Schwertmannite Adherence to the Reactor Wall during the Bio-Synthesis Process and Deterioration of Its Structural Characteristics and Arsenic(III) Removal Efficiency
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of A. ferrooxidans Cell Suspensions
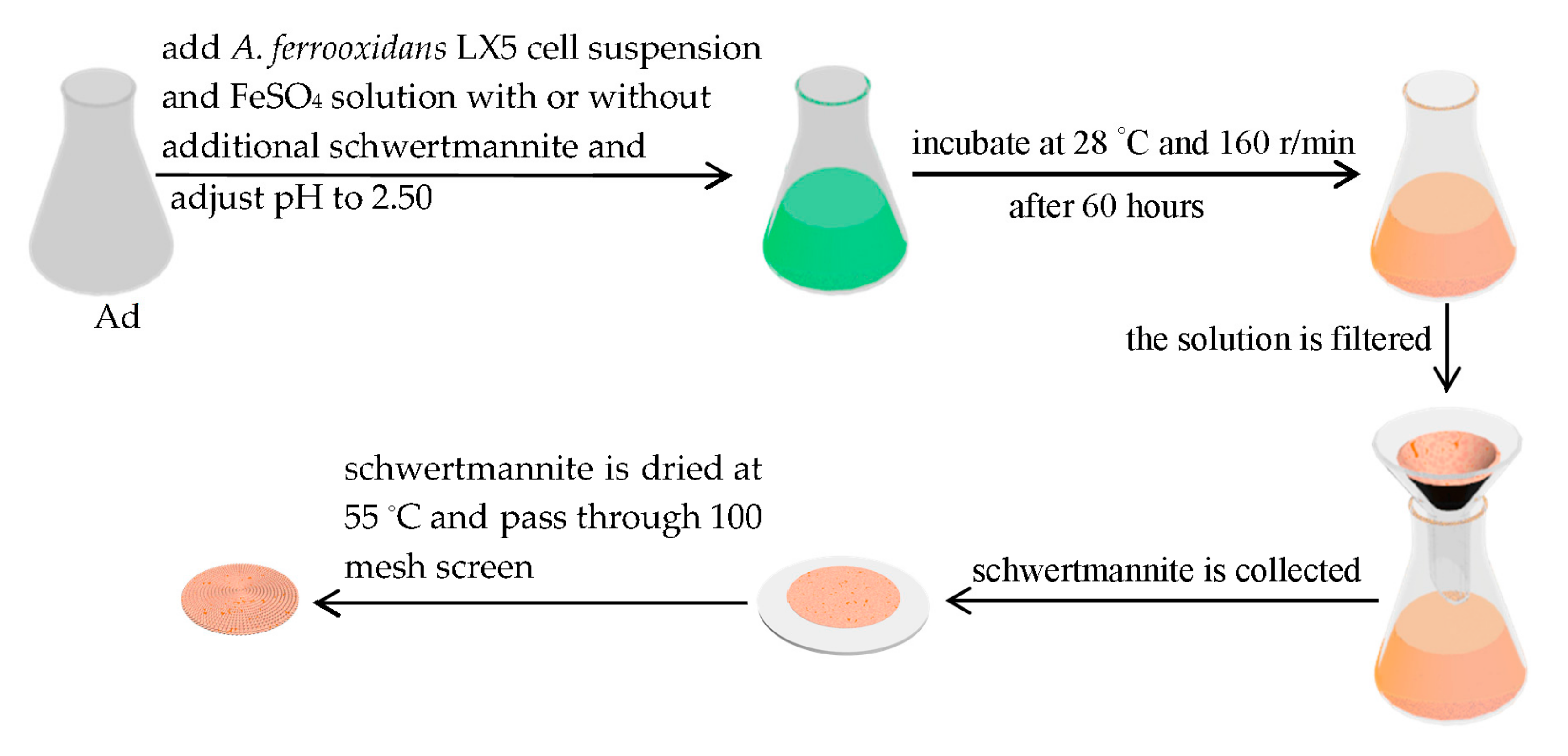
2.2. Preparation of Additional Schwertmannite in a 25 L Bioreactor
2.3. Schwertmannite Bio-Synthesis Experiment Introducing Additional Schwertmannite before Bio-Synthesis
2.4. Effect of Adhered, Suspended-Schwertmannite on Arsenic(III) Adsorption
2.5. Analytical Procedures
3. Results and Discussion
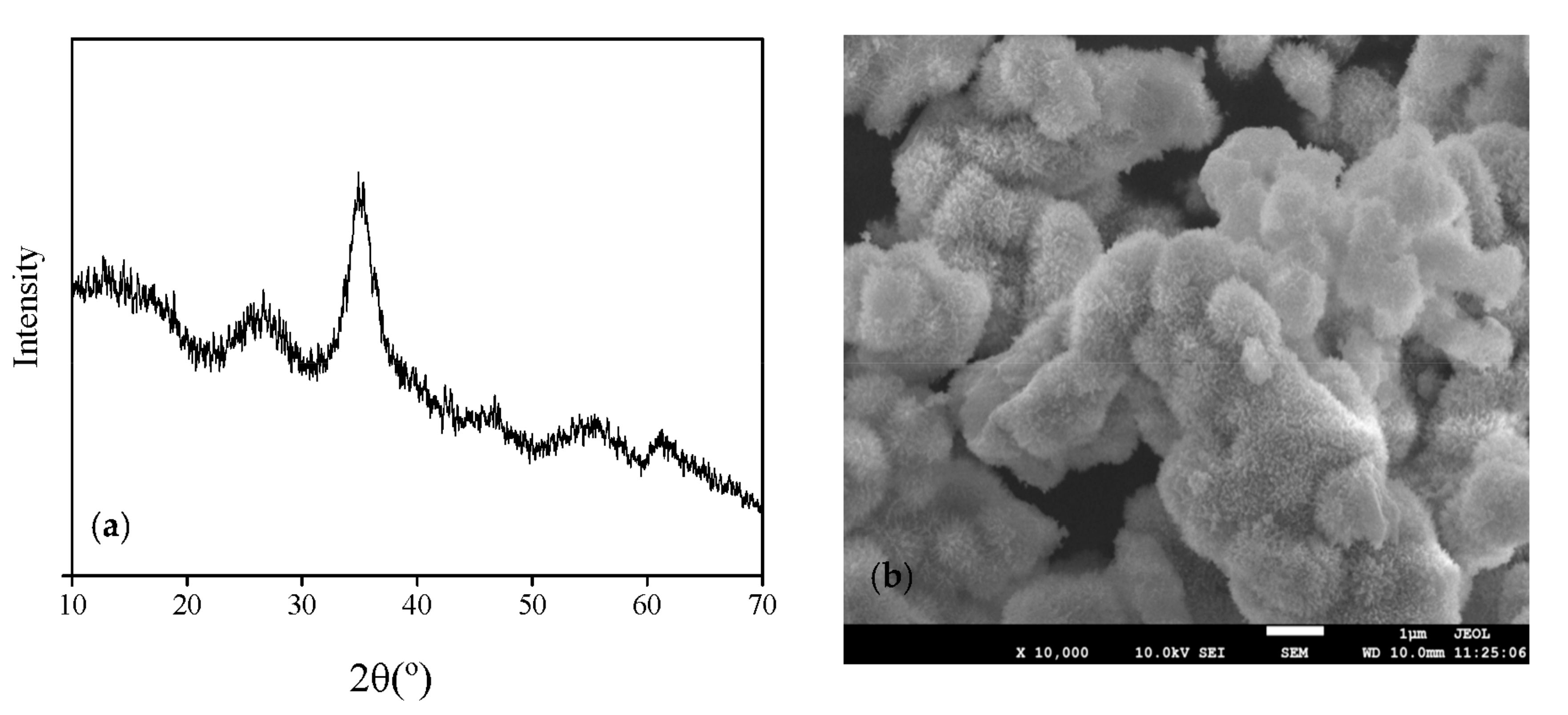
3.1. Schwertmannite Bio-Synthesized in 25 L Bioreactor
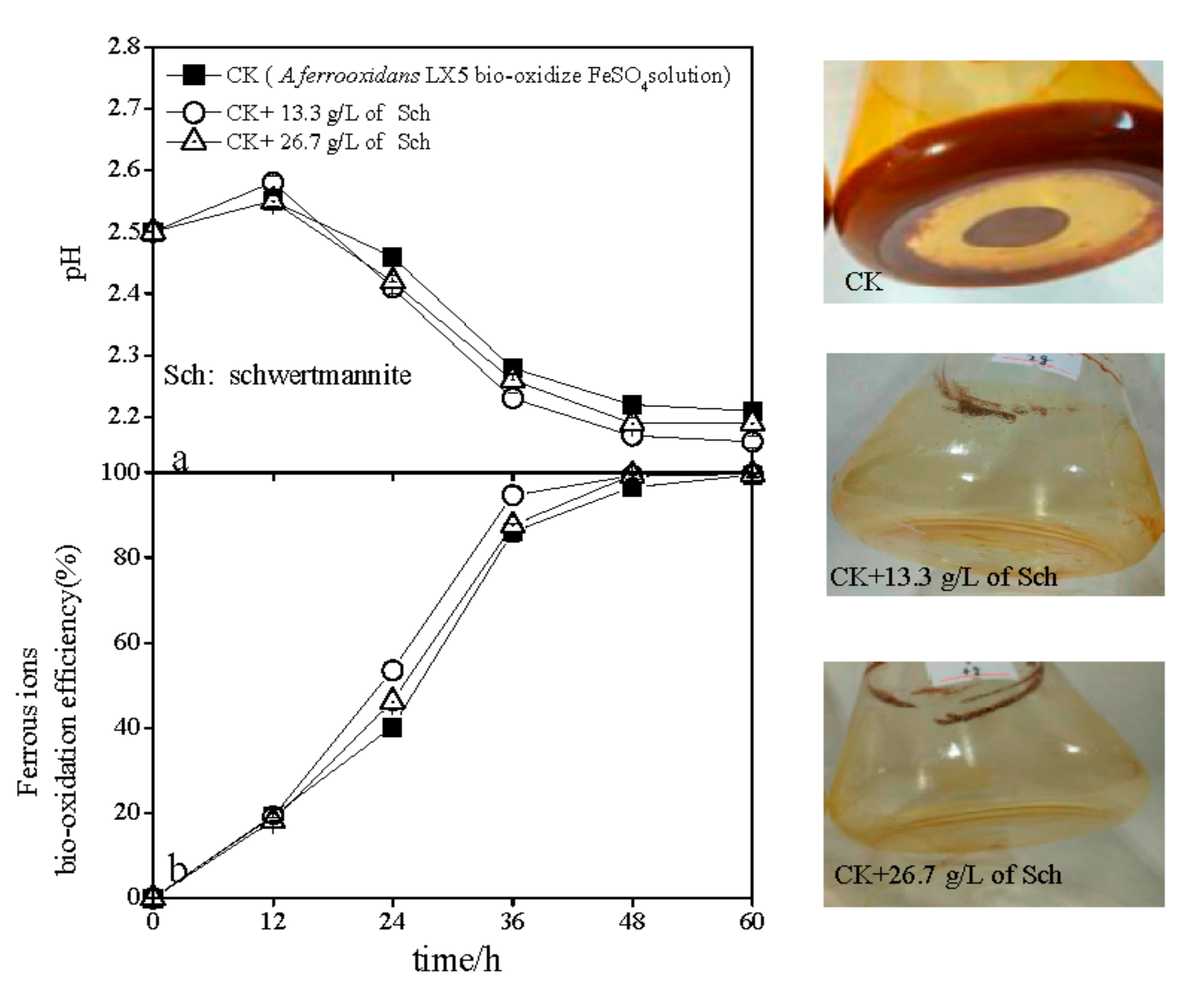
3.2. The pH and Ferrous Ions’ Efficiency Variations during Schwertmannite Bio-Synthesis with the Introduction of Additional Schwertmannite
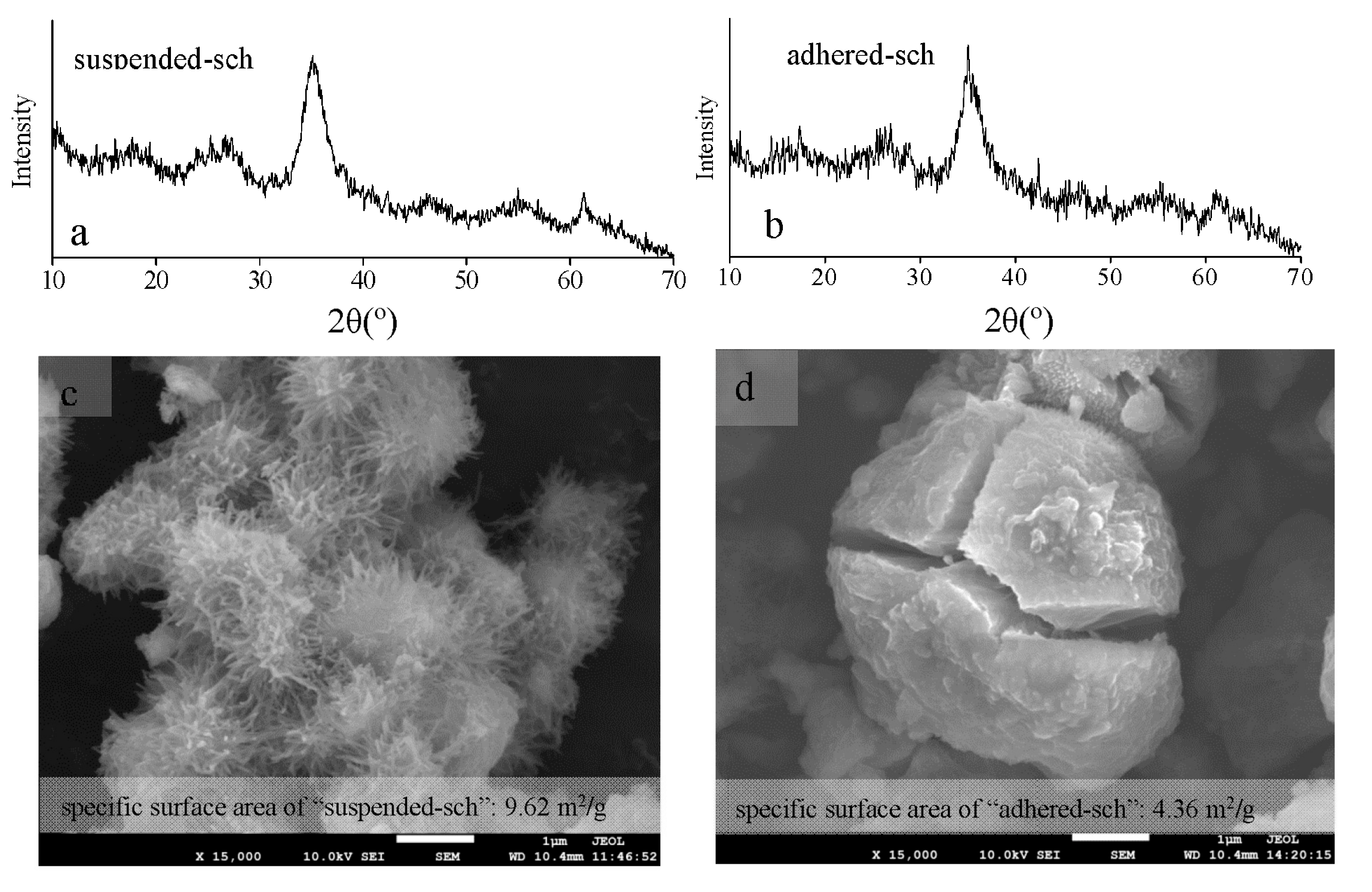
3.3. The Structural Characteristics of Adhered-Sch and Suspended-Sch
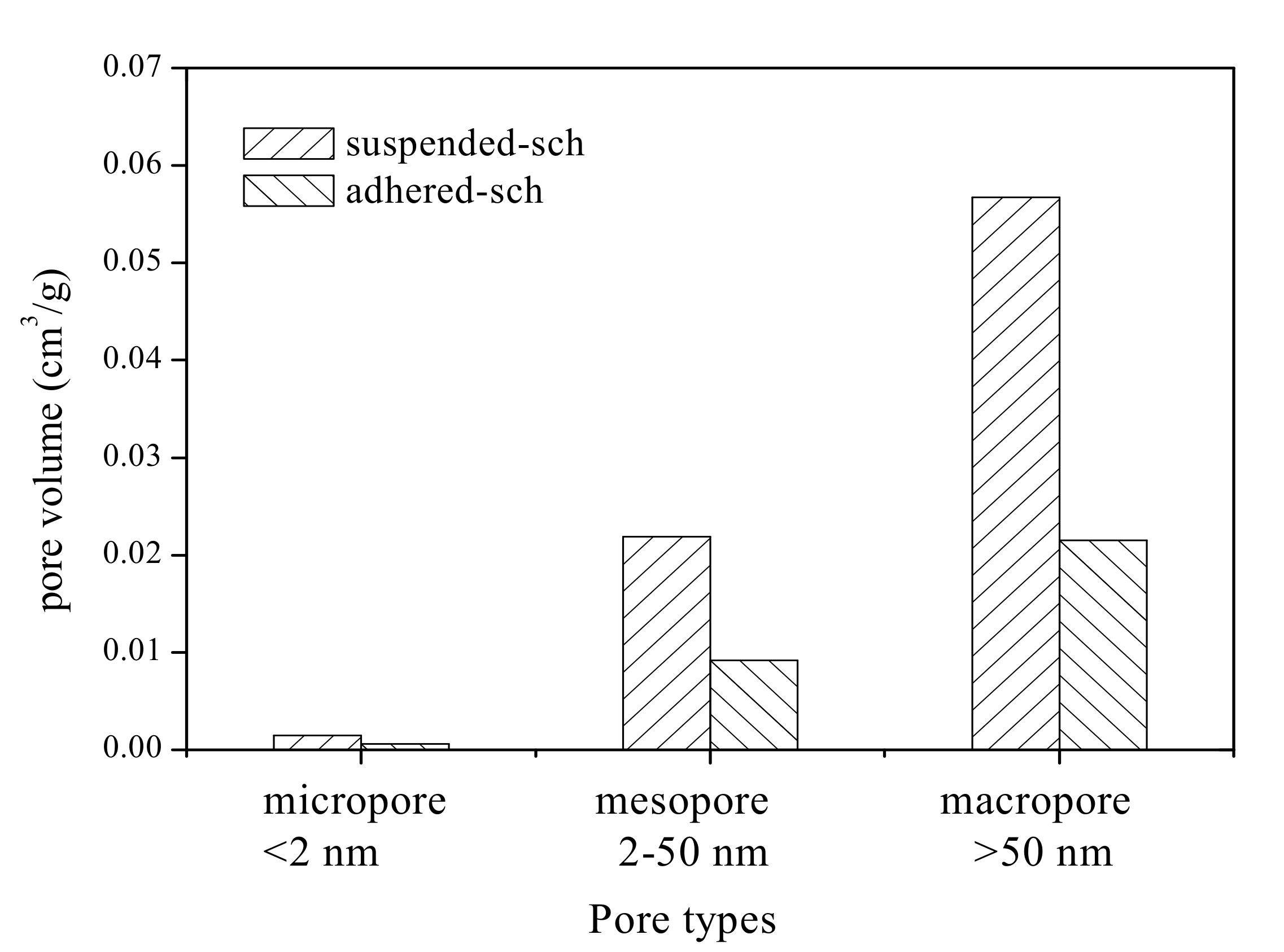
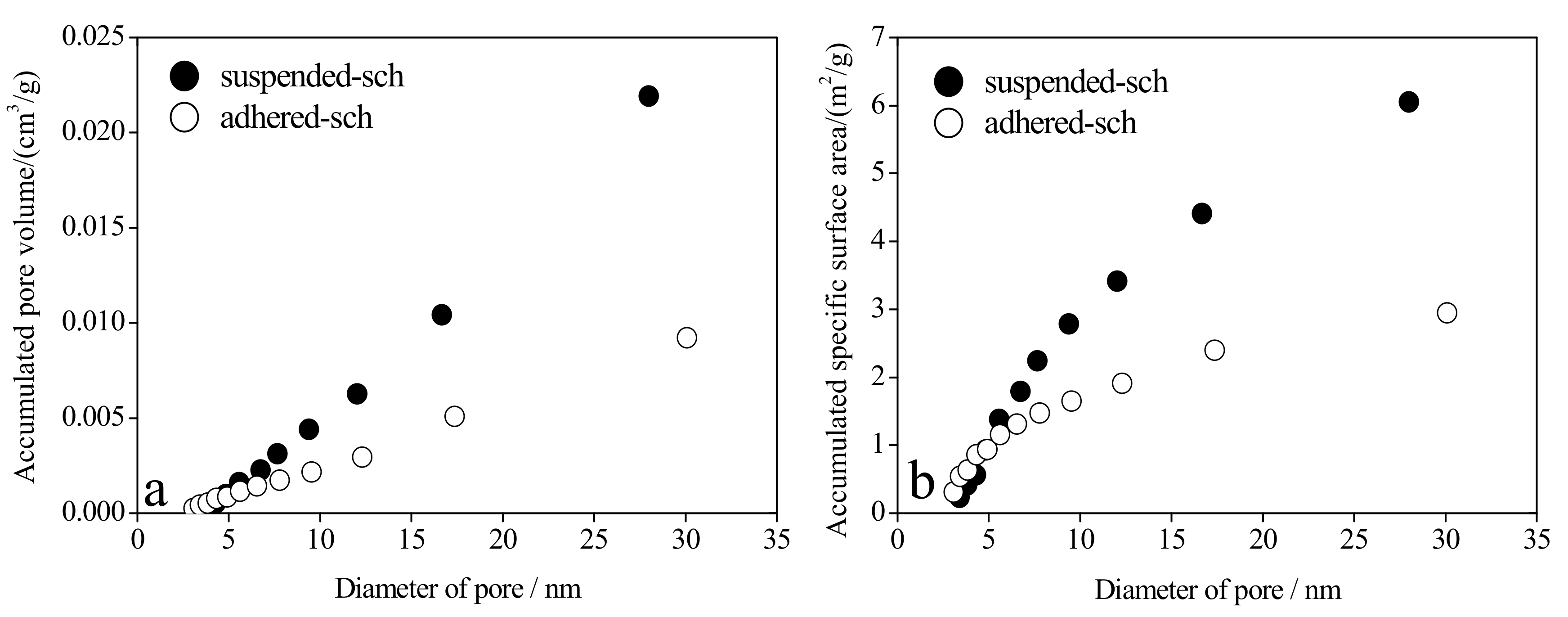
3.4. The Pore Volume and Pore Size Distribution of Adhered-Sch and Suspended-Sch
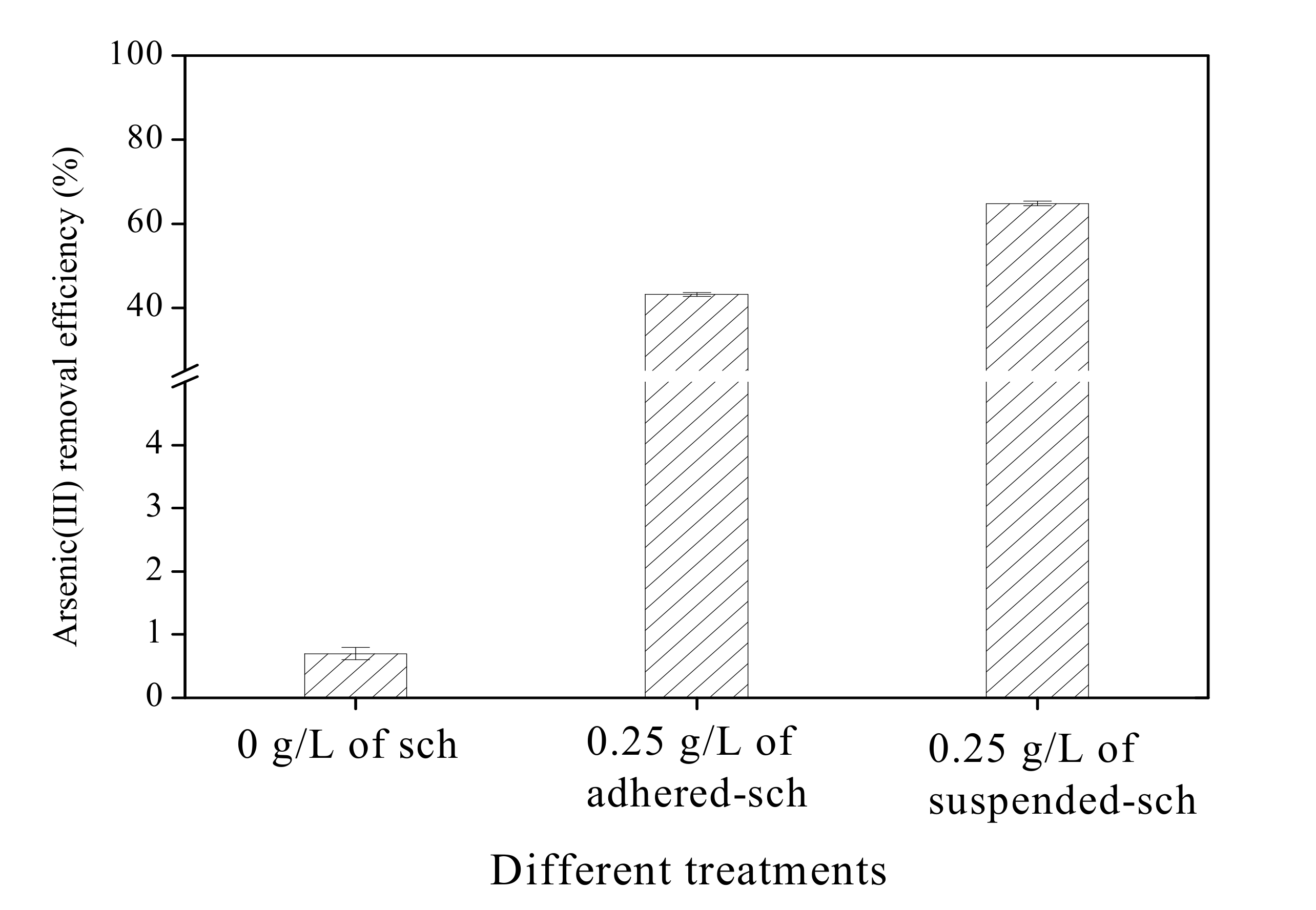
3.5. Arsenic(III) Removal Efficiency of Adhered-Sch and Suspended-Sch
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Podder, M.S.; Majumder, C.B. Phycoremediation of arsenic from wastewaters by Chlorella pyrenoidosa. Groundw. Sustain. Dev. 2015, 1, 8–91. [Google Scholar] [CrossRef]
