ore with weathering. Vanadium and iron mainly occur in iron minerals and silicate minerals, and ilmenite is the main form of Ti-containing mineral. Most of the vanadium-titanium magnetite is replaced by martite and sphene. The replacement of vanadium-titanium magnetite by martite weakens the susceptibility of vanadium-titanium magnetite, and the vanadium content of sphene is relatively high. The differences between the separation characteristics of sphene and pyroxene are not significant, and they will be lost together in tailing, thus affecting the recovery of valuable elements.
- The intergrowth relationship between vanadium-titanium magnetite and sphene is very complex and the grain size of sphene is generally fine, and cannot be completely liberated even by fine grinding, whereas the content of vanadium and titanium in sphene is higher than that in vanadium-titanium magnetite, resulting in vanadium-titanium magnetite concentrates with the characteristics of high vanadium and titanium content.
- A high efficiency, low cost and environmentally friendly pre-concentration process by magnetic separation was investigated for enriching iron minerals and ilmenite in order to realize the enrichment of valuable elements. Through the process, 73.52% of the feed ore are directly discarded, greatly reducing the ore processing capacity of the follow-up processing. In addition, a vanadium-titanium magnetite concentrate with 1.14% V2O5, 22.22% TiO2, and 42.51% Fe, and a rough concentrate of ilmenite with 16.05% TiO2 and 20.77% Fe were obtained from a low-grade vanadium-titanium magnetite ore which contained 0.050% V2O5, 1.67% TiO2 and 8.53% Fe. The total recovery of V2O5, TiO2 and Fe were 48.21%, 61.84% and 18.45%, respectively. However, the recovery of V2O5, TiO2 and Fe will be 71.87%, 75.18% and 33.38%, respectively, if the magnetic minerals obtained from the pre-discarding tailing are used as feed materials.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Y.M.; Bao, S.X.; Liu, T.; Chen, T.J.; Huang, J. The technology of extracting vanadium from stone coal in China: History, current status and future prospects. Hydrometallurgy 2011, 109, 116–124. [Google Scholar] [CrossRef]
- Chen, D.S.; Zhao, H.X.; Hu, G.P.; Qi, T.; Yu, H.D. An extraction process to recover vanadium from low-grade vanadium-bearing titanomagnetite. J. Hazard. Mater. 2015, 294, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.L.; Guo, Y.F.; Jiang, T.; Qiu, G.Z. Reduction kinetics of oxidized vanadium titano-magnetite pellets using carbon monoxide and hydrogen. J. Alloys Compd. 2017, 706, 546–553. [Google Scholar] [CrossRef]
- Chen, S.Y.; Fu, X.J.; Chu, M.S.; Liu, Z.G.; Tang, J. Life cycle assessment of the comprehensive utilization of vanadium titano-magnetite. J. Clean. Prod. 2015, 101, 122–128. [Google Scholar] [CrossRef]
- Zhang, J.L.; Xing, X.D.; Cao, M.M.; Jiao, K.X.; Wang, C.L.; Ren, S. Reduction kinetics of vanadium titano-magnetite carbon composite pellets adding catalysts under high temperature. J. Iron Steel Res. Int. 2013, 20, 1–7. [Google Scholar] [CrossRef]
- Cheng, G.J.; Gao, Z.X.; Lv, M.Y.; Yang, H.; Xue, X.X. Coal-based reduction and magnetic separation behavior of low-grade vanadium-titanium magnetite pellets. Minerals 2017, 7, 86. [Google Scholar] [CrossRef]
