Advances and Opportunities in Ore Mineralogy
Abstract
:1. Introduction
2. Advances
2.1. Microanalytical Methods
2.1.1. Laser-Ablation Inductively-Coupled Plasma Mass Spectrometry (LA-ICP-MS)
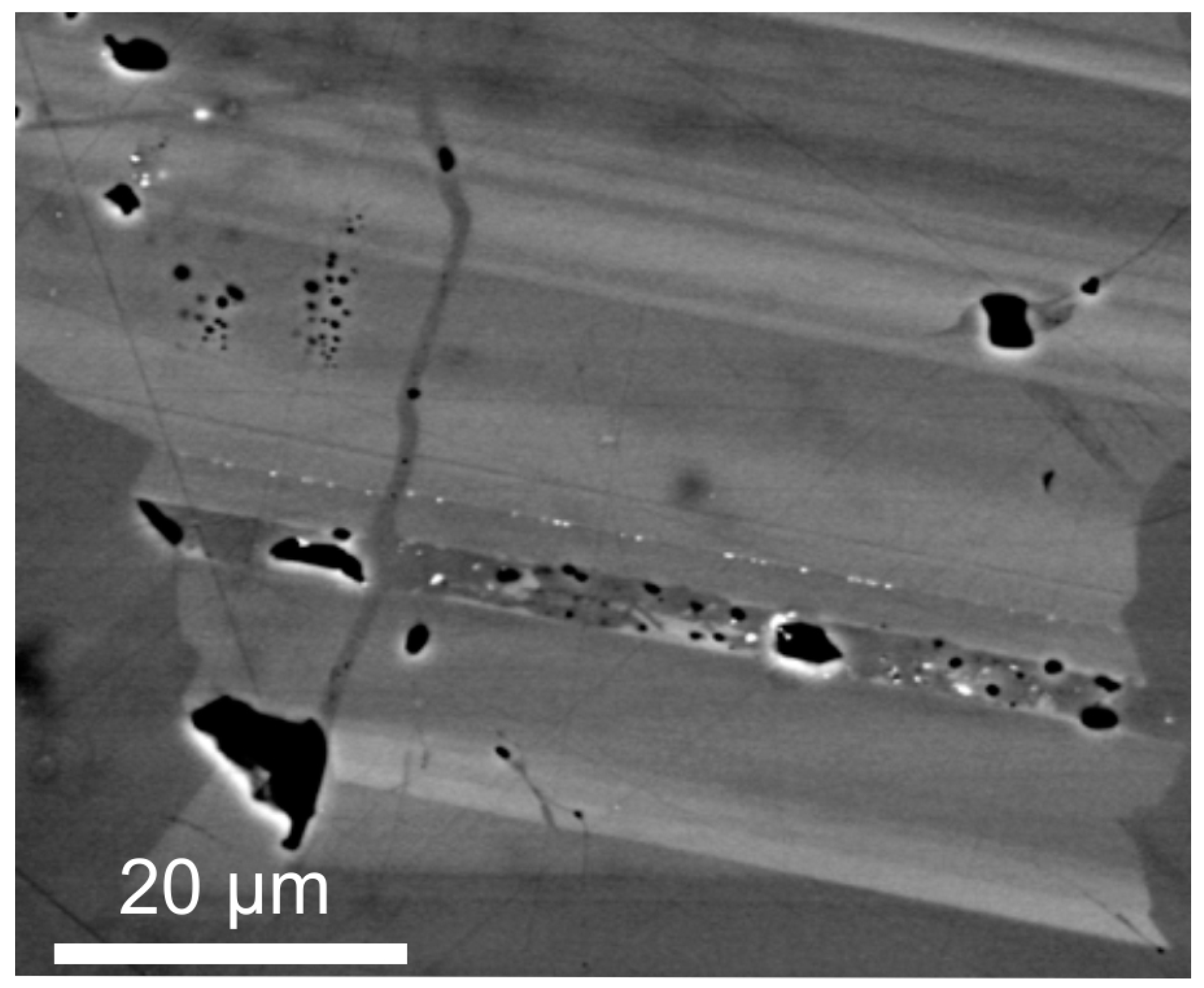
2.1.2. Focussed Ion Beam-Scanning Electron Microscopy (FIB-SEM)
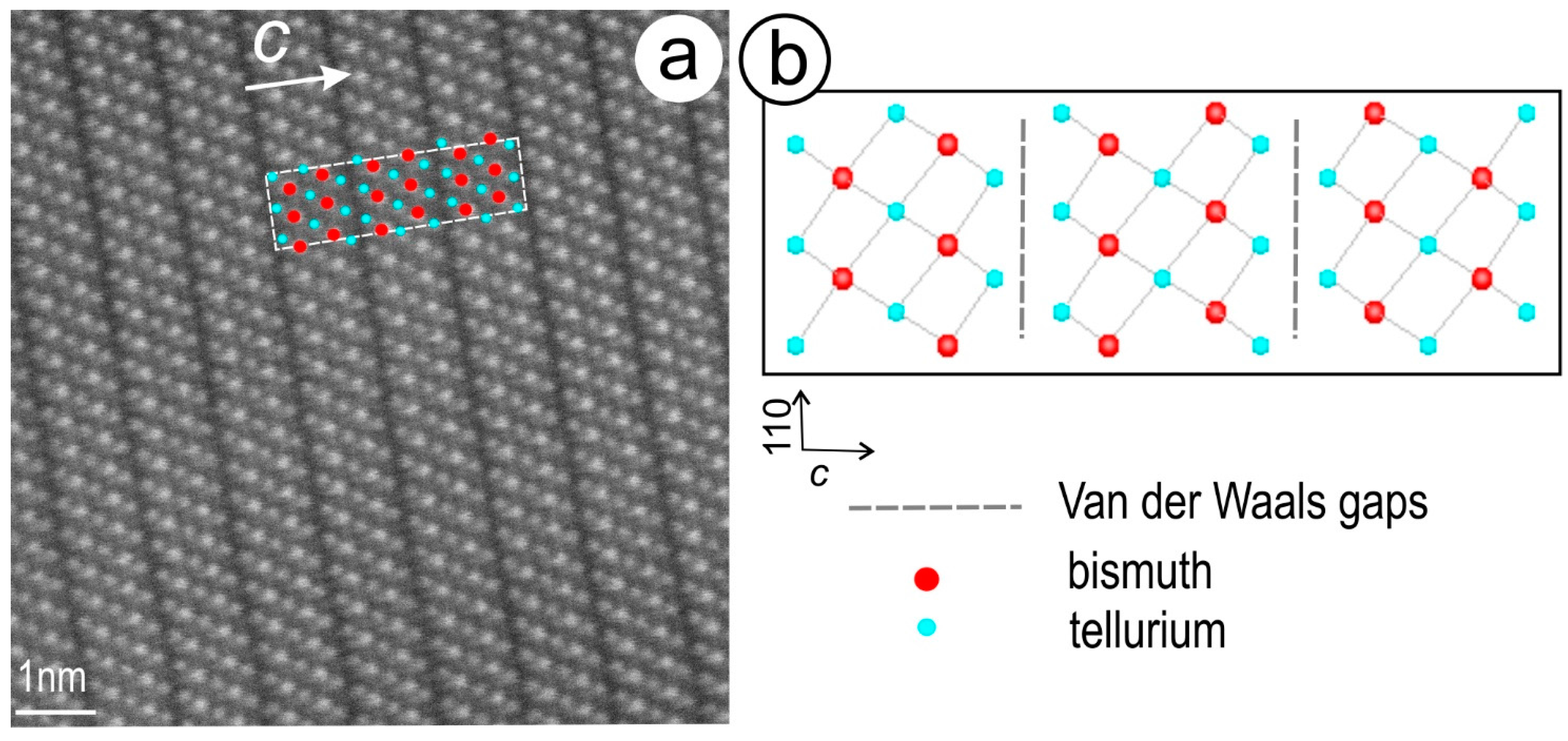
2.1.3. HAADF STEM Imaging and STEM-EDS Mapping
2.1.4. Electron Back-Scatter Diffraction (EBSD)
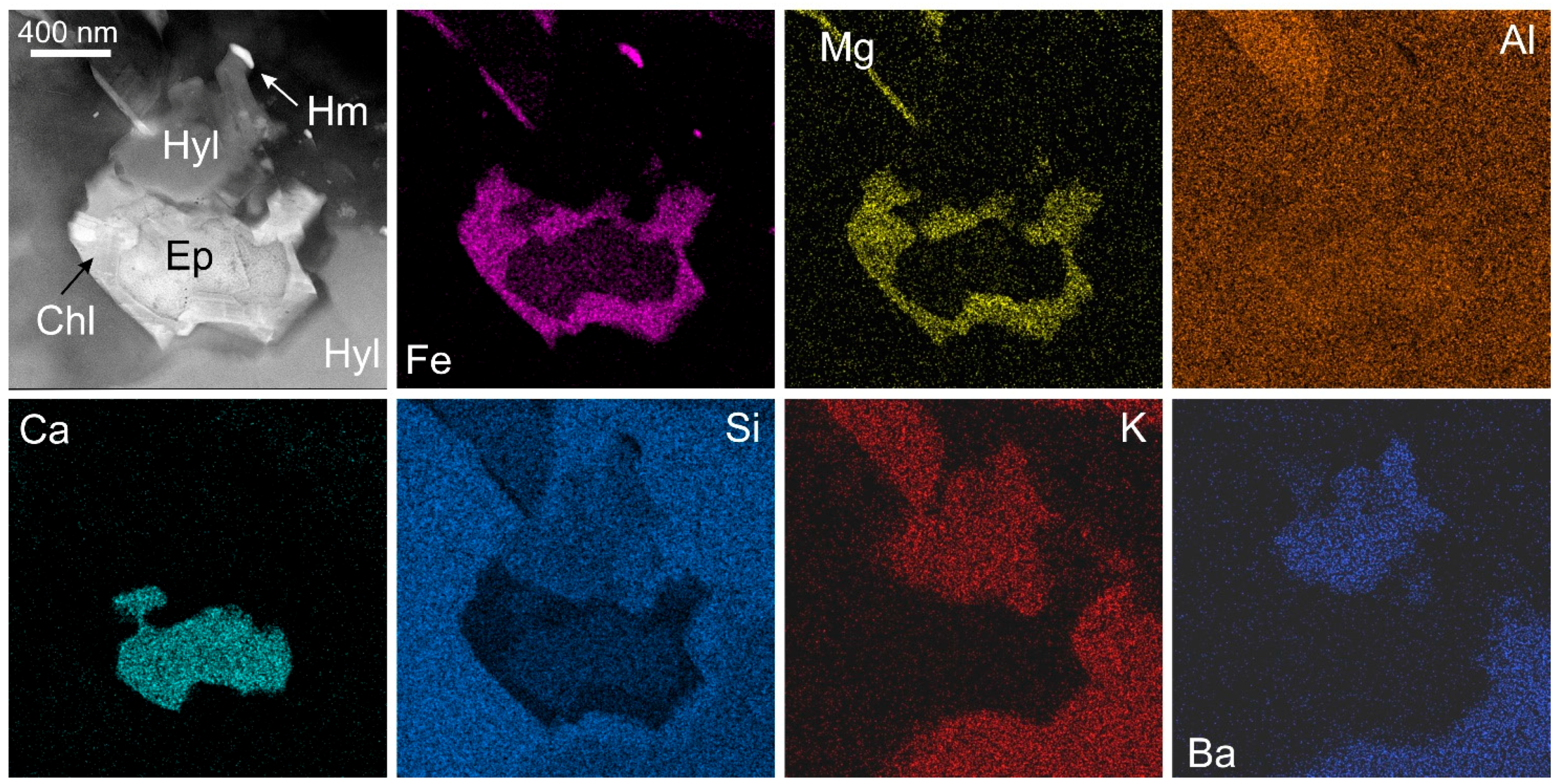
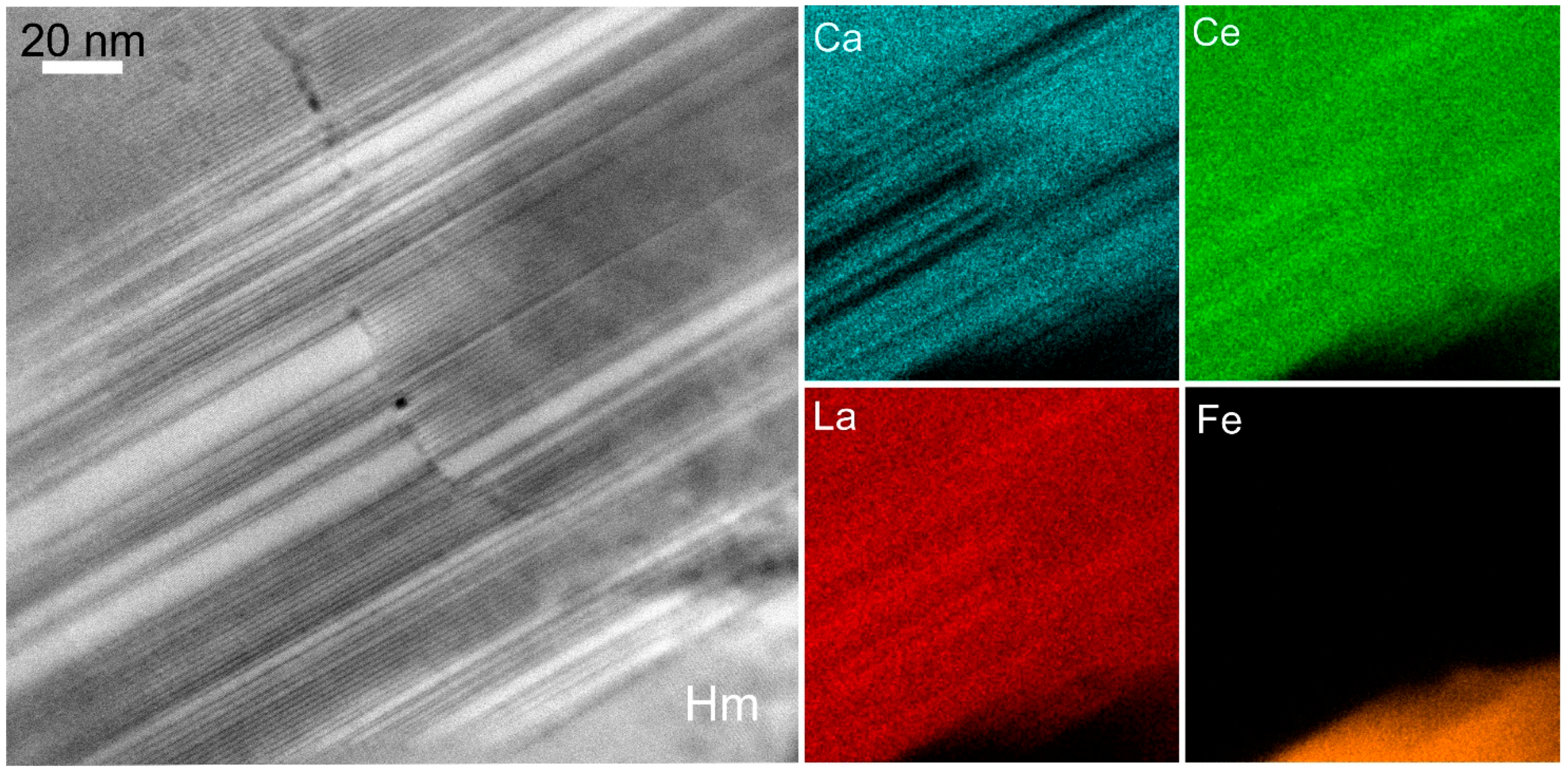
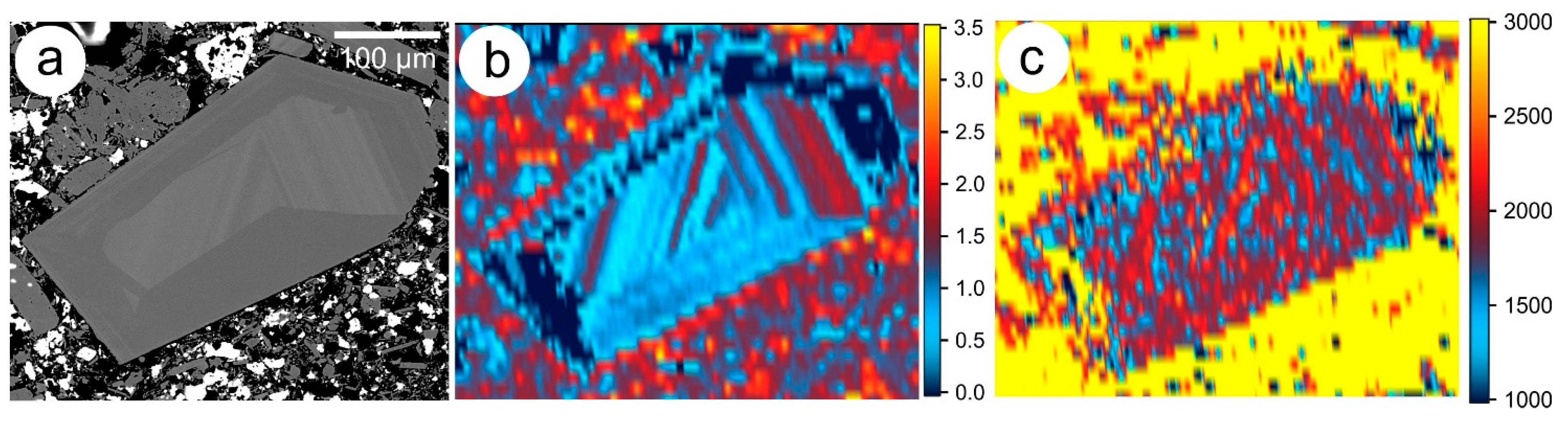
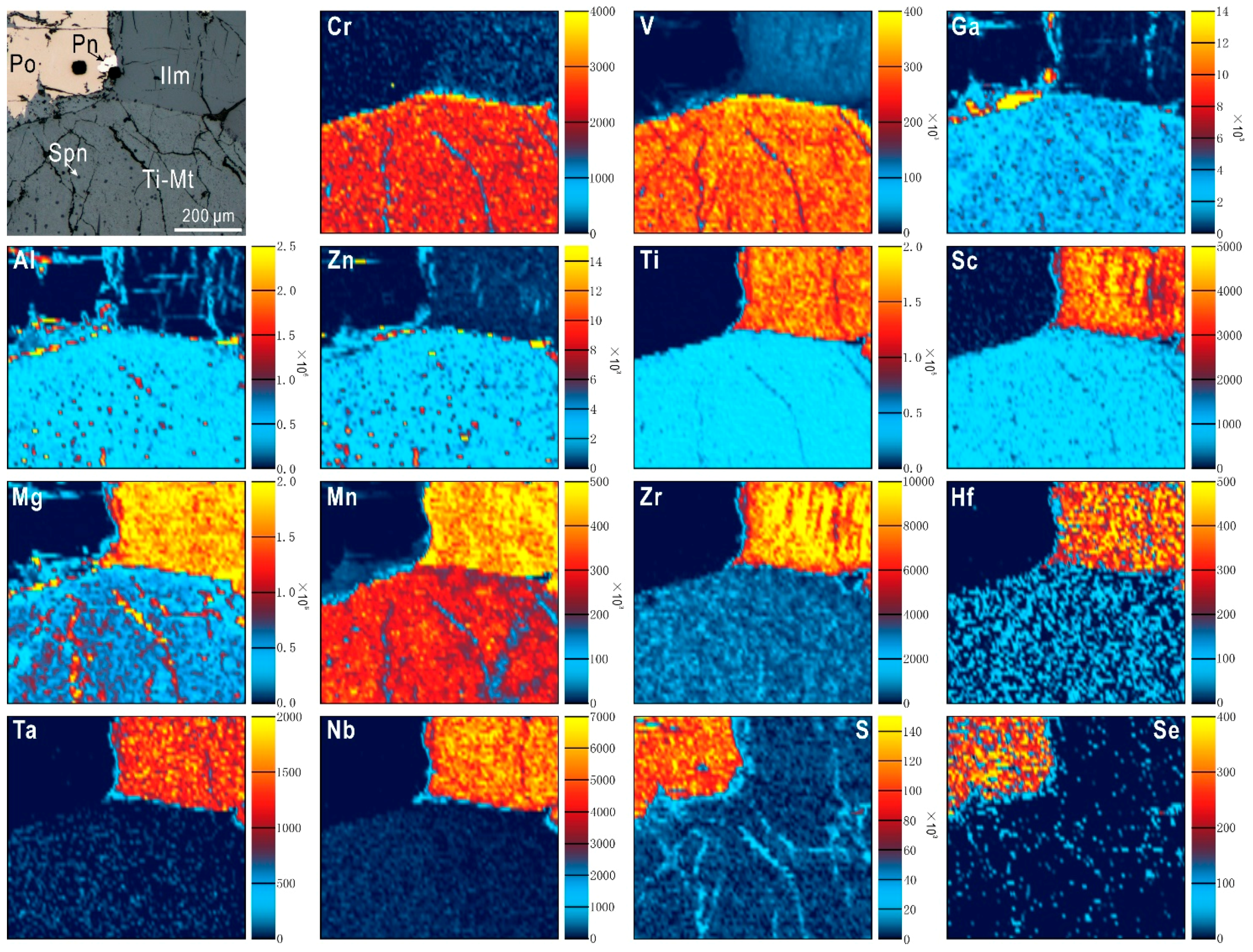
2.1.5. Trace Element X-ray Fluorescence (SXRF) Mapping Using Synchrotron Radiation
2.1.6. Automated Mineralogy
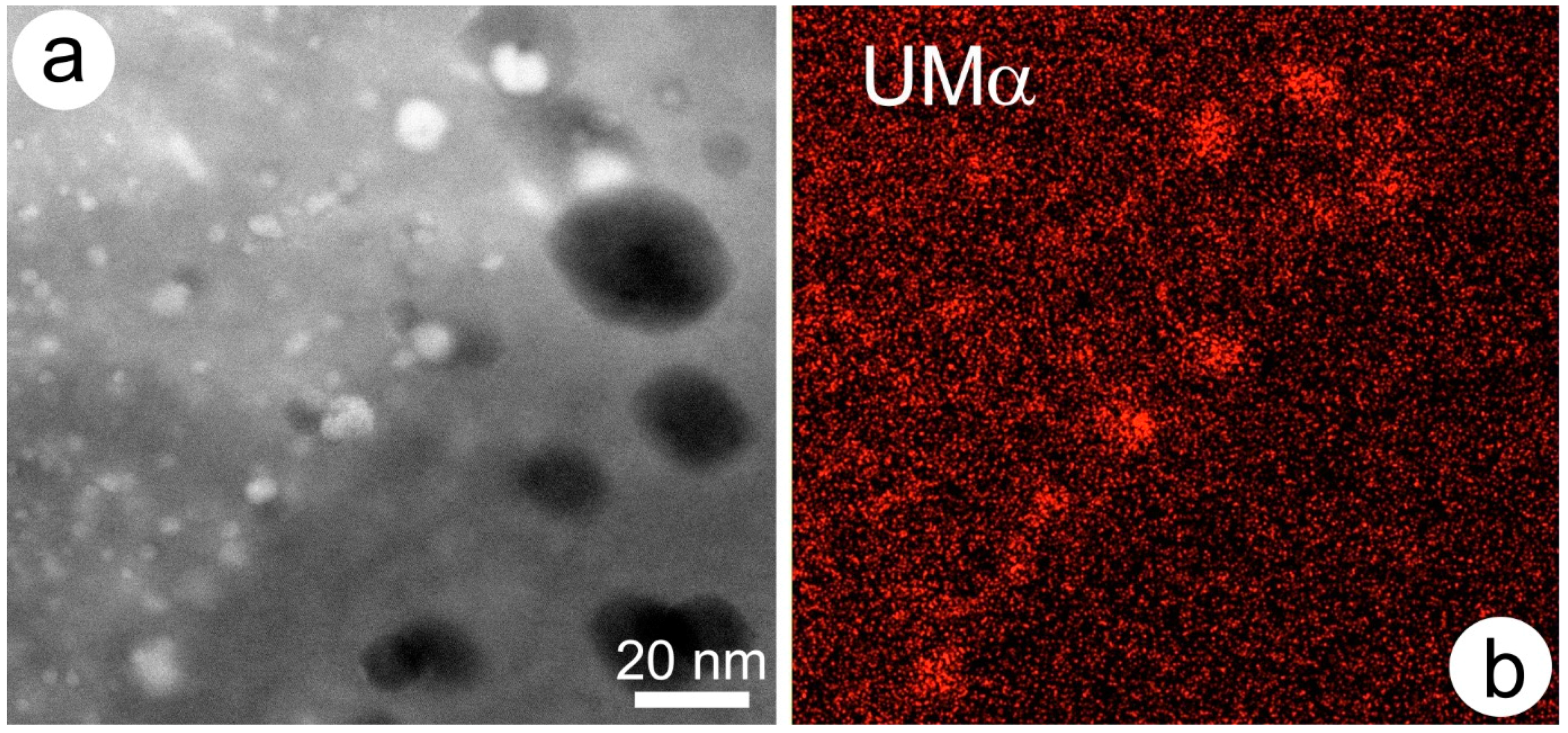
2.1.7. Nanoscale Secondary Ion Mass Spectrometry (nanoSIMS)
2.1.8. Atom Probe Tomography (APT)
2.1.9. Geochronology Using Ore Minerals
2.1.10. Non-Traditional Stable Isotopes
2.2. New Approaches and Sub-Disciplines
2.2.1. Trace Elements in Ore Minerals
2.2.2. The Significance of Gangue Minerals—A Holistic Approach to Ore Deposits
2.2.3. Solid Solutions and Nanoparticles
2.2.4. New Sub-Disciplines
2.3. Discovery of New Minerals
3. Challenges—A Short Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ramdohr, P. The Ore Minerals and Their Intergrowths, English translation of the 3rd ed.; Pergamon Press: Oxford, UK, 1969; p. 1174. [Google Scholar]
- Picot, P.; Johan, Z.; Watkinson, D.H. Atlas of Ore Minerals; Elsevier: Amsterdam, The Netherlands, 1982. [Google Scholar]
- Craig, J.R.; Vaughan, D.J. Ore Microscopy and Ore Petrography, 2nd ed.; Wiley-Interscience: New York, NY, USA, 1994; p. 368. [Google Scholar]
- Vaughan, D.J.; Corkhill, C.L. Mineralogy of sulfides. Elements 2017, 13, 81–87. [Google Scholar] [CrossRef]
- McCuaig, T.C.; Vann, J.E.; Sykes, J.P. Mines versus Mineralisation—Deposit Quality, Mineral Exploration Strategy and the Role of ‘Boundary Spanners’. In Proceedings of the 9th International Mining Geology Conference, Adelaide, Australia, 18–20 August 2014; pp. 33–41. [Google Scholar]
- Gunn, G. (Ed.) Critical Metals Handbook; American Geophysical Union: Washington, DC, USA, 2014; 454p. [Google Scholar]
- Roedder, E. Fluid inclusions. Rev. Mineral. 1984, 12, 646. [Google Scholar]
- Ohmoto, H. Stable isotope geochemistry of ore deposits. Rev. Mineral. 1986, 16, 491–559. [Google Scholar]
- Sylvester, P. Laser Ablation ICP-MS in the Earth Sciences: Current Practices and Outstanding Issues; Mineralogical Association of Canada: Québec, QC, Canada, 2008; Volume 40. [Google Scholar]
- Sylvester, P.J.; Jackson, S.E. A Brief History of Laser Ablation Inductively Coupled Plasma Mass Spectrometry (LA–ICP–MS). Elements 2016, 12, 307–310. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Horstwood, M.S.A.; Cottle, J.M. Advances in Isotope Ratio Determination by LA–ICP–MS. Elements 2016, 12, 317–322. [Google Scholar] [CrossRef]
- Wagner, T.; Fusswinkel, T.; Wälle, M.; Heinrich, C.A. Microanalysis of Fluid Inclusions in Crustal Hydrothermal Systems using Laser Ablation Methods. Elements 2016, 12, 323–328. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; George, L.; Zhu, Z.-Y.; Wade, B.; Ehrig, K. Trace Element Analysis of Minerals in Magmatic-Hydrothermal Ores by Laser Ablation Inductively-Coupled Plasma Mass Spectrometry: Approaches and Opportunities. Minerals 2016, 6, 111. [Google Scholar] [CrossRef]
- Woodhead, J.; Hergt, J.; Meffre, S.; Large, R.R.; Danyushevsky, L.; Gilbert, S. In situ Pb-isotope analysis of pyrite by laser ablation (multi-collector and quadrupole) ICPMS. Chem. Geol. 2009, 262, 344–354. [Google Scholar] [CrossRef]
- Darling, J.R.; Storey, C.D.; Hawkesworth, C.J.; Lightfoot, P.C. In-situ Pb isotope analysis of Fe–Ni–Cu sulphides by laser ablation multi-collector ICPMS: New insights into ore formation in the Sudbury impact melt sheet. Geochim. Cosmochim. Acta 2012, 99, 1–17. [Google Scholar] [CrossRef]
- Gilbert, S.E.; Danyushevsky, L.V.; Rodemann, T.; Shimizu, N.; Gurenko, A.; Meffre, S.; Thomas, H.; Large, R.R.; Death, D. Optimisation of laser parameters for the analysis of sulphur isotopes in sulphide minerals by laser ablation ICP-MS. J. Anal. At. Spectrom. 2014, 29, 1042–1051. [Google Scholar] [CrossRef]
- McFarlane, C.R.M.; Soltani Dehnavi, A.; Lentz, D. Pb-Isotopic Study of Galena by LA-Q-ICP-MS: Testing a New Methodology with Applications to Base-Metal Sulphide Deposits. Minerals 2016, 6, 96. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Yang, T.; Ciobanu, C.L.; Cook, N.J.; Zhao, K.D.; Jiang, S.Y. Mapping of S isotopes and trace elements in sulfides by LA-(MC)-ICP-MS: Potential problems and implications. Minerals 2016, 6, 110. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Jiang, S.Y.; Yang, T.; Ciobanu, C.L.; Cook, N.J. Sulfur isotope fractionation in pyrite during laser ablation: Implications for laser ablation multiple collector inductively coupled plasma mass spectrometry mapping. Chem. Geol. 2017, 450, 223–234. [Google Scholar] [CrossRef]
