Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide
Abstract
:1. Introduction
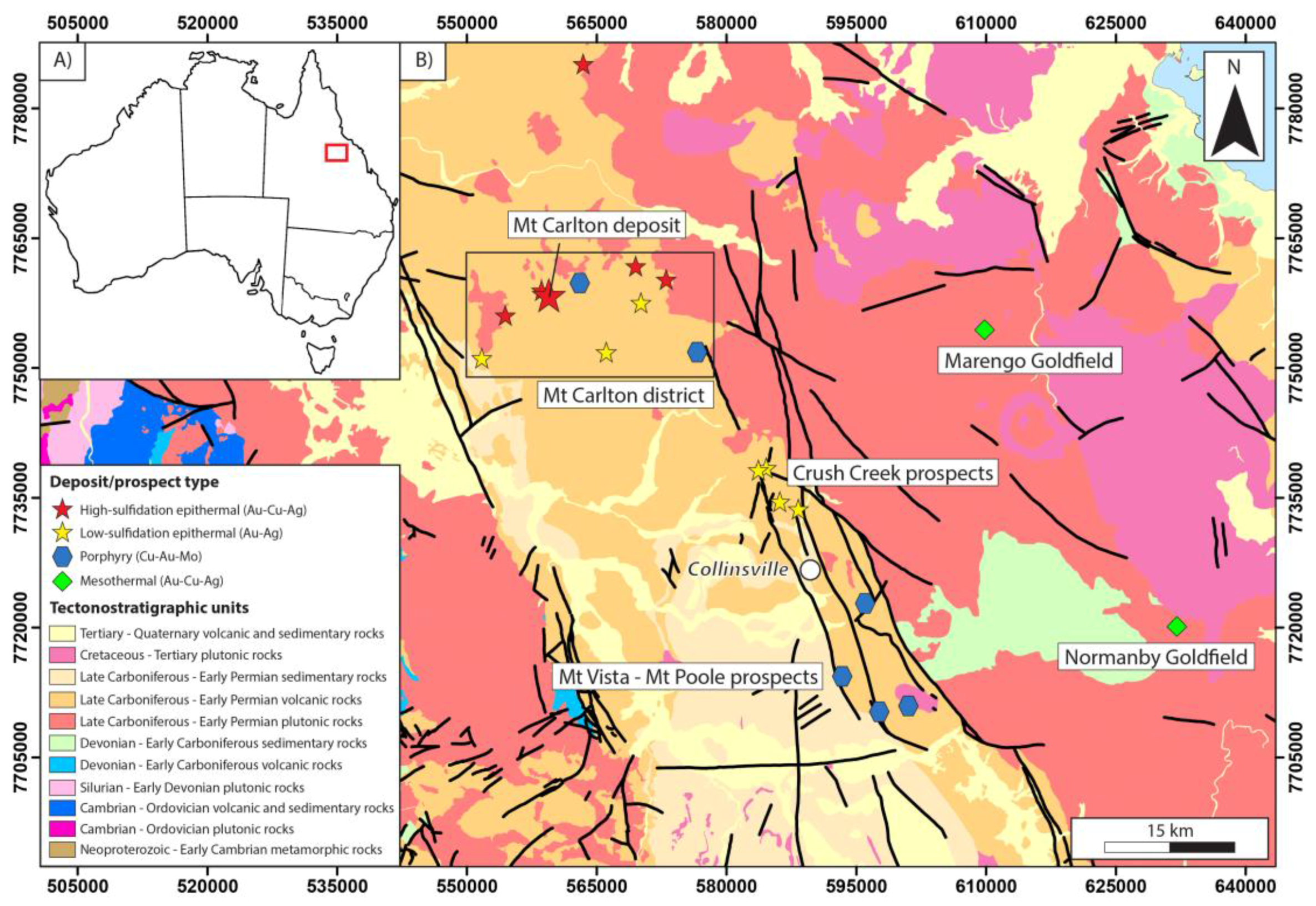
2. Geologic Setting of the Mt Carlton Deposit
2.1. Regional Geology
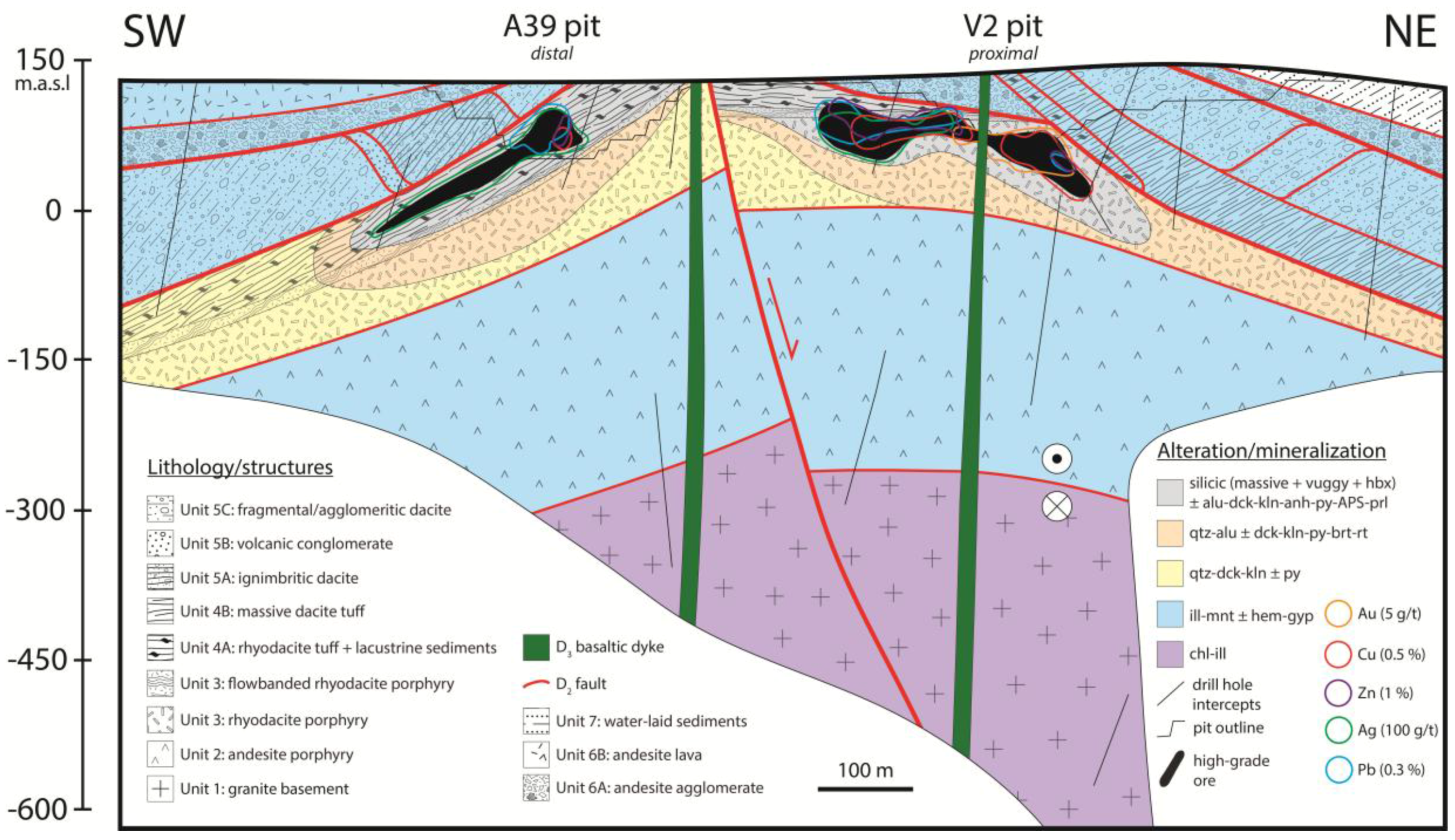
2.2. Stratigraphy and Deformation Sequence at Mt Carlton
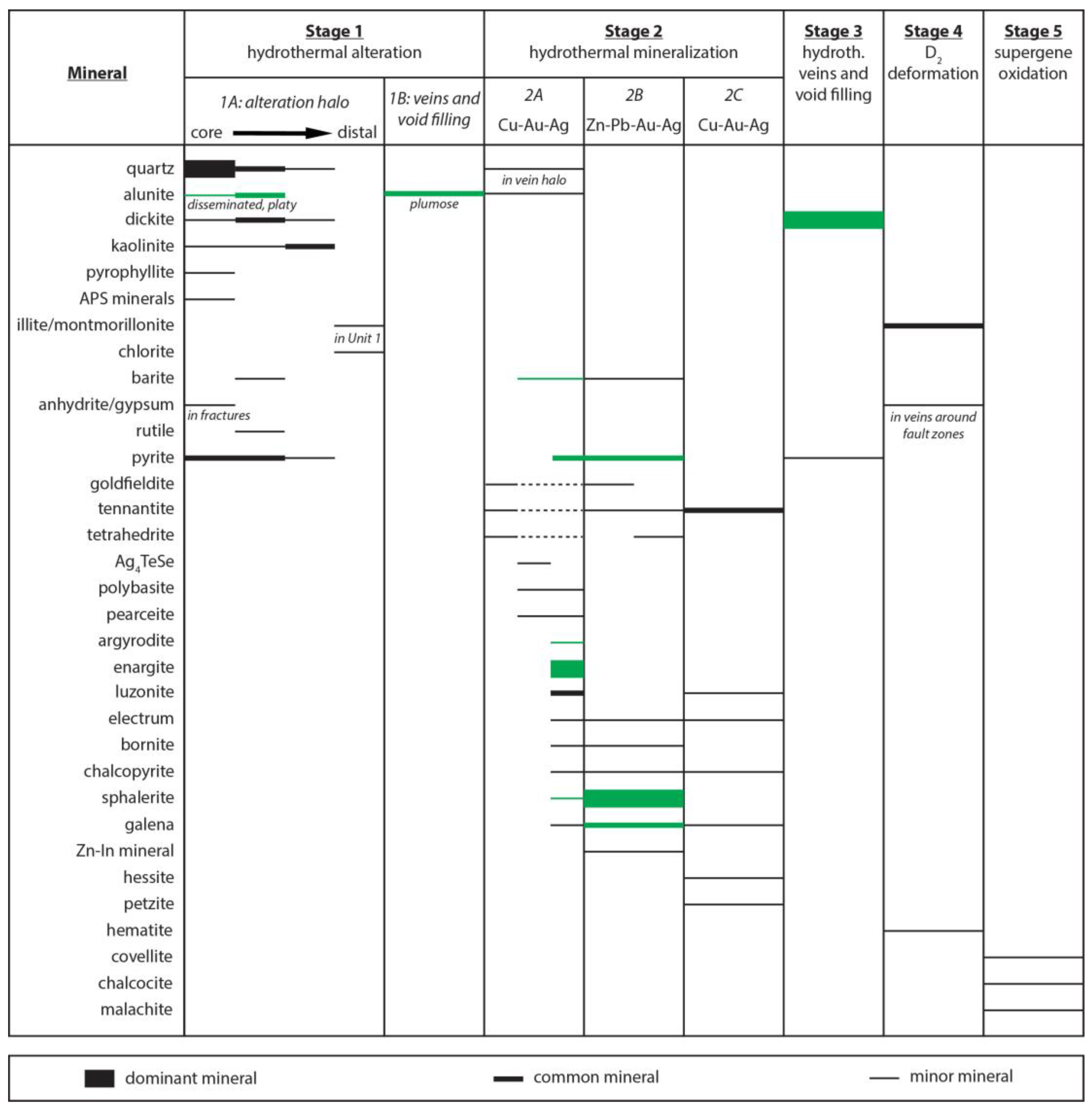
3. Mineral Paragenesis
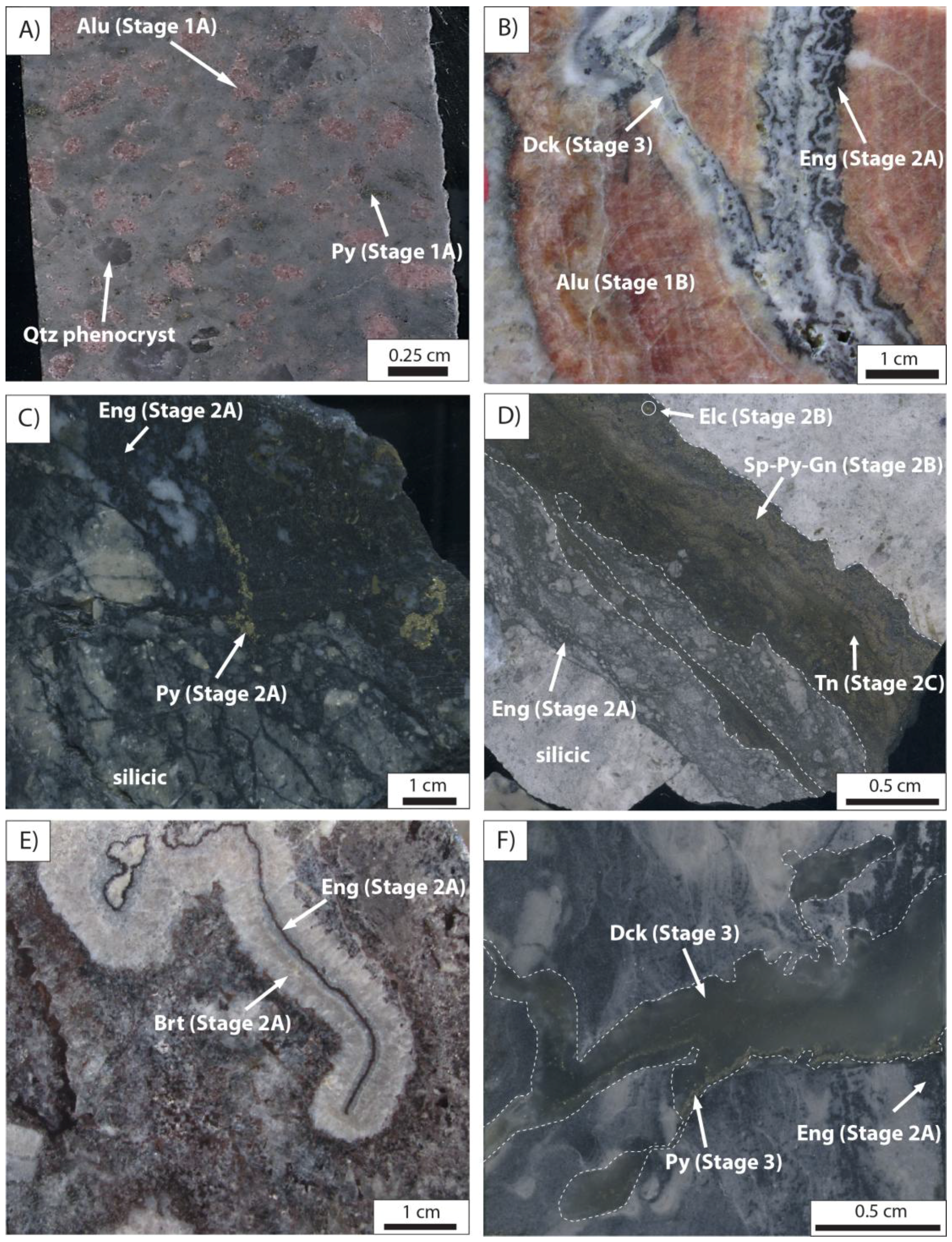
3.1. Hydrothermal Alteration
3.2. Hydrothermal Mineralization and Void Fill
3.3. Syn-Tectonic Alteration and Supergene Oxidation
4. Methods
4.1. Sampling
4.2. WDS Spot Analyses
4.3. WDS Element Mapping
4.4. LA-ICP-MS Spot Analyses
5. Occurrence and Composition of Key Minerals at Mt Carlton
5.1. Alunite
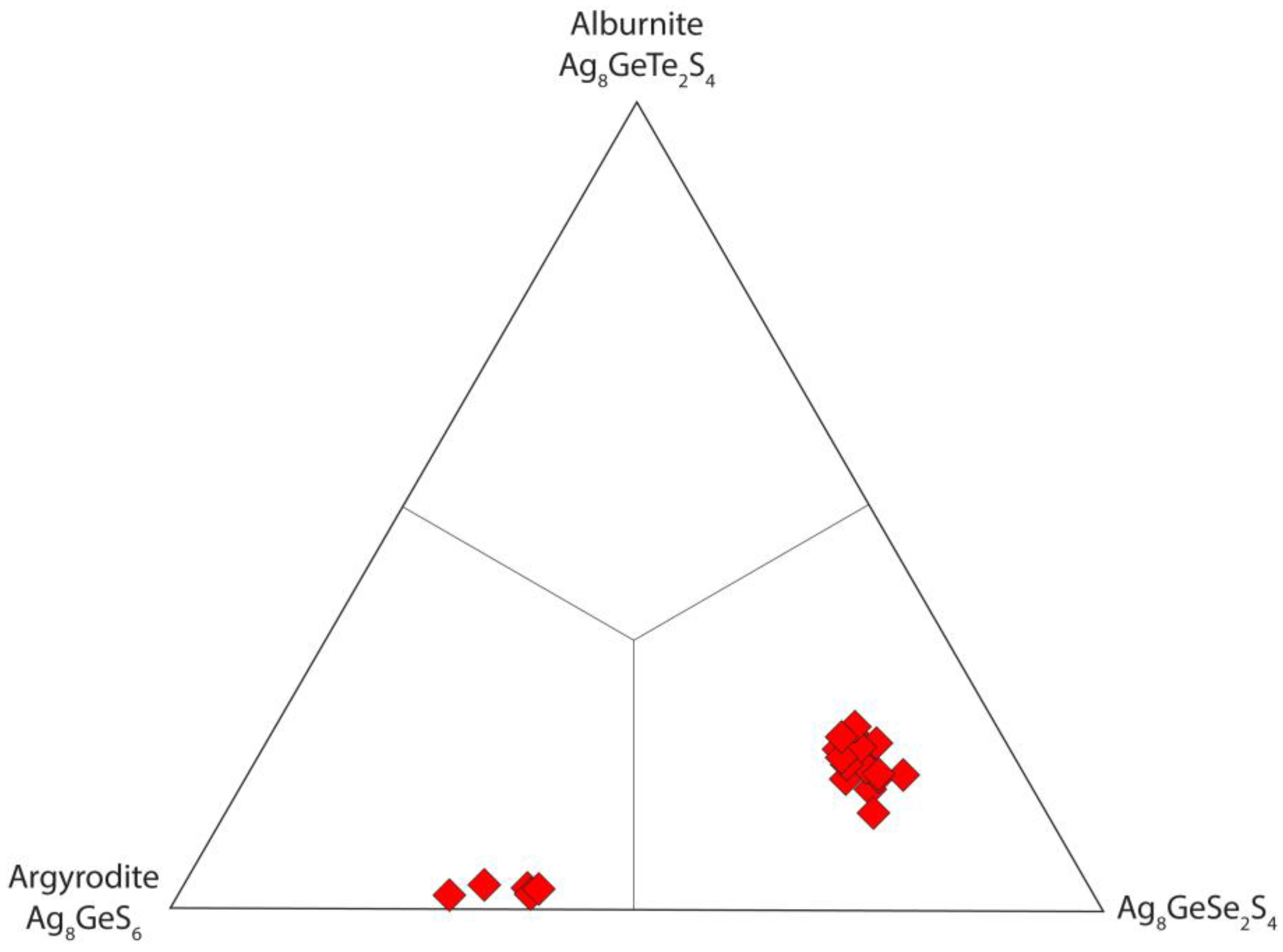
5.2. Argyrodite
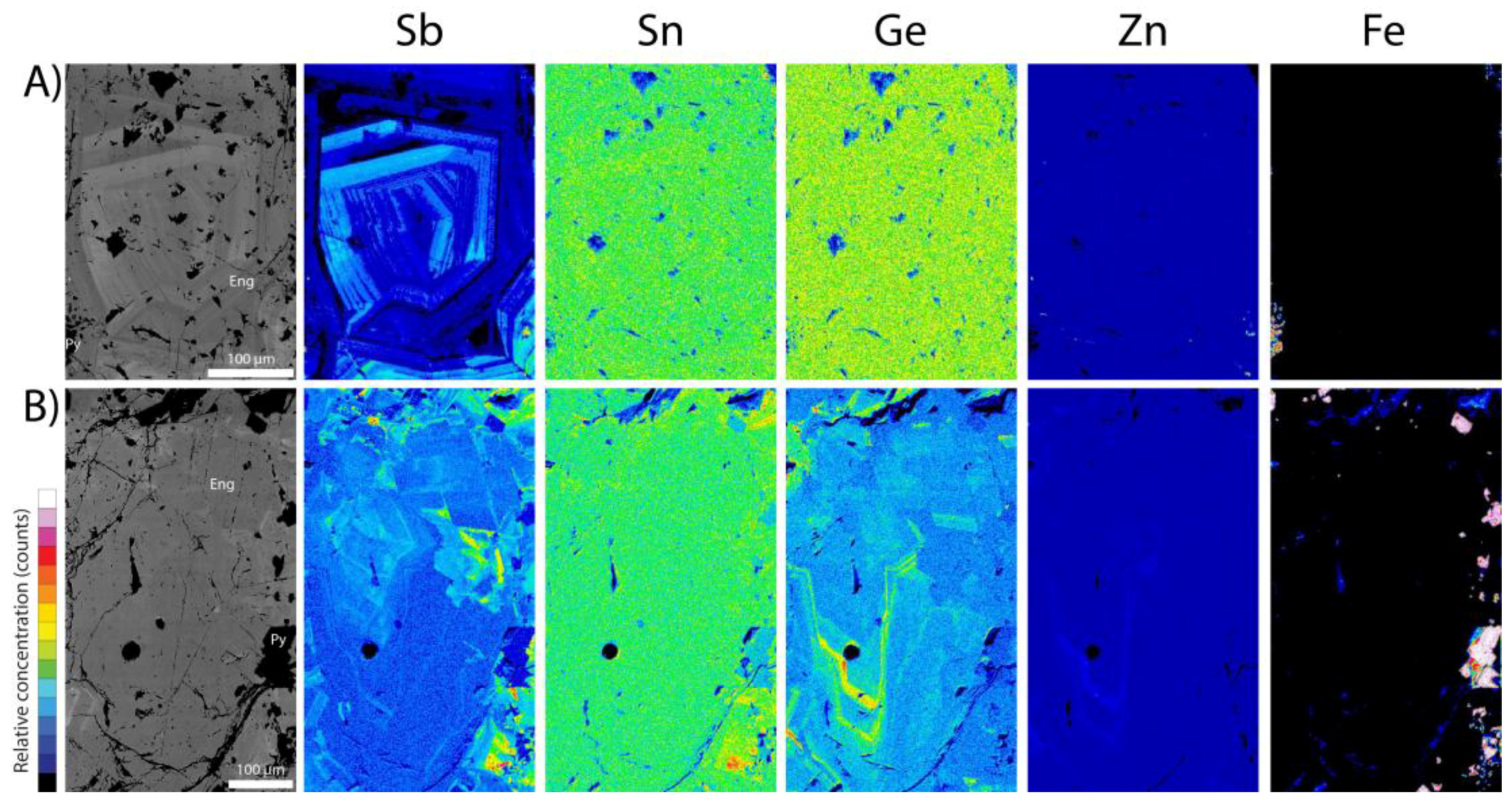
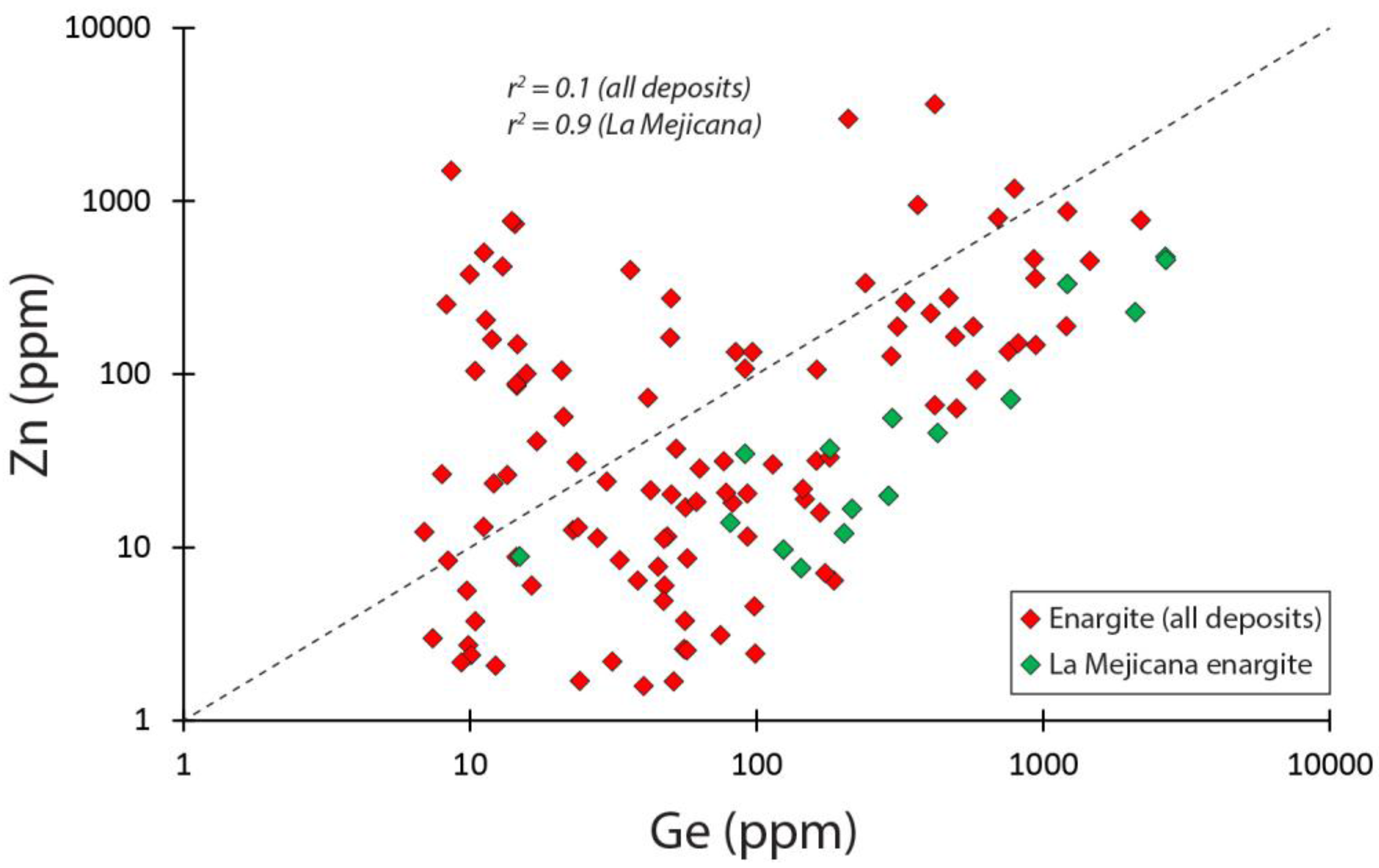
5.3. Enargite
5.4. Barite
5.5. Pyrite
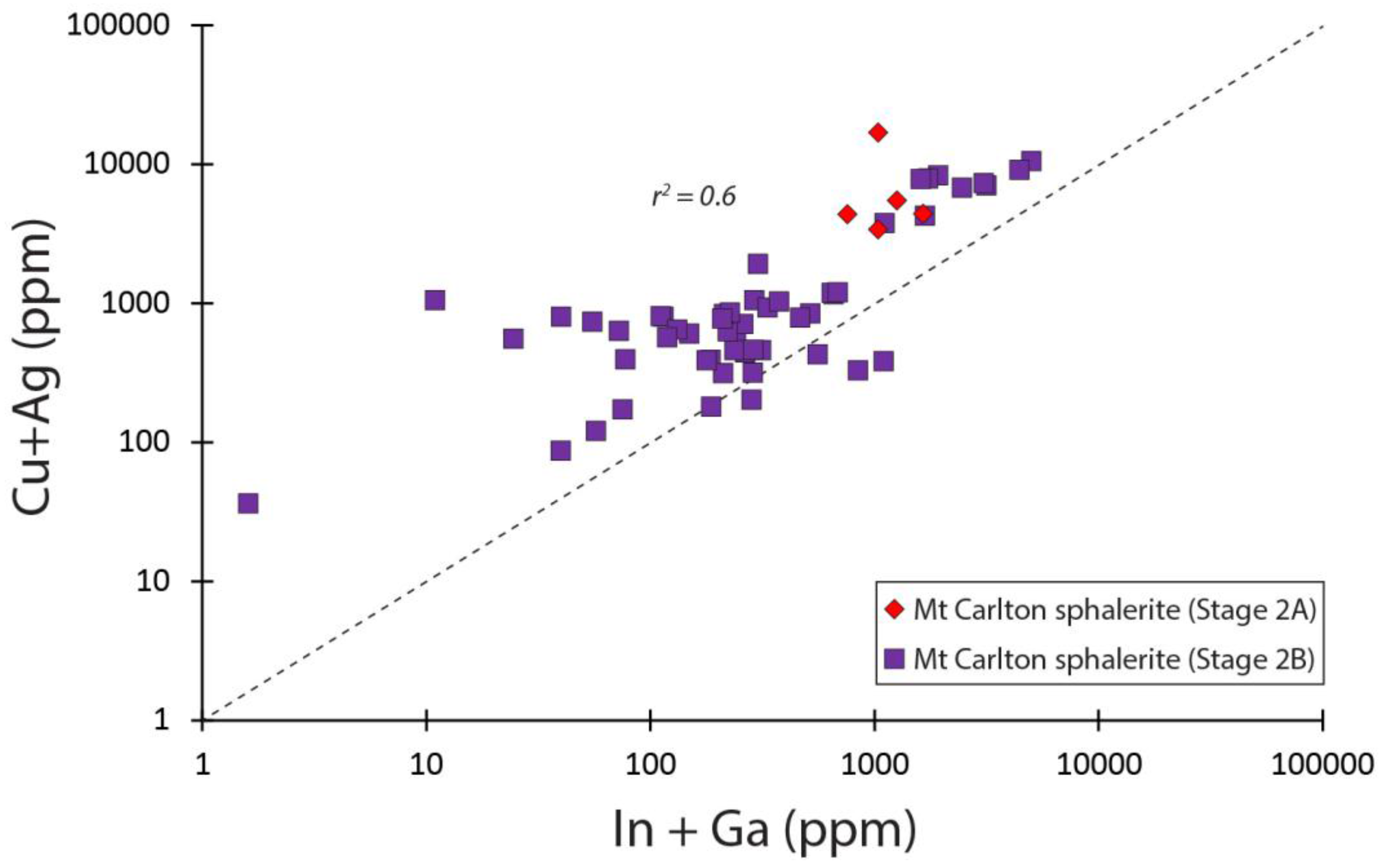
5.6. Sphalerite
5.7. Galena
5.8. Dickite
6. Composition of Enargite from Reference Deposits
7. Discussion
7.1. Ga Enrichment in Al-Bearing Sulfates and Silicates
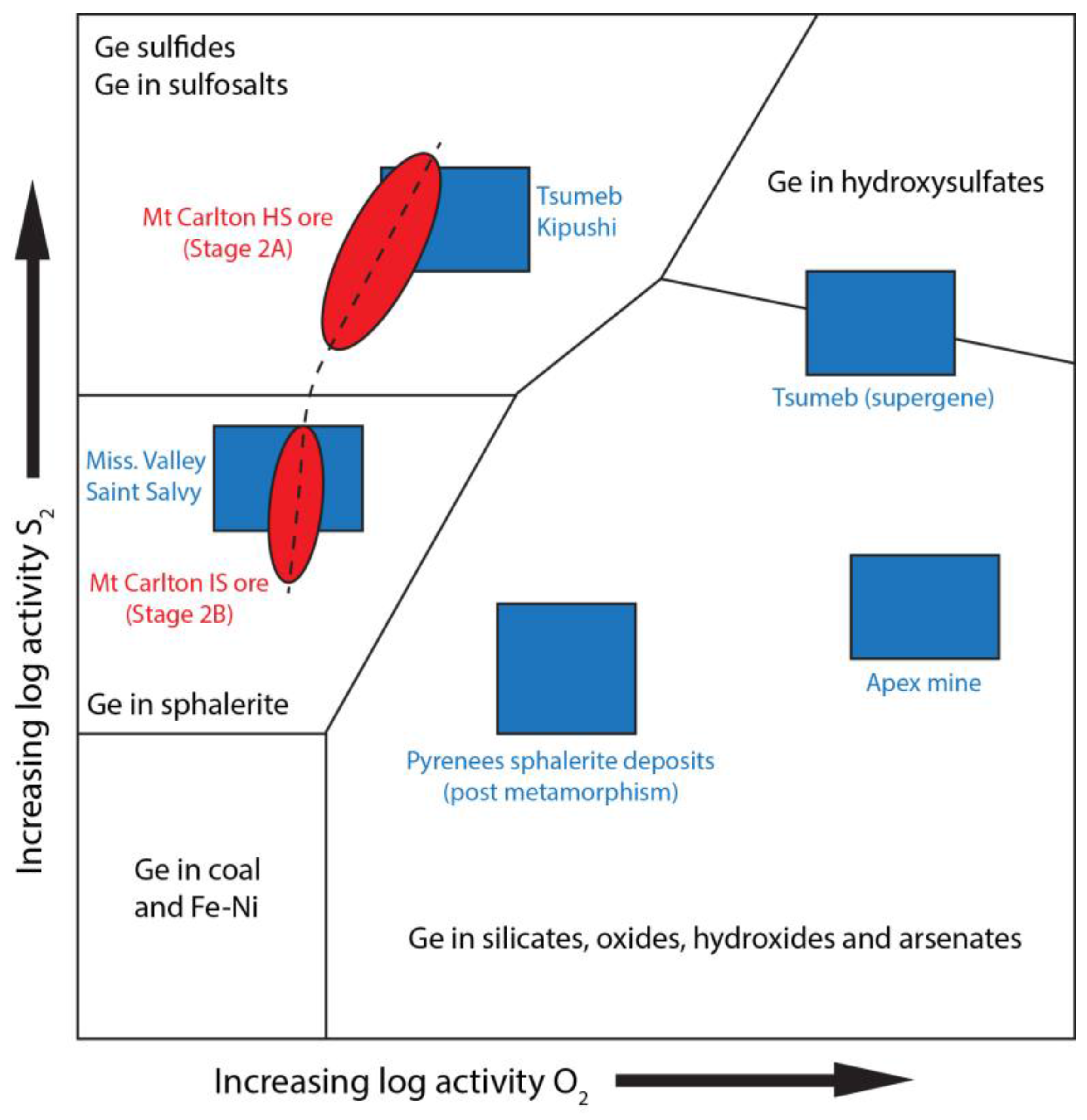
7.2. Ge ± In-Ga Mineralization in High-Sulfidation Enargite Ores
7.3. In-Ga ± Ge Mineralization in Intermediate-Sulfidation Sphalerite Ores