- Sui, Y.L.; Guo, Y.F.; Travyanov, A.Y.; Jiang, T.; Chen, F.; Qiu, G.Z. Reduction roasting–magnetic separation of vanadium tailings in presence of sodium sulfate and its mechanisms. Rare Met. 2016, 35, 954–960. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, H.Y.; Dong, Y.; Jiang, X.; Shen, Y.S.; Shen, F.M. Melting and separation behavior of slag and metal phases in metallized pellets obtained from the direct-reduction process of vanadium-bearing titanomagnetite. Int. J. Miner. Process. 2015, 142, 119–124. [Google Scholar] [CrossRef]
- Liu, S.S.; Guo, Y.F.; Qiu, G.Z. Jiang, T.; Chen, F. Solid-state reduction kinetics and mechanism of pre-oxidized vanadium-titanium magnetite concentrate. Trans. Nonferrous Met. Soc. China 2014, 24, 3372–3377. [Google Scholar] [CrossRef]
- Zhu, X.B.; Li, W.; Sun, Z.Y. Process Mineralogy of vanadium Titano-magnetite from Shanxi. Iron Steel Vanadium Titanium 2016, 37, 31–36. (In Chinese) [Google Scholar]
- Wang, W.Z.; Meng, Q.L.; Yang, C.G. Experimental research on comprehensive recovery of iron and titanium from the vanadium-titanium magnetite ore. Adv. Mater. Res. 2013, 641–642, 381–384. [Google Scholar] [CrossRef]
- Laxmi, T.; Srikant, S.S.; Rao, D.S.; Rao, R.B. Beneficiation studies on recovery and in-depth characterization of ilmenite from red sediments of badlands topography of Ganjam District, Odisha, India. Int. J. Min. Sci. Technol. 2013, 23, 725–731. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, J.; Wang, W.Q.; Deng, J.; Chen, B.Y.; Yan, W.; Xiong, S.Q.; Huang, Y.; Liu, J. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Liu, G.Z.; Dai, S.J.; Bai, L.M.; Ma, Y.X.; Zhang, Y. Experimental studies on a mineral containing titanium in Baoding area. Adv. Mater. Res. 2013, 826, 34–37. [Google Scholar] [CrossRef]
- Laxmi, T.; Behera, J.R.; Rao, R.B. Beneficiation studies on recovery of ilmenite from red sediments of badlands topography, Andhra Pradesh. Adv. Sci. Lett. 2016, 22, 344–348. [Google Scholar] [CrossRef]
- Lv, J.F.; Zhang, H.P.; Tong, X.; Fan, C.L.; Yang, W.T. Innovative methodology for recovering titanium and chromium from a raw ilmenite concentrate by magnetic separation after modifying magnetic properties. J. Hazard. Mater. 2017, 325, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Ferrovanadium–Determination of Vanadium Content–The Ammonium Ferrous Sulfate Titrimetric Method and the Potentiometric Titrimetric Method; Chinese National Standard: GB/T 8704.5; Standard Press of China: Beijing, China, 2007. (In Chinese)
- Editorial Board of Mineral Processing Handbook. Mineral Processing Handbook; Metallurgical Industry Press: Beijing, China, 2008; Volume 3, Fascicle 3; pp. 25–175. (In Chinese) [Google Scholar]
- Beijing General Research Institute of Mining & Metallurgy. Chemical Phase Analyses; Metallurgical Industry Press: Beijing, China, 1979; pp. 141–150. (In Chinese) [Google Scholar]
- Wang, L.Z.; Liu, Y.; Zhong, B.; Cao, J.H.; Qu, S.S.; Jiang, C.L. Mineralogical characteristics and separation performance of ilmenomagnetite ore. Min. Metall. Eng. 2016, 36, 57–59. (In Chinese) [Google Scholar]