- Heaney, P.J.; Vicenzi, E.P.; Giannuzzi, L.A.; Livi, K.J.T. Focused ion beam milling: A method of site-specific sample extraction for microanalysis of Earth and planetary materials. Am. Mineral. 2001, 86, 1094–1099. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 2009, 261, 217–229. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Pring, A.; Green, L. Focussed ion beam–transmission electron microscopy applications in ore mineralogy: Bridging micro- and nanoscale observations. Ore Geol. Rev. 2011, 42, 6–31. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Maunders, C.; Wade, B.P.; Ehrig, K. Focused Ion Beam and Advanced Electron Microscopy for Minerals: Insights and Outlook from Bismuth Sulphosalts. Minerals 2016, 6, 112. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Pring, A.; Cook, N.J.; Self, P.; Jefferson, D.; Dima, G.; Melnikov, V. Chemical-structural modularity in the tetradymite group: A HRTEM study. Am. Mineral. 2009, 94, 517–534. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Birch, W.D.; Cook, N.J.; Pring, A.; Grundler, P.V. Petrogenetic significance of Au-Bi-Te-S associations: The example of Maldon, Central Victorian gold province, Australia. Lithos 2010, 116, 1–17. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Utsunomiya, S.; Kogagwa, M.; Green, L.; Gilbert, S.; Wade, B. Gold-telluride nanoparticles revealed in arsenic-free pyrite. Am. Mineral. 2012, 97, 1515–1518. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Ore minerals down to the nanoscale: Cu-(Fe)-sulphides from the Iron Oxide Copper Gold deposit at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 81, 1218–1235. [Google Scholar] [CrossRef]
- Wirth, R.; Reid, D.; Schreiber, A. Nanometer-sized platinum-group minerals (PGM) in base metal sulfides: New evidence for an orthomagmatic origin of the Merensky Reef PGE ore deposit, bushveld complex, South Africa. Can. Mineral. 2013, 51, 143–155. [Google Scholar] [CrossRef]
- Junge, M.; Wirth, R.; Oberthür, T.; Melcher, F. Mineralogical siting of platinum-group elements in pentlandite from the Bushveld Complex, South Africa. Miner. Depos. 2015, 50, 41–54. [Google Scholar] [CrossRef]
- Harries, D.; Pollok, K.; Langenhorst, F. Translation interface modulation in NC-pyrrhotites: Direct imaging by TEM and a model toward understanding partially disordered structural states. Am. Mineral. 2011, 96, 716–731. [Google Scholar] [CrossRef]
- Harries, D.; Pollok, K.; Langenhorst, F. Oxidative dissolution of 4C- and NC-pyrrhotite: Intrinsic reactivity differences, pH dependence, and the effect of anisotropy. Geochim. Cosmochim. Acta 2013, 102, 23–44. [Google Scholar] [CrossRef]
- Reith, F.; Fairbrother, L.; Nolze, G.; Wilhelmi, O.; Clode, P.L.; Gregg, A.; Parsons, J.E.; Wakelin, S.A.; Pring, A.; Hough, R.; et al. Nanoparticle factories: Biofilms hold the key to gold dispersion and nugget formation. Geology 2010, 38, 843–846. [Google Scholar] [CrossRef]
- Shuster, J.; Reith, F.; Cornelis, G.; Parsons, J.E.; Parsons, J.M.; Southam, G. Secondary gold structures: Relics of past biogeochemical transformations and implications for colloidal gold dispersion in subtropical environments. Chem. Geol. 2017, 450, 154–164. [Google Scholar] [CrossRef]
- Liu, Y.; King, H.E.; van Huis, M.A.; Drury, M.R.; Plümper, O. Nano-tomography of porous geological materials using Focused Ion Beam-Scanning Electron Microscopy. Minerals 2016, 6, 104. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Apatite at Olympic Dam, South Australia: A petrogenetic tool. Lithos 2016, 262, 470–485. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Krneta, S.; Kamenetsky, V.S. Feldspar evolution in the Roxby Downs Granite, host to Fe-oxide Cu-Au-(U) mineralisation at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 80, 838–859. [Google Scholar] [CrossRef]
- Hraško, L.; Kotov, A.B.; Salinikova, E.B.; Kovach, V.P. Enclaves in the Rochovce granite intrusion as indicators of the temperature and origin of the magma. Geol. Carpathica 1998, 49, 125–138. [Google Scholar]
- Nieto, F.; Livi, K.J.T. (Eds.) Minerals at the Nanoscale; EMU Notes in Mineralogy 14; European Mineralogical Union: London, UK, 2013; p. 440. [Google Scholar]
- Cook, N.J.; Ciobanu, C.L.; Wagner, T.; Stanley, C.J. Minerals of the system Bi-Te-Se-S related to the tetradymite archetype: Review of classification and compositional variation. Can. Mineral. 2007, 45, 665–708. [Google Scholar] [CrossRef]
- Feutelais, Y.; Legendre, B.; Rodier, N.; Agafonov, V.A. Study of the phases in the bismuth—Tellurium system. Mater. Res. Bull. 1993, 28, 591–596. [Google Scholar] [CrossRef]
- Balassone, G.; Nieto, F.; Arfè, G.; Boni, M.; Mondillo, N. Zn-clay minerals in the Skorpion Zn nonsulfide deposit (Namibia): Identification and genetic clues revealed by HRTEM and AEM study. Appl. Clay Sci. 2017, 150, 309–322. [Google Scholar] [CrossRef]
- Mondillo, N.; Nieto, F.; Balassone, G. Micro- and nano-characterization of Zn-clays in nonsulfide supergene ores of southern Peru. Am. Mineral. 2015, 100, 2484–2496. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in mixed-layer compounds: A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Z.; Konishi, H. Si-magnetite nano-precipitates in silician magnetite from banded iron formation: Z-contrast imaging and ab initio study. Am. Mineral. 2014, 99, 2196–2202. [Google Scholar] [CrossRef]
- Utsunomiya, S.; Kogawa, M.; Kamiishi, E.; Ewing, R.C. Scanning Transmission Electron Microscopy and Related Techniques for Research on Actinide and Radionuclide Nanomaterials. In Actinide Nanoparticle Research; Kalmykov, S.N., Denecke, M.A., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 33–62. [Google Scholar]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Ismail, R.; Krneta, S.; Basak, A. Feldspar mineralogy and rare earth element (re)mobilization in iron-oxide copper gold systems from South Australia: A nanoscale study. Mineral. Mag. 2017, in press. [Google Scholar] [CrossRef]
- Barton, M.D. Iron oxide (-Cu-Au-REE-P-Ag-U-Co) systems. In Treatise on Geochemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 13, pp. 515–541. [Google Scholar]
- Apukhtina, O.B.; Kamenetsky, V.S.; Ehrig, K.; Kamenetsky, M.B.; Maas, R.; Thompson, J.; McPhie, J.; Ciobanu, C.L.; Cook, N.J. Early, deep magnetite-fluorapatite mineralization at the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Econ. Geol. 2017, 112, 1531–1542. [Google Scholar] [CrossRef]