7.4. Petrogenetic Considerations
7.5. Concluding Remarks and Future Developments
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwarz-Schampera, U.; Herzig, P.M. Indium: Geology, Mineralogy, and Economics; Springer: Heidelberg, Germany, 2002. [Google Scholar]
- Frenzel, M.; Hirsch, T.; Gutzmer, J. Gallium, germanium, indium, and other trace and minor elements in sphalerite as a function of deposit type—A meta-analysis. Ore Geol. Rev. 2016, 76, 52–78. [Google Scholar] [CrossRef]
- Guberman, D. Germanium. In US Geological Survey Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2017; pp. 70–71. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/germanium/mcs-2017-germa.pdf (accessed on 15 September 2017).
- Jaskula, B. Gallium. In US Geological Survey Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2017; pp. 64–65. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/gallium/mcs-2017-galli.pdf (accessed on 15 September 2017).
- Tolcin, A.C. Indium. In US Geological Survey Mineral Commodity Summaries; US Geological Survey: Reston, VA, USA, 2017; pp. 80–81. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/indium/mcs-2017-indiu.pdf (accessed on 15 September 2017).
- Erdmann, L.; Graedel, T.E. Criticality of non-fuel minerals: A review of major approaches and analyses. Environ. Sci. Technol. 2011, 45, 7620–7630. [Google Scholar] [CrossRef] [PubMed]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Paradis, S. Indium, germanium and gallium in volcanic and sediment-hosted base-metal sulphide deposits. In Proceedings of the Symposium on Critical and Strategic Materials, Victoria, BC, Canada, 13–14 November 2015; Simandl, G.J., Neetz, M., Eds.; British Columbia Ministry of Energy and Mines: Victoria, BC, Canada, 2015; pp. 23–29. [Google Scholar]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L. Mineral hosts for critical metals in hydrothermal ores. In Mineral Resources in a Sustainable World, Proceedings of the 13th SGA Biennial Meeting, Nancy, France, 24–27 August 2015; André-Meyer, A.S., Cathelineau, M., Muchez, P.H., Pirard, E., Sindern, S., Eds.; Society for Geology Applied to Mineral Deposits: Geneva, Switzerland, 2015; pp. 707–710. [Google Scholar]
- Ishihara, S.; Hoshino, K.; Murakami, H.; Endo, Y. Resource evaluation and some genetic aspects of indium in the Japanese ore deposits. Resour. Geol. 2006, 56, 347–364. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Williams, T. The mineralogy and mineral chemistry of indium in sulphide deposits and implications for mineral processing. Hydrometallurgy 2011, 108, 226–228. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.P.; Gutzmer, J. On the geological availability of germanium. Mineralium Deposita 2014, 49, 471–486. [Google Scholar] [CrossRef]
- Frenzel, M.; Ketris, M.P.; Seifert, T.; Gutzmer, J. On the current and future availability of gallium. Resour. Policy 2016, 47, 38–50. [Google Scholar] [CrossRef]
- Shimizu, T.; Morishita, Y. Petrography, chemistry, and near-infrared microthermometry of indium-bearing sphalerite from the Toyoha polymetallic deposit, Japan. Econ. Geol. 2012, 107, 723–735. [Google Scholar] [CrossRef]
- Murakami, H.; Ishihara, S. Trace elements of indium-bearing sphalerite from tin-polymetallic deposits in Bolivia, China and Japan: A femto-second LA-ICPMS study. Ore Geol. Rev. 2013, 53, 223–243. [Google Scholar] [CrossRef]
- Sillitoe, R.H.; Hedenquist, J.W. Linkages between volcanotectonic settings, ore-fluid compositions, and epithermal precious metal deposits. SEG Spec. Publ. 2003, 10, 315–343. [Google Scholar]