- Podder, M.S.; Majumder, C.B. Study of the kinetics of arsenic removal from wastewater using Bacillus arsenicus biofilms supported on a Neem leaves/MnFe2O4 composite. Ecol. Eng. 2016, 88, 195–216. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef]
- Li, H.; Zeng, X.C.; He, Z.; Chen, X.; Guoji, E.; Han, Y.; Wang, Y. Long-term performance of rapid oxidation of arsenite in simulated groundwater using a population of arsenite-oxidizing microorganisms in a bioreactor. Water Res. 2016, 101, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Kanel, S.R.; Manning, B.; Charlet, L.; Choi, H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Halem, D.V.; Verberk, J. Review of high arsenic groundwater in China. In Proceedings of the 4th International Conference on Bioinformatics & Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar]
- He, J.; Ma, T.; Deng, Y.; Yang, H.; Wang, Y. Environmental geochemistry of high arsenic groundwater at western Hetao plain, Inner Mongolia. Front. Earth Sci. 2009, 3, 63–72. [Google Scholar] [CrossRef]
- Xie, X.J.; Ellis, A.; Wang, Y.; Xie, Z.; Duan, M.; Su, C. Geochemistry of redox-sensitive elements and sulfur isotopes in the high arsenic groundwater system of Datong Basin, China. Sci. Total Environ. 2009, 407, 3823–3835. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Wang, J.P.; Zheng, Y.J.; Ng, J.C. Biomarkers for the evaluation of population health status 16 years after the intervention of arsenic-contaminated groundwater in Xinjiang, China. J. Hazard. Mater. 