- Knipping, J.L.; Bilenker, L.D.; Simon, A.C.; Reich, M.; Barra, F.; Deditius, A.P.; Lundstrom, C.; Bindeman, I.; Munizaga, R. Giant Kiruna-type deposits form by efficient flotation of magmatic magnetite suspensions. Geology 2015, 43, 591–594. [Google Scholar] [CrossRef]
- Prior, D.J.; Boyle, A.P.; Brenker, F.; Cheadle, M.C.; Day, A.; Lopez, G.; Potts, G.J.; Reddy, S.M.; Spiess, R.; Trimby, P.W.; et al. The application of electron backscatter diffraction and orientation contrast imaging in the SEM to textural problems in rocks. Am. Mineral. 1999, 84, 1741–1759. [Google Scholar] [CrossRef]
- Prior, D.J.; Mariani, E.; Wheeler, J. EBSD in the earth sciences: Applications, common practice, and challenges. In Electron Backscatter Diffraction in Materials Science; Springer: Berlin/Heidelberg, Germany, 2009; pp. 345–360. [Google Scholar]
- Wilkinson, A.J.; Britton, B. Strains, planes, and EBSD in materials science. Mater. Today 2012, 15, 366–376. [Google Scholar] [CrossRef]
- Boyle, A.P.; Prior, D.J.; Banham, M.H.; Timms, N.E. Plastic deformation of metamorphic pyrite: New evidence from electron-backscatter diffraction and forescatter orientation-contrast imaging. Miner. Depos. 1998, 34, 71–81. [Google Scholar] [CrossRef]
- Freitag, K.; Boyle, A.P.; Nelson, E.; Hitzman, M.; Churchill, J.; Lopez-Pedrosa, M. The use of electron backscatter diffraction and orientation contrast imaging as tools for sulphide textural studies: Example from the Greens Creek deposit (Alaska). Miner. Depos. 2004, 39, 103. [Google Scholar]
- Barrie, C.D.; Boyle, A.P.; Prior, D.J. An analysis of the microstructures developed in experimentally deformed polycrystalline pyrite and minor sulphide phases using electron backscatter diffraction. J. Struct. Geol. 2007, 29, 1494–1511. [Google Scholar] [CrossRef]
- Barrie, C.D.; Boyle, A.P.; Salter, M. How low can you go?—Extending downwards the limits of plastic deformation in pyrite. Mineral. Mag. 2009, 73, 895–913. [Google Scholar] [CrossRef]
- Barrie, C.D.; Boyle, A.P.; Cox, S.F.; Prior, D.J. Slip systems and critical resolved shear stress in pyrite: An electron backscatter diffraction (EBSD) investigation. Mineral. Mag. 2009, 72, 1181–1199. [Google Scholar] [CrossRef]
- Barrie, C.D.; Boyle, A.P.; Cook, N.J.; Prior, D.J. Pyrite deformation textures in the massive sulfide ore deposits of the Norwegian Caledonides. Tectonophysics 2010, 483, 269–286. [Google Scholar] [CrossRef]
- Barrie, C.D.; Cook, N.J.; Boyle, A.P. Textural variation in the pyrite-rich ore deposits of the Røros district, Trondheim Region, Norway: Implications for pyrite deformation mechanisms. Miner. Depos. 2010, 45, 51–68. [Google Scholar] [CrossRef]
- Barrie, C.D.; Pearce, M.A.; Boyle, A.P. Reconstructing the pyrite deformation mechanism map. Ore Geol. Rev. 2011, 39, 265–276. [Google Scholar] [CrossRef]
- Ohfuji, H.; Boyle, A.P.; Prior, D.J.; Rickard, D. Structure of framboidal pyrite: An electron backscatter diffraction study. Am. Mineral. 2005, 90, 1693–1704. [Google Scholar] [CrossRef]
- Barrie, C.D.; Boyce, A.J.; Boyle, A.P.; Williams, P.J.; Blake, K.; Ogawara, T.; Akai, J.; Prior, D.J. Growth controls in colloform pyrite. Am. Mineral. 2009, 94, 415–429. [Google Scholar] [CrossRef]
- Gao, S.; Huang, F.; Gu, X.; Chen, Z.; Xing, M.; Li, Y. Research on the growth orientation of pyrite grains in the colloform textures in Baiyunpu Pb–Zn polymetallic deposit, Hunan, China. Mineral. Petrol. 2017, 111, 69–79. [Google Scholar] [CrossRef]
- Reddy, S.M.; Hough, R.M. Microstructural evolution and trace element mobility in Witwatersrand pyrite. Contrib. Mineral. Petrol. 2013, 166, 1269–1284. [Google Scholar] [CrossRef]
- Feinberg, J.M.; Wenk, H.-R.; Renne, P.R.; Scott, G.R. Epitaxial relationships of clinopyroxene-hosted magnetite determined using electron backscatter diffraction (EBSD) technique. Am. Mineral. 2004, 89, 462–466. [Google Scholar] [CrossRef]
- Mamtani, M.A.; Piazolo, S.; Greiling, R.O.; Kontny, A.; Hrouda, F. Process of magnetite fabric development during granite deformation. Earth Plan. Sci. Lett. 2011, 308, 77–89. [Google Scholar] [CrossRef]
- Franke, C.; Pennock, G.M.; Drury, M.R.; Engelmann, R.; Lattard, D.; Garming, J.F.L.; von Dobeneck, T.; Dekkers, M.J. Identification of magnetic Fe-Ti oxides in marine sediments by electron backscatter diffraction in scanning electron microscopy. Geophys. J. Int. 2007, 170, 545–555. [Google Scholar] [CrossRef]
- Barbosa, P.F.; Lagoeiro, L. Crystallographic texture of the magnetite-hematite transformation: Evidence for topotactic relationships in natural samples from Quadrilátero Ferrífero, Brazil. Am. Mineral. 2010, 95, 118–125. [Google Scholar] [CrossRef]
- Rosière, C.A.; Garcia, O.L.; Siemes, H.; Schaeben, H. Domainal fabrics of hematite in schistose, shear zone-hosted high-grade Fe ores: The product of the interplay between deformation and mineralization. J. Struct. Geol. 2013, 55, 150–166. [Google Scholar] [CrossRef]
- Till, J.L.; Moskowitz, B.M. Deformation microstructures and magnetite texture development in synthetic shear zones. Tectonophysics 2014, 629, 211–223. [Google Scholar] [CrossRef]
- Vukmanovic, Z.; Reddy, S.M.; Godel, B.; Barnes, S.J.; Fiorentini, M.L.; Barnes, S.-J.; Kilburn, M.R. Relationship between microstructures and grain-scale trace element distribution in komatiite-hosted magmatic sulphide ores. Lithos 2014, 184–187, 42–61. [Google Scholar] [CrossRef]
- Huberty, J.M.; Kita, N.T.; Kozdon, R.; Heck, P.R.; Fournelle, J.H.; Spicuzza, M.J.; Xu, H.; Valley, J.W. Crystal orientation effects in δ18O for magnetite and hematite by SIMS. Chem. Geol. 2010, 276, 269–283. [Google Scholar] [CrossRef]