- Broska, I.; Harlov, D.; Tropper, P.; Siman, P. Formation of magmatic titanite and titanite-ilmenite phase relations during granite alteration in the Tribec Mountains, Western Carpathians, Slovakia. Lithosphere 2007, 95, 58–71. [Google Scholar] [CrossRef]
- Single Mineral Separation Laboratory, Institute of Geochemistry, Chinese Academy of Sciences. Single Mineral Separation; Geological Publishing House: Beijing, China, 1981; pp. 50–64. (In Chinese) [Google Scholar]

| Element | V2O5 | TiO2 | TFe | SiO2 | Al2O3 | CaO | MgO | P | S |
|---|---|---|---|---|---|---|---|---|---|
| Content | 0.050 | 1.67 | 8.53 | 47.25 | 12.89 | 10.32 | 5.96 | 0.06 | 0.01 |
| Mineral | Magnetite | Martite | Ilmenite | Sphene | Feldspar |
|---|---|---|---|---|---|
| Content | 0.87 | 6.33 | 1.94 | 1.37 | 51.78 |
| Specific Susceptibility (10−8 m3/kg) | >4000 | 60~440 | 280~450 | 5~10 | −1~5 |
| Mineral | Pyroxene | Amphibole | Talc | Montmorillonite | Else |
| Content | 22.34 | 4.00 | 5.03 | 5.34 | 1.00 |
| Specific Susceptibility (10−8 m3/kg) | 20~400 | 50~300 | −1~5 | - | - |
| Phase | Magnetite | Martite | Ilmenite | Carbonate | Silicate | Total |
|---|---|---|---|---|---|---|
| Mass fraction | 0.58 | 3.76 | 0.66 | 0.19 | 3.33 | 8.52 |
| Distribution | 6.81 | 44.13 | 7.75 | 2.23 | 39.08 | 100.00 |
| Phase | Ilmenite | Iron Minerals | Rutile | Silicate | Total |
|---|---|---|---|---|---|
| Mass fraction | 0.97 | 0.35 | 0.02 | 0.34 | 1.68 |
| Distribution | 57.74 | 20.83 | 1.19 | 20.24 | 100.00 |
| Phase | Iron Minerals | Ilmenite | Silicate | Total |
|---|---|---|---|---|
| Mass fraction | 0.0273 | 0.0016 | 0.0211 | 0.0500 |
| Distribution | 54.60 | 3.20 | 42.20 | 100.00 |
| V2O5 | TiO2 | Fe | CaO | MgO | Al2O3 | SiO2 | Mineral |
|---|---|---|---|---|---|---|---|
| 0.59 | 5.21 | 63.46 | 0.00 | 0.45 | 1.37 | 0.48 | V-Ti Magnetite |
| 0.10 | 51.64 | 36.73 | 0.00 | 0.66 | 0.00 | 0.00 | Ilmenite |
| 1.53 | 25.53 | 22.05 | 18.50 | 0.70 | 3.09 | 19.15 | Sphene |
| Magnetic Intensity/T | Products | Yield (wt %) | Grade | Recovery | ||||
|---|---|---|---|---|---|---|---|---|
| V2O5 | TiO2 | Fe | V2O5 | TiO2 | Fe | |||
| 0.4 | Concentrate | 7.78 | 0.113 | 10.98 | 18.24 | 16.73 | 21.57 | 8.86 |
| Tailing | 92.22 | 0.048 | 3.37 | 15.82 | 83.27 | 78.43 | 91.14 | |
| Feed | 100.00 | 0.053 | 3.96 | 16.01 | 100.00 | 100.00 | 100.00 | |
| 0.6 | Concentrate | 15.88 | 0.091 | 16.05 | 20.77 | 27.37 | 64.86 | 20.57 |
| Tailing | 84.12 | 0.045 | 1.65 | 15.13 | 72.63 | 35.14 | 79.43 | |
| Feed | 100.00 | 0.052 | 3.95 | 16.03 | 100.00 | 100.00 | 100.00 | |
| 0.8 | Concentrate | 42.71 | 0.075 | 7.26 | 17.92 | 61.34 | 78.10 | 47.78 |
| Tailing | 57.29 | 0.035 | 1.52 | 14.60 | 38.66 | 21.90 | 52.22 | |
| Feed | 100.00 | 0.053 | 3.97 | 16.02 | 100.00 | 100.00 | 100.00 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Zhang, Y.; Liu, T.; Huang, J. Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore. Minerals 2017, 7, 137. https://doi.org/10.3390/min7080137
Xu C, Zhang Y, Liu T, Huang J. Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore. Minerals. 2017; 7(8):137. https://doi.org/10.3390/min7080137
Chicago/Turabian StyleXu, Chengbao, Yimin Zhang, Tao Liu, and Jing Huang. 2017. "Characterization and Pre-Concentration of Low-Grade Vanadium-Titanium Magnetite Ore" Minerals 7, no. 8: 137. https://doi.org/10.3390/min7080137