2013, 262, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, S.; Sengupta, M.K.; Mukherjee, A.; Hossain, M.A.; Das, B.; Nayak, B.; Pal, A.; Mukherjee, S.C.; Pati, S.; Dutta, R.N. Arsenic groundwater contamination and its health effects in the state of Uttar Pradesh (UP) in upper and middle Ganga plain, India: A severe danger. Sci. Total Environ. 2006, 370, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.M. Arsenic in groundwater and health problems in Bangladesh. Water Res. 2000, 34, 304–310. [Google Scholar] [CrossRef]
- Zhao, D.D.; Yang, Y.; Chen, J.P. Zirconium/polyvinyl alcohol modified flat-sheet polyvinyldene fluoride membrane for decontamination of arsenic: Material design and optimization, study of mechanisms, and application prospects. Chemosphere 2016, 155, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Pessoa-Lope, M.; Crespo, J.G.; Velizarov, S. Arsenate removal from sulphate-containing water streams by an ion-exchange membrane process. Sep. Purif. Technol. 2016, 166, 125–134. [Google Scholar] [CrossRef]
- Ali, I.; Khan, T.A.; Asim, M. Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sep. Purif. Rev. 2011, 40, 25–42. [Google Scholar] [CrossRef]
- Peng, B.; Song, T.T.; Wang, T.; Chai, L.; Yang, W.; Li, X.; Li, C.; Wang, H. Facile synthesis of Fe3O4@Cu(OH)2 composites and their arsenic adsorption application. Chem. Eng. J. 2016, 299, 15–22. [Google Scholar] [CrossRef]