- Taylor, R.; Clark, C.; Reddy, S.M. The effect of grain orientation on secondary ion mass spectrometry (SIMS) analysis of rutile. Chem. Geol. 2012, 300–301, 81–87. [Google Scholar] [CrossRef]
- Carr, P.A.; Norman, M.D.; Bennett, V.C. Assessment of crystallographic orientation effects on secondary ion mass spectrometry (SIMS) analysis of cassiterite. Chem. Geol. 2017, 467, 122–133. [Google Scholar] [CrossRef]
- West, G.D.; Thompson, R.C. Combined EBSD/EDS tomography in a dual-beam FIB/FEG–SEM. J. Microsc. 2009, 233, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Chemical zoning and lattice distortion: Uraninite from Olympic Dam, South Australia. Am. Mineral. 2016, 101, 2351–2354. [Google Scholar] [CrossRef]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Replacement of uraninite by bornite via coupled dissolution-reprecipitation: Evidence from texture and microstructure. Can. Mineral. 2016, 54, 1369–1383. [Google Scholar] [CrossRef]
- Ryan, C.; Kirkham, R.; Hough, R.; Moorhead, G.; Siddons, D.; De Jonge, M.; Paterson, D.; De Geronimo, G.; Howard, D.; Cleverley, J. Elemental X-ray imaging using the Maia detector array: The benefits and challenges of large solid-angle. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 619, 37–43. [Google Scholar] [CrossRef]
- Ryan, C.G.; Siddons, D.P.; Kirkham, R.; Li, Z.Y.; de Jonge, M.D.; Paterson, D.; Cleverley, J.S.; Kuczewski, A.; Dunn, P.A.; Jensen, M.; et al. The Maia detector array and X-ray fluorescence imaging system: Locating rare precious metal phases in complex samples. In Proceedings of the SPIE 8851, X-ray Nanoimaging: X-ray Nanoimaging: Instruments and Methods, San Diego, CA, USA, 25–29 August 2013. [Google Scholar] [CrossRef]
- Ryan, C.J.; Ryan, C.; Siddons, D.; Kirkham, R.; Dunn, P.; Kuczewski, A.; Moorhead, G.; De Geronimo, G.; Paterson, D.; de Jonge, M.; et al. The New Maia Detector System: Methods for High Definition Trace Element Imaging Of Natural Material. In Proceedings of the International Conference on X-ray Optics and Microanalysis, Karlsruhe, Germany, 15–18 September 2009; Volume 1221, pp. 9–17. [Google Scholar]
- Ryan, C.; Siddons, D.; Kirkham, R.; Li, Z.; de Jonge, M.; Paterson, D.; Kuczewski, A.; Howard, D.; Dunn, P.; Falkenberg, G. MAIA X-ray fluorescence imaging: Capturing detail in complex natural samples. J. Phys. Conf. Ser. 2014, 499, 12002. [Google Scholar] [CrossRef]
- Barnes, S.; Godel, B.; Locmelis, M.; Fiorentini, M.; Ryan, C. Extremely Ni-rich Fe-Ni sulfide assemblages in komatiitic dunite at Betheno, Western Australia: Results from synchrotron X-ray fluorescence mapping. Aust. J. Earth Sci. 2011, 58, 691–709. [Google Scholar] [CrossRef]
- Fisher, L.; Fougerouse, D.; Cleverley, J.; Ryan, C.; Micklethwaite, S.; Halfpenny, A.; Hough, R.; Gee, M.; Paterson, D.; Howard, D.; et al. Quantified, multi-scale X-ray fluorescence element mapping using the Maia detector array: Application to mineral deposit studies. Miner. Depos. 2014, 50, 665–674. [Google Scholar] [CrossRef]
- Li, K.; Etschmann, B.; Rae, N.; Reith, F.; Ryan, C.G.; Kirkham, R.; Howard, D.; Rosa, D.R.N.; Zammit, C.; Pring, A.; et al. Ore Petrography Using Megapixel X-Ray Imaging: Rapid Insights into Element Distribution and Mobilization in Complex Pt and U-Ge-Cu Ores. Econ. Geol. 2016, 111, 487–501. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.J.; de Jonge, M.; Ryan, C.G.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via μ-XANES spectroscopy. Am. Mineral. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Cook, N.J.; Etschmann, B.; Ciobanu, C.L.; Geraki, K.; Howard, D.L.; Williams, T.; Rae, N.; Pring, A.; Chen, G.; Johannessen, B.; et al. Distribution and substitution mechanism of Ge in a Ge-(Fe)-bearing sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef]
- Bonnet, J.; Cauzid, J.; Testemale, D.; Kieffer, I.; Proux, O.; Lecomte, A.; Bailly, L. Characterization of germanium speciation in sphalerite (ZnS) from Central and Eastern Tennessee, USA, by X-ray absorption spectroscopy. Minerals 2017, 7, 79. [Google Scholar] [CrossRef]
- Frandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J.; Butcher, A.R. The use of QEMSCAN and diagnostic leaching in the characterisation of visible gold. Miner. Eng. 2005, 18, 877–886. [Google Scholar] [CrossRef]
- Goodall, W.R.; Scales, P.J. An overview of the advantages and disadvantages of the determination of gold mineralogy by automated mineralogy. Miner. Eng. 2007, 20, 506–517. [Google Scholar] [CrossRef]
- Gu, Y.; Schouwstra, R.; Rule, C. The value of automated mineralogy. Miner. Eng. 2007, 58, 100–103. [Google Scholar] [CrossRef]
- Ehrig, K.; McPhie, J.; Kamenetsky, V.S. Geology and mineralogical zonation of the Olympic Dam iron oxide Cu-U-Au-Ag deposit, South Australia. In Geology and Genesis of Major Copper Deposits and Districts of the World, a Tribute to Richard Sillitoe; Hedenquist, J.W., Harris, M., Camus, F., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2012; Volume 16, pp. 237–268. [Google Scholar]
- Bachmann, K.; Frenzel, M.; Krause, J.; Gutzmer, J. Advanced identification and quantification of In-bearing minerals by scanning electron microscope-based image analysis. Microsc. Microanal. 2017, 23, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Kilburn, M.R.; Wacey, D. Nanoscale secondary ion mass spectrometry (NanoSIMS) as an analytical tool in the geosciences. In Principles and Practice of Analytical Techniques in Geosciences; Royal Society of Chemistry: London, UK, 2015; pp. 1–34. [Google Scholar]