- Simmons, S.F.; White, N.C.; John, D.A. Geological characteristics of epithermal precious and base metal deposits. In Economic Geology One Hundredth Anniversary Volume: 1905–2005; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2005; pp. 485–522. [Google Scholar]
- Hedenquist, J.W.; Simmons, S.F.; Giggenbach, W.F.; Eldridge, C.S. White Island, New Zealand, volcanic-hydrothermal system represents the geochemical environment of high-sulfidation Cu and Au ore deposition. Geology 1993, 21, 731–734. [Google Scholar] [CrossRef]
- Arribas, A. Characteristics of high-sulfidation epithermal deposits, and their relation to magmatic fluid. Miner. Assoc. Can. 1995, 23, 419–454. [Google Scholar]
- Sillitoe, R.H. Gold-rich porphyry deposits: Descriptive and genetic models and their role in exploration and discovery. Rev. Econ. Geol. 2000, 13, 315–345. [Google Scholar]
- Hedenquist, J.W.; Arribas, A.; Gonzalez-Urien, E. Exploration for epithermal gold deposits. Rev. Econ. Geol. 2000, 13, 245–277. [Google Scholar]
- Sillitoe, R.H. Porphyry copper systems. Econ. Geol. 2010, 105, 3–41. [Google Scholar] [CrossRef]
- Chang, Z.; Hedenquist, J.W.; White, N.C.; Cooke, D.R.; Roach, M.; Deyell, C.L.; Garcia, J.; Gemmell, J.B.; McKnight, S.; Cuison, A.L. Exploration tools for linked porphyry and epithermal deposits: Example from the Mankayan intrusion-centered Cu-Au district, Luzon, Philippines. Econ. Geol. 2011, 106, 1365–1398. [Google Scholar] [CrossRef]