- Kumar, A.S.K.; Jiang, S.J. Chitosan-functionalized graphene oxide: A novel adsorbent an efficient adsorption of arsenic from aqueous solution. J. Environ. Chem. Eng. 2016, 4, 1698–1713. [Google Scholar] [CrossRef]
- Kofa, G.P.; Ndikoungou, S.; Kayem, G.J.; Kamga, R. Adsorption of arsenic by natural pozzolan in a fixed bed: Determination of operating conditions and modeling. J. Water Process Eng. 2015, 6, 166–173. [Google Scholar] [CrossRef]
- Dou, X.M.; Mohan, D.; Pittman, C.U., Jr. Arsenate adsorption on three types of granular schwertmannite. Water Res. 2013, 47, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Dey, A.; Singh, R.; Purkait, M.K. Cobalt ferrite nanoparticles aggregated schwertmannite: A novel adsorbent for the efficient removal of arsenic. J. Water Process Eng. 2014, 3, 1–9. [Google Scholar] [CrossRef]
- Liu, F.W.; Zhou, J.; Zhang, S.S.; Liu, L.; Zhou, L.; Fan, W. Schwertmannite synthesis through ferrous ion chemical oxidation under different H2O2 supply rates and its removal efficiency for arsenic from contaminated groundwater. PLoS ONE 2015, 10, e0138891. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Liang, J.R.; Zhou, L.X. Adsorptive removal of As(III) by biogenic schwertmannite from simulated As-contaminated groundwater. Chemosphere 2011, 83, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Mokadem, N.; Demdoum, A.; Hamed, Y.; Bouri, S.; Hadji, R.; Boyce, A.; Laouar, R.; Saad, A. Hydrogeochemical and stable isotope data of groundwater of a multi-aquifer system: Northern Gafsa basin—Central Tunisia. J. Afr. Earth Sci. 2016, 114, 174–191. [Google Scholar] [CrossRef]