- Barker, S.L.L.; Hickey, K.A.; Cline, J.S.; Dipple, G.M.; Kilburn, M.; Vaughan, J.R.; Longo, A.A. Uncloaking invisible gold: Use of nanosims to evaluate gold, trace elements, and sulfur isotopes in pyrite from Carlin-type gold deposits. Econ. Geol. 2009, 104, 897–904. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Halfpenny, A.; Reddy, S.M.; Cliff, J.B.; Martin, L.A.J.; Kilburn, M.; Guagliardo, P.; Ulrich, S. The golden ark: Arsenopyrite crystal plasticity and the retention of gold through high strain and metamorphism. Terra Nova 2016, 28, 181–187. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Tomkins, A.G.; Mei, Y.; Kilburn, M.; Guagliardo, P.; Fisher, L.A.; Halfpenny, A.; Gee, M.; Paterson, D.; et al. Gold remobilisation and formation of high grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochim. Cosmochim. Acta 2016, 178, 143–159. [Google Scholar] [CrossRef]
- Wacey, D.; Kilburn, M.R.; Saunders, M.; Cliff, J.B.; Kong, C.; Liu, A.G.S.C.; Matthews, J.J.; Brasier, M.D. Uncovering framboidal pyrite biogenicity using nano-scale CNorg mapping. Geology 2015, 43, 27–30. [Google Scholar] [CrossRef]
- Rollog, M.; Cook, N.J.; Guagliardo, P.; Kilburn, M.; Ehrig, K.; Ciobanu, C.L. NanoSIMS mapping of 210RN and 226Ra in South Australian copper concentrates. Abstract of Goldschmidt 2017, Paris, France, 13–18 August 2017. [Google Scholar]
- Didier, A.; Bosse, V.; Bouloton, J.; Mostefaoui, S.; Viala, M.; Paquette, J.L.; Devidal, J.L.; Duhamel, R. NanoSIMS mapping and LA-ICP-MS chemical and U-Th-Pb data in monazite from a xenolith enclosed in andesite (Central Slovakia Volcanic Field). Contrib. Mineral. Petrol. 2016, 170, 45. [Google Scholar] [CrossRef]
- Wang, W.; Lin, Y.; Hao, J.; Zhang, J.; Hu, S.; Ni, H. Phosphorus-controlled trace element distribution in zircon revealed by NanoSIMS. Contrib. Mineral. Petrol. 2016, 171, 28. [Google Scholar] [CrossRef]
- Kelly, T.F.; Larson, D.J. Atom Probe Tomography 2012. Ann. Rev. Mater. Res. 2012, 42, 1–31. [Google Scholar] [CrossRef]
- Valley, J.W.; Reinhard, D.A.; Cavosie, A.J.; Ushikubo, T.; Lawrence, D.F.; Larson, D.J.; Kelly, T.F.; Snoeyenbos, D.R.; Strickland, A. Nano-and micro-geochronology in Hadean and Archean zircons by atom-probe tomography and SIMS: New tools for old minerals. Am. Mineral. 2015, 100, 1355–1377. [Google Scholar] [CrossRef]
- Peterman, E.M.; Reddy, S.M.; Saxey, D.W.; Snoeyenbos, D.R.; Rickard, W.D.A.; Fougerouse, D.; Kylander-Clark, A.R.C. Nanogeochronology of discordant zircon measured by atom probe microscopy of Pb-enriched dislocation loops. Sci. Adv. 2016, 2, e1601318. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.M.; van Riessen, A.; Saxey, D.W.; Johnson, T.E.; Rickard, W.D.A.; Fougerouse, D.; Fischer, S.; Prosa, T.J.; Rice, K.P.; Reinhard, D.A.; et al. Mechanisms of deformation-induced trace element migration in zircon resolved by atom probe and correlative microscopy. Geochim. Cosmochim. Acta 2016, 195, 158–170. [Google Scholar] [CrossRef]
- Piazolo, S.; Belousova, E.; La Fontaine, A.; Corcoran, C.; Cairney, J.M. Trace element homogeneity from micron- to atomic scale: Implication for the suitability of the zircon GJ-1 as a trace element reference material. Chem. Geol. 2017, 456, 10–18. [Google Scholar] [CrossRef]
- Weber, J.; Barthel, J.; Brandt, F.; Klinkenberg, M.; Breuer, U.; Kruth, M.; Bosbach, D. Nano-structural features of barite crystals observed by electron microscopy and atom probe tomography. Chem. Geol. 2016, 424, 51–59. [Google Scholar] [CrossRef]
- Parman, S.W.; Diercks, D.R.; Gorman, B.P.; Cooper, R.F. Atom probe tomography of isoferroplatinum. Am. Mineral. 2015, 100, 852–860. [Google Scholar] [CrossRef]
- Parman, S.W. Highlights and Breakthroughs. Going small: Nanoscale geochronology using atom probe tomography. Am. Mineral. 2015, 100, 1333–1334. [Google Scholar] [CrossRef]
- Stein, H.J.; Markey, R.J.; Morgan, J.W.; Hannah, J.L.; Schersten, A. The remarkable Re-Os chronometer in molybdenite: How and why it works. Terra Nova 2001, 13, 479–486. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Wade, B.; Cook, N.J.; Schmidt Mumm, A.; Giles, D. Uranium-bearing hematite from the Olympic Dam Cu-U-Au deposit, South Australia; a geochemical tracer and reconnaissance Pb-Pb geochronometer. Precambr. Res. 2013, 238, 129–147. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Zhu, Z.; Ciobanu, C.L.; Wade, B.P.; Cook, N.J.; Ehrig, K.; Cabral, A.R.; Kennedy, A. Matrix-matched iron-oxide laser ablation ICP-MS U-Pb geochronology using mixed solutions standards. Minerals 2016, 6, 85. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Ciobanu, C.L.; Tapster, S.; Cook, N.J.; Kennedy, A.; Ehrig, K.; Condon, D.; Wade, B.P.; Gilbert, S.; Verdugo-Ihl, M.; et al. ID-TIMD U-Pb dating of oscillatory zoned U-bearing hematite from the Olympic Dam deposit (South Australia): A glimpse at the mineralization timeframe. Geology 2017. in review. [Google Scholar]
- Chew, D.M.; Petrus, J.A.; Kenny, G.G.; McEvoy, N. Rapid high-resolution U–Pb LA-Q-ICPMS age mapping of zircon. J. Anal. At. Spectrom. 2017, 32, 262–276. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Ciobanu, C.L.; Cook, N.J.; Verdugo-Ihl, M.; Tapster, S.R.; Condon, D.J.; Kennedy, A.K.; Ehrig, K.; Wade, B.P.; Gilbert, S.E. Oscillatory-zoned hematite: A reliable U-Pb mineral geochronometer. Abstract of Goldschmidt 2017, Paris, France, 13–18 August 2017. [Google Scholar]
- LaFlamme, C.; Martin, L.; Jeon, H.; Reddy, S.M.; Selvaraja, V.; Caruso, S.; Bui, T.H.; Roberts, M.P.; Voute, F.; Hagemann, S.; et al. In situ multiple sulfur isotope analysis by SIMS of pyrite, chalcopyrite, pyrrhotite, and pentlandite to refine magmatic ore genetic models. Chem. Geol. 2016, 444, 1–15. [Google Scholar] [CrossRef]
- Dauphas, N.; John, S.G.; Rouxel, O. Iron isotope systematics. Rev. Mineral. Geochem. 2017, 82, 415–510. [Google Scholar] [CrossRef]