- Hedenquist, J.W. Mineralization associated with volcanic-related hydrothermal systems in the Circum-Pacific Basin. In Proceedings of the 4th Circum-Pacific Energy and Mineral Resources Conference, Singapore, 17–22 August 1986; American Association of Petroleum Geologists: Tulsa, OK, USA, 1986; pp. 513–524. [Google Scholar]
- Einaudi, M.T.; Hedenquist, J.W.; Inan, E.E. Sulfidation state of fluids in acti ve and extinct hydrothermal systems: Transitions from porphyry to epithermal environments. SEG Spec. Publ. 2003, 10, 285–314. [Google Scholar]
- Jannas, R.R.; Beane, R.E.; Ahler, B.A.; Brosnahan, D.R. Gold and copper mineralization at the El Indio deposit, Chile. J. Geochem. Explor. 1990, 36, 233–266. [Google Scholar] [CrossRef]
- Arribas, A.; Hedenquist, J.W.; Itaya, T.; Okada, T.; Concepción, R.A.; Garcia, J.S. Contemporaneous formation of adjacent porphyry and epithermal Cu-Au deposits over 300 ka in northern Luzon, Philippines. Geology 1995, 23, 337–340. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion-centered hydrothermal system: Far Southeast-Lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Sahlström, F.; Dirks, P.; Chang, Z.; Arribas, A.; Corral, I.; Obiri-Yeboah, M.; Hall, C. The Paleozoic Mt Carlton deposit, Bowen Basin, NE Australia: Shallow high-sulfidation epithermal Au-Ag-Cu mineralization formed during rifting. Econ. Geol. 2018. (under review). [Google Scholar]
- Donchak, P.; Purdy, D.J.; Withnall, I.; Blake, P.; Jell, P.A. New England Orogen. In Geology of Queensland; Jell, P.A., Ed.; Geological Survey of Queensland: Brisbane, Australia, 2013; pp. 305–472. [Google Scholar]
- Hutton, L.; Withnall, I.; Rienks, I.; Bultitude, R.J.; Hayward, M.; von Gneilinski, F.; Fordham, B.; Simpson, G. A preliminary Carboniferous to Permian magmatic framework for the Auburn and Connors Arches, central Queensland. In Proceedings of the 1999 New England Orogen Conference, Armidale, Australia, 1–3 February 1999; pp. 223–232. [Google Scholar]
- Esterle, J.S.; Sliwa, R.; Smith, G.L.B.; Yago, J.V.R.; Williams, R.; Li, S.; Dimitrakopoulos, R. Bowen Basin Supermodel 2000; CSIRO Exploration & Mining: Kenmore, Australia, 2002. [Google Scholar]
- Korsch, R.; Totterdell, J.; Cathro, D.; Nicoll, M. Early Permian east Australian rift system. Aust. J. Earth Sci. 2009, 56, 381–400. [Google Scholar] [CrossRef]