- Li, X.Q.; Hou, X.W.; Zhou, Z.C.; Liu, L.X. Geochemical provenance and spatial distribution of fluoride in groundwater of Taiyuan basin, China. Environ. Earth. Sci. 2011, 62, 1635–1642. [Google Scholar] [CrossRef]
- Paikaray, S.; Göttlicher, J.; Peiffer, S. Removal of As(III) from acidic waters using schwertmannite: Surface speciation and effect of synthesis pathway. Chem. Geol. 2011, 283, 134–142. [Google Scholar] [CrossRef]
- Qiao, X.X.; Liu, L.L.; Shi, J.; Zhou, L.X.; Guo, Y.H.; Ge, Y.Y.; Fan, W.H.; Liu, F.W. Heating changes bio-schwertmannite microstructure and arsenic(III) removal efficiency. Minerals 2017, 7, 9. [Google Scholar] [CrossRef]
- Xu, Y.Q.; Yang, M.; Yao, T.; Xiong, H. Isolation, identification and arsenic-resistance of Acidithiobacillus ferrooxidans HX3 producing schwertmannite. J. Environ. Sci. 2014, 26, 1463–1470. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.W.; Zhou, J.; Zhou, L.X.; Zhang, S.S.; Liu, L.L.; Wang, M. Effect of neutralized solid waste generated in lime neutralization on the ferrous ion bio-oxidation process during acid mine drainage treatment. J. Hazard. Mater. 2015, 299, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.H.; Zhou, L.X.; Liang, J.R.; Xiong, H.X. Biosynthesis of schwertmannite by Acidithiobacillus ferrooxidans cell suspensions under different pH condition. Mater. Sci. Eng. C 2009, 29, 211–215. [Google Scholar] [CrossRef]