- Rouxel, O.; Shanks, W.C.; Bach, W.; Edwards, K.J. Integrated Fe- and S-isotope study of seafloor hydrothermal vents at East Pacific Rise 9–10° N. Chem. Geol. 2008, 252, 214–227. [Google Scholar] [CrossRef]
- Hofmann, A.; Bekker, A.; Rouxel, O.; Rumble, D.; Master, S. Multiple sulphur and iron isotope composition of detrital pyrite in Archaean sedimentary rocks: A new tool for provenance analysis. Earth Plan. Sci. Lett. 2009, 286, 436–445. [Google Scholar] [CrossRef]
- Syverson, D.D.; Pester, N.J.; Craddock, P.R.; Seyfried, W.E. Fe isotope fractionation during phase separation in the NaCl–H2O system: An experimental study with implications for seafloor hydrothermal vents. Earth Plan. Sci. Lett. 2014, 406, 223–232. [Google Scholar] [CrossRef]
- Nie, N.X.; Dauphas, N.; Greenwood, R.C. Iron and oxygen isotope fractionation during iron UV photo-oxidation: Implications for early earth and mars. Earth Plan. Sci. Lett. 2017, 458, 179–191. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Jiang, S.-Y.; Mathur, R.; Cook, N.J.; Yang, T.; Wang, M.; Ma, L.; Ciobanu, C.L. Iron isotope behavior during fluid/rock interaction in K-feldspar alteration zone—A model for pyrite in gold deposits from the Jiaodong Peninsula, East China. Geochim. Cosmochim. Acta 2018, 222, 94–116. [Google Scholar] [CrossRef]
- Elliott, T.; Steele, R.C.J. The Isotope Geochemistry of Ni. Rev. Mineral. Geochem. 2017, 82, 511–542. [Google Scholar] [CrossRef]
- Hofmann, A.; Bekker, A.; Dirks, P.; Gueguen, B.; Rumble, D.; Rouxel, O.J. Comparing orthomagmatic and hydrothermal mineralization models for komatiite-hosted nickel deposits in Zimbabwe using multiple-sulfur iron and nickel isotope data. Miner. Depos. 2014, 49, 75–100. [Google Scholar] [CrossRef]
- Ratié, G.; Quantin, C.; Jouvin, D.; Calmels, D.; Ettler, V.; Sivry, Y.; Cruz Viera, L.; Ponzevera, E.; Garnier, J. Nickel isotope fractionation during laterite Ni ore smelting and refining: Implications for tracing the sources of Ni in smelter-affected soils. Appl. Geochem. 2016, 64, 136–145. [Google Scholar] [CrossRef]
- Moynier, F.; Vance, D.; Fujii, T.; Savage, P. The Isotope Geochemistry of Zinc and Copper. Rev. Mineral. Geochem. 2017, 82, 543–600. [Google Scholar] [CrossRef]
- Larson, P.B.; Maher, K.; Ramos, F.C.; Chang, Z.; Gaspar, M.; Meinert, L.D. Copper isotope ratios in magmatic and hydrothermal ore forming environments. Chem. Geol. 2003, 201, 337–350. [Google Scholar] [CrossRef]
- Mason, T.F.D.; Weiss, D.J.; Chapman, J.B.; Wilkinson, J.J.; Tessalina, S.G.; Spiro, B.; Horstwood, M.S.A.; Spratt, J.; Coles, B.J. Zn and Cu isotopic variability in the Alexandrinka volcanic-hosted massive sulphide (VHMS) ore deposit, Urals, Russia. Chem. Geol. 2005, 221, 170–187. [Google Scholar] [CrossRef]
- Markl, G.; Lahaye, Y.; Schwinn, G. Copper isotopes as monitors of redox processes in hydrothermal mineralization. Geochim. Cosmochim. Acta 2006, 70, 4215–4228. [Google Scholar] [CrossRef]
- Mathur, R.; Titley, S.; Barra, F.; Brantley, S.; Wilson, M.; Phillips, A.; Munizaga, F.; Maksaev, V.; Vervoort, J.; Hart, G. Exploration potential of Cu isotope fractionation in porphyry copper deposits. J. Geochem. Explor. 2009, 102, 1–6. [Google Scholar] [CrossRef]
- Mathur, R.; Dendas, M.; Titley, S.; Phillips, A. Patterns in the copper isotope composition of minerals in porphyry copper deposits in southwestern United States. Econ. Geol. 2010, 105, 1457–1467. [Google Scholar] [CrossRef]
- Wilkinson, J.J.; Weiss, D.J.; Mason, T.F.D.; Coles, B.J. Zinc isotope variation in hydrothermal systems: Preliminary evidence from the Irish Midlands ore field. Econ. Geol. 2005, 110, 583–590. [Google Scholar] [CrossRef]
- Gagnevin, D.; Boyce, A.J.; Barrie, C.D.; Menuge, J.F.; Blakemann, R.J. Zn, Fe and S isotope fractionation in a large hydrothermal system. Geochim. Cosmochim. Acta 2012, 88, 183–198. [Google Scholar] [CrossRef]
- Rouxel, O.; Luais, B. Germanium Isotope Geochemistry. Rev. Mineral. Geochem. 2017, 82, 601–656. [Google Scholar] [CrossRef]
- Escoube, R.; Rouxel, O.J.; Edwards, K.; Glazer, B.; Donard, O. Coupled Ge/Si and Ge isotope ratios as geochemical tracers of seafloor hydrothermal systems: Case studies at Loihi Seamount and East Pacific Rise 9°50′ N. Geochim. Cosmochim. Acta 2015, 167, 93–112. [Google Scholar] [CrossRef]
- Johnson, T.M. A review of mass-dependent fractionation of selenium isotopes and implications for other heavy stable isotopes. Chem. Geol. 2004, 204, 201–214. [Google Scholar] [CrossRef]
- Layton-Matthews, D.; Leybourne, M.I.; Peter, J.M.; Scott, S.D.; Cousens, B.; Eglington, B.M. Multiple sources of selenium in ancient seafloor hydrothermal systems: Compositional Se, S and Pb-isotopic evidence from volcanic-hosted and volcanic-sediment-hosted massive sulphide deposits of the Finlayson Lake District, Yukon, Canada. Geochim. Cosmochim. Acta 2013, 117, 318–331. [Google Scholar] [CrossRef]
- Kendall, B.; Dahl, T.W.; Anbar, A.D. Good Golly, Why Moly? The Stable isotope geochemistry of molybdenum. Rev. Mineral. Geochem. 2017, 82, 683–732. [Google Scholar] [CrossRef]
- Hannah, J.L.; Stein, H.J.; Wieser, M.E.; de Laeter, J.R.; Varner, M.D. Molybdenum isotope variations in molybdenite: Vapor transport and Rayleigh fractionation of Mo. Geology 2007, 35, 703–706. [Google Scholar] [CrossRef]
- Mathur, R.; Brantley, S.; Anbar, A.; Munizaga, F.; Maksaev, V.; Newburry, R.; Vervoort, J.; Hart, G. Variation of Mo isotopes from molybdenite in high-temperature hydrothermal ore deposits. Miner. Depos. 2010, 45, 43–50. [Google Scholar] [CrossRef]