- Malone, E.J.; Olgers, F.; Kirkegaard, A. The Geology of the Duaringa and Saint-Lawrence 1:250,000 Sheet Areas, Queensland; Bureau of Mineral Resources: Canberra, Australia, 1969.
- Allen, J.P.; Fielding, C.R. Sequence architecture within a low-accommodation setting: An example from the Permian of the Galilee and Bowen basins, Queensland, Australia. AAPG Bull. 2007, 91, 1503–1539. [Google Scholar] [CrossRef]
- Korsch, R.; Totterdell, J. Subsidence history and basin phases of the Bowen, Gunnedah and Surat Basins, eastern Australia. Aust. J. Earth Sci. 2009, 56, 335–353. [Google Scholar] [CrossRef]
- Fielding, C.; Falkner, A.; Kassan, J.; Draper, J. Permian and Triassic depositional systems in the Bowen Basin. In Proceedings of the Bowen Basin Symposium, Mackay, Australia, 1990; Beeston, J.W., Ed.; Geological Society of Australia—Queensland Division: Brisbane, Australia, 1990; pp. 21–25. [Google Scholar]
- Fergusson, C.L. Thin-skinned thrusting in the northern New England Orogen, central Queensland, Australia. Tectonics 1991, 10, 797–806. [Google Scholar] [CrossRef]
- Holcombe, R.; Stephens, C.; Fielding, C.; Gust, D.; Little, T.; Sliwa, R.; Kassan, J