- Liu, F.W.; Zhou, J.; Jing, T.J.; Zhang, S.S.; Liu, L.L. Effect of calcium oxide on the efficiency of ferrous ion oxidation and total iron precipitation during ferrous ion oxidation in simulated acid mine drainage treatment with inoculation of Acidithiobacillus ferrooxidan. Water Sci. Technol. 2016, 73, 1442–1452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, Y.B.; Zhao, X.; Liu, Z.L.; Chen, W. Porous perovskite LaNiO3 nanocubes as cathode catalysts for Li-O2 batteries with Low charge potential. Sci. Rep. 2014, 4, 6005. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Aguilerasigalat, J.; Elhankari, S.; Bradshaw, D. Magnetic MOF microreactors for recyclable size-selective biocatalysis. Chem. Sci. 2014, 6, 1938–1943. [Google Scholar] [CrossRef]
- Bian, C.; Ji, L.Y.; Liu, P. Determination of As and Hg Simultaneously in Wheat Flour by Dual Channel Atomic Fluorescence Spectrometry. J. Chin. Cereals Oils Assoc. 2013, 28, 108–111. [Google Scholar]
- Joint Committee on Powder Diffraction Standards (JCPDS). Mineral Powder Diffraction Files; International Center for Diffraction Data: Swarthmore, PA, USA, 2002. [Google Scholar]