- Breillat, N.; Guerrot, C.; Marcoux, E.; Négrel, P. A new global database of δ98Mo in molybdenites: A literature review and new data. J. Geochem. Explor. 2016, 161, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Fornadel, A.P.; Spry, P.G.; Haghnegahdar, M.A.; Schauble, E.A.; Jackson, S.E.; Mills, S.J. Stable Te isotope fractionation in tellurium-bearing minerals from precious metal hydrothermal ore deposits. Geochim. Cosmochim. Acta 2017, 202, 215–230. [Google Scholar] [CrossRef]
- Blum, J.D.; Johnson, M.W. Recent Developments in Mercury Stable Isotope Analysis. Rev. Mineral. Geochem. 2017, 82, 733–757. [Google Scholar] [CrossRef]
- Smith, C.N.; Kesler, S.E.; Klaue, B.; Blum, J.D. Mercury isotope fractionation in fossil hydrothermal systems. Geology 2005, 33, 825–828. [Google Scholar] [CrossRef]
- Smith, C.N.; Kesler, S.E.; Blum, J.D.; Rytuba, J.J. Isotope geochemistry of mercury in source rocks, mineral deposits and spring deposits of the California Coast Ranges, USA. Earth Plan. Sci. Lett. 2008, 269, 399–407. [Google Scholar] [CrossRef]
- Sonke, J.E.; Schäfer, J.; Chmeleff, J.; Audry, S.; Blanc, G.; Dupré, B. Sedimentary mercury stable isotope records of atmospheric and riverine pollution from two major European heavy metal refineries. Chem. Geol. 2010, 279, 90–100. [Google Scholar] [CrossRef]
- Blum, J.D.; Sherman, L.S.; Johnson, M.W. Mercury isotopes in earth and environmental sciences. Ann. Rev. Earth Plan. Sci. 2014, 42, 249–269. [Google Scholar] [CrossRef]
- Yin, R.; Feng, X.; Hurley, J.P.; Krabbenhoft, D.P.; Lepak, R.F.; Hu, R.; Zhang, Q.; Li, Z.; Bi, X. Mercury isotopes as proxies to identify sources and environmental impacts of mercury in sphalerites. Sci. Rep. 2016, 6, 18686. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.G.; Rehkämper, M.; Prytulak, J. Investigation and application of thallium isotope fractionation. Rev. Mineral. Geochem. 2017, 82, 759–798. [Google Scholar] [CrossRef]
- Baker, G.G.A.; Rehkämper, M.; Ihlenfeld, C.; Oates, C.J.; Coggon, R. Thallium isotope variations in an ore-bearing continental igneous setting: Collahuasi Formation, northern Chile. Geochim. Cosmochim. Acta 2010, 74, 4405–4416. [Google Scholar] [CrossRef]
- Coggon, R.M.; Rehkämper, M.; Atteck, C.; Teagle, D.A.H.; Alt, J.C.; Cooper, M.J. Controls on thallium uptake during hydrothermal alteration of the upper ocean crust. Geochim. Cosmochim. Acta 2014, 144, 25–42. [Google Scholar] [CrossRef]
- Hettmann, K.; Marks, M.A.W.; Kreissig, K.; Zack, T.; Wenzel, T.; Rehkämper, M.; Jacob, D.E.; Markl, G. The geochemistry of Tl and its isotopes during magmatic and hydrothermal processes: The peralkaline Ilimaussaq complex, southwest Greenland. Chem. Geol. 2014, 366, 1–13. [Google Scholar] [CrossRef]
- Hettmann, K.; Kreissig, K.; Rehkämper, M.; Wenzel, T.; Mertz-Kraus, R.; Markl, G. Thallium geochemistry in the metamorphic Lengenbach sulfide deposit, Switzerland: Thallium-isotope fractionation in a sulfide melt. Am. Mineral. 2014, 99, 793–803. [Google Scholar] [CrossRef]
- Reich, M.; Large, R.; Deditius, A.P. New advances in trace element geochemistry of ore minerals and accessory phases. Ore Geol. Rev. 2017, 81, 1215–1217. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Williams, T. The mineralogy and mineral chemistry of indium in sulphide deposits and implications for mineral processing. Hydrometallurgy 2011, 108, 226–228. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Ismail, R.; Ciobanu, C.L.; Cook, N.J.; Teale, G.S.; Giles, D.; Schmidt Mumm, A.; Wade, B. Rare earths and other trace elements in minerals from skarn assemblages, Hillside iron oxide–copper–gold deposit, Yorke Peninsula, South Australia. Lithos 2014, 184–187, 456–477. [Google Scholar] [CrossRef]
- Xu, J.; Ciobanu, C.L.; Cook, N.J.; Zheng, Y.; Sun, X.; Wade, B.P. Skarn formation and trace elements in garnet and associated minerals from Zhibula copper deposit, Gangdese Belt, southern Tibet. Lithos 2016, 262, 213–231. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Rare earth element behaviour in apatite from the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 135. [Google Scholar] [CrossRef]
- Krneta, S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Kontonikas-Charos, A. The Wirrda Well and Acropolis prospects Gawler Craton, South Australia: Insights into evolving fluid conditions through apatite chemistry. J. Geochem. Explor. 2017, 181, 276–291. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare earth element fluorocarbonate minerals from the Olympic Dam Cu-U-Au-Ag deposit, South Australia. Minerals 2017, 7, 202. [Google Scholar] [CrossRef]
- Verdugo-Ihl, M.R.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L.; Gilbert, S. Textures and U-W-Sn-Mo signatures in hematite from the Cu-U-Au-Ag orebody at Olympic Dam, South Australia: Defining the archetype for IOCG deposits. Ore Geol. Rev. 2017, in press. [Google Scholar] [CrossRef]
- George, L.; Cook, N.J.; Ciobanu, C.L.; Wade, B.P. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Partitioning of trace elements in co-crystallized sphalerite–galena–chalcopyrite hydrothermal ores. Ore Geol. Rev. 2016, 77, 97–116. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Crowe, B.B.P.; Ciobanu, C.L. Trace elements in hydrothermal chalcopyrite. Mineral. Mag. 2017, in press. [Google Scholar] [CrossRef]
- George, L.L.; Cook, N.J.; Ciobanu, C.L. Minor and trace elements in natural tetrahedrite-tennantite: Effects on element partitioning among base metal sulphides. Minerals 2017, 7, 17. [Google Scholar] [CrossRef]
- Gao, W.; Ciobanu, C.L.; Cook, N.J.; Huang, F.; Mang, L.; Gao, S. Petrography and trace element signatures in silicates and Fe-Ti-oxides from the Lanjiahuoshan deposit, Panzhihua layered intrusion, Southwest China. Lithos 2017, 294–295, 164–183. [Google Scholar] [CrossRef]
- Navrotsky, A. Nanoscale Effects on Thermodynamics and Phase Equilibria in Oxide Systems. ChemPhysChem 2011, 12, 2207–2215. [Google Scholar] [CrossRef] [PubMed]
- Navrotsky, A.; Mazeina, L.; Majzlan, J. Size-Driven Structural and Thermodynamic Complexity in Iron Oxides. Science 2008, 319, 1635–1638. [Google Scholar] [CrossRef] [PubMed]
- Putnis, A. Mineral replacement reactions: From macroscopic observations to microscopic mechanisms. Mineral. Mag. 2002, 66, 689–708. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Altree-Williams, A.; Pring, A.; Ngothai, Y.; Brugger, J. Textural and compositional complexities resulting from coupled dissolution-reprecipitation reactions in geomaterials. Earth-Sci. Rev. 2015, 150, 628–651. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Bowell, R.J.; Grogan, J.; Hutton-Ashkenny, M.; Brough, C.; Penman, K.; Sapsford, D.J. Geometallurgy of uranium deposits. Miner. Eng. 2011, 24, 1305–1313. [Google Scholar] [CrossRef]
- Hunt, J.; Berry, R.; Bradshaw, D. Characterising chalcopyrite liberation and flotation potential: Examples from an IOCG deposit. Miner. Eng. 2011, 24, 1271–1276. [Google Scholar] [CrossRef]
- Lotter, N.O.; Kormos, L.J.; Oliveira, J.; Fragomeni, D.; Whiteman, E. Modern Process Mineralogy: Two case studies. Miner. Eng. 2011, 24, 638–650. [Google Scholar] [CrossRef]
- Lund, C.; Lamberg, P. Geometallurgy—A tool for better resource efficiency. Eur. Geol. 2014, 37, 39–43. [Google Scholar]
- Pérez-Barnuevo, L.; Pirard, E.; Castroviejo, R. Automated characterisation of intergrowth textures in mineral particles. A case study. Miner. Eng. 2013, 52, 136–142. [Google Scholar] [CrossRef] [Green Version]
- Lund, C.; Lamberg, P.; Lindberg, T. Development of a geometallurgical framework to quantify mineral textures for process prediction. Miner. Eng. 2015, 82, 61–77. [Google Scholar] [CrossRef]
- Tungpalan, K.; Wightman, E.; Manlapig, E. Relating mineralogical and textural characteristics to flotation behaviour. Minerals Eng. 2015, 82, 136–140. [Google Scholar] [CrossRef]
- Christy, A.G. Causes of anomalous mineralogical diversity in the Periodic Table. Mineral. Mag. 2015, 79, 33–49. [Google Scholar] [CrossRef]
- Moëlo, Y.; Makovicky, E.; Mozgova, N.N.; Jambor, J.L.; Cook, N.J.; Pring, A.; Paar, W.; Nickel, E.H.; Graeser, G.; Karup-Møller, S.; et al. Sulfosalt systematics: A review. Report of the Sulfosalt Sub-committee of the IMA Commission on Ore Mineralogy. Eur. J. Mineral. 2008, 20, 7–46. [Google Scholar] [CrossRef]
- Hazen, R.M.; Hystad, G.; Downs, R.T.; Golden, J.; Pires, A.J.; Grew, E.S. Earth’s “missing” minerals. Am. Mineral. 2015, 100, 2344–2347. [Google Scholar] [CrossRef]
- Mindat.org. Most Prolific Type Localities. 2017. Available online: https://www.mindat.org/toptypelocs.php (accessed on 24 October 2017).
- Ciobanu, C.L.; Brugger, J.; Cook, N.J.; Mills, S.J.; Elliott, P.; Damian, G.; Damian, F. Graţianite, MnBi2S4, a new mineral from the Bǎiţa Bihor skarn, Romania. Am. Mineral. 2014, 99, 1163–1170. [Google Scholar] [CrossRef]
- Hazen, R.M.; Grew, E.S.; Origlieri, M.J.; Downs, R.T. On the mineralogy of the “Anthropocene Epoch”. Am. Mineral. 2017, 102, 595–611. [Google Scholar] [CrossRef]
- Schreyer, W. Memorial of Paul Ramdohr, January 1, 1890–March 8, 1985. Am. Mineral. 1986, 71, 839–840. [Google Scholar]

| Isotope System | Selected References | Key Ore-Related Questions Addressed |
|---|---|---|
| Fe | [117] and references therein, [118,119,120,121,122] | Origin of BIFs; metal-silicate, sulphide-melt, fluid-mineral and various mineral-mineral fractionations; δ56Fe as a tracer of ore-forming processes, including seafloor hydrothermal vents; discrimination of hydrothermal vs. magmatic processes; origin of detrital pyrite in Archaean sedimentary rocks |
| Ni | [123] and references therein, [124,125] | The origin of komatiite- and laterite-hosted nickel ores |
| Cu | [126] and references therein, [127,128,129,130,131] | Redox-induced fractionations wide large variability in supergene ores; large potential for application to complex deposits, e.g., IOCGs |
| Zn | [126] and references therein, [130,132,133] | Less data than for Cu-isotopes. Evidence for modest fractionation during evolution of hydrothermal fluids |
| Ge | [134] and references therein, [135] | Ge concentrated in sphalerite; potential for tracing processes in seafloor hydrothermal systems. |
| Se | [136,137] | Selenium source; distinguishing magmatic and hydrothermal input in ancient deposits |
| Mo | [138,139,140,141] | Significant application to ore-forming processes in deposits containing molybdenite; fractionation induced by redox reactions, fluid boiling. |
| Te | [142] | Ore-forming processes in Te-bearing epithermal and orogenic precious metal deposits |
| Hg | [143] and references therein, [144,145,146,147,148] | Relatively little used in ore geology to date but with potential application to hydrothermal systems. |
| Tl | [149] and references therein, [150,151,152,153] | Significant isotopic variability in natural samples points to the potential of Tl-isotopes for understanding formation of hydrothermal deposits; more baseline data are needed. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.; Verdugo-Ihl, M.R.; Courtney-Davies, L.; Gao, W. Advances and Opportunities in Ore Mineralogy. Minerals 2017, 7, 233. https://doi.org/10.3390/min7120233
Cook NJ, Ciobanu CL, Ehrig K, Slattery A, Verdugo-Ihl MR, Courtney-Davies L, Gao W. Advances and Opportunities in Ore Mineralogy. Minerals. 2017; 7(12):233. https://doi.org/10.3390/min7120233
Chicago/Turabian StyleCook, Nigel J., Cristiana L. Ciobanu, Kathy Ehrig, Ashley Slattery, Max R. Verdugo-Ihl, Liam Courtney-Davies, and Wenyuan Gao. 2017. "Advances and Opportunities in Ore Mineralogy" Minerals 7, no. 12: 233. https://doi.org/10.3390/min7120233