- Veríssimo, L.M.P.; Ribeiro, A.C.F.; Lobo, V.M.M.; Esteso, M.A. Effect of hydrolysis on the diffusion of ferric sulphate in aqueous solutions at T = 298.15 K. J. Chem. Thermodyn. 2012, 55, 56–59. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.X. Simultaneous oxidation and precipitation of iron using jarosite immobilized Acidithiobacillus ferrooxidans and its relevance to acid mine drainage. Hydrometallurgy 2012, 125–126, 152–156. [Google Scholar] [CrossRef]
- Liu, J.Y.; Tao, X.X.; Cai, P. Study of formation of jarosite mediated by thiobacillus ferrooxidans in 9K medium. Procedia Earth Planet. Sci. 2009, 1, 706–712. [Google Scholar] [CrossRef]
- Su, G.Z.; Lu, J.J.; Lu, X.C.; Li, J.; Tu, B.W. An experimental study of the adsorption of Cu2+ and Acidothiobacillus ferrooxidans on schwertmannite. Acta Petrol. Mineral. 2009, 28, 575–580. [Google Scholar]
- Wang, K.B.; Fang, D.; Xu, Z.H.; Shi, Y.; Zheng, G.Y.; Zhou, L.X. Biosynthetic schwertmannite as catalyst in Fenton-like reactions for degradation of methyl orange. Environ. Sci. 2015, 36, 995–999. [Google Scholar]
- Bigham, J.M.; Schwertmann, U.; Carlson, L.; Murad, E. A poorly crystallized oxyhydroxysulfate of iron formed by bacterial oxidation of Fe(II) in aid mine waters. Geochim. Cosmochim. Acta 1990, 54, 2743–2758. [Google Scholar] [CrossRef]
- Oseto, K.; Okabe, H.; Okatsu, K. Evaluation of waterflooding performance utilizing core analysis data for carbonate reservoir, offshore Abu Dhabi. J. Jpn. Assoc. Pet. Technol. 2007, 72, 594–600. [Google Scholar] [CrossRef]
- Lee, D.; Jung, J.Y.; Jung, M.J.; Lee, Y.S. Hierarchical porous carbon fibers prepared using a SiO2 template for high-performance EDLCs. Chem. Eng. J. 2015, 263, 62–70. [Google Scholar] [CrossRef]
- Peng, J.H.; Zhang, L.B.; Zhang, S.M.; Tu, J.H.; Xia, H.Y.; Fan, X.X.; Guo, S.H. Study on Preparation of Micropore Activated Carbons from Tobacco Stems Impregnated with Zinc Chloride by Microwave Heating. J. Mater. Sci. Eng. 2006, 24, 52–57. [Google Scholar]
- Hsieh, C.T.; Teng, H. Influence of mesopore volume and adsorbate size on adsorption capacities of activated carbons in aqueous solutions. Carbon 2000, 38, 863–869. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Shi, J.; Zhang, S.; Zhou, L.; Xu, J.; Ge, Y.; Fan, W.; Liu, F. Schwertmannite Adherence to the Reactor Wall during the Bio-Synthesis Process and Deterioration of Its Structural Characteristics and Arsenic(III) Removal Efficiency. Minerals 2017, 7, 64. https://doi.org/10.3390/min7040064
Zhang J, Shi J, Zhang S, Zhou L, Xu J, Ge Y, Fan W, Liu F. Schwertmannite Adherence to the Reactor Wall during the Bio-Synthesis Process and Deterioration of Its Structural Characteristics and Arsenic(III) Removal Efficiency. Minerals. 2017; 7(4):64. https://doi.org/10.3390/min7040064
Chicago/Turabian StyleZhang, Jian, Jing Shi, Shasha Zhang, Lixiang Zhou, Jianmin Xu, Yuanying Ge, Wenhua Fan, and Fenwu Liu. 2017. "Schwertmannite Adherence to the Reactor Wall during the Bio-Synthesis Process and Deterioration of Its Structural Characteristics and Arsenic(III) Removal Efficiency" Minerals 7, no. 4: 64. https://doi.org/10.3390/min7040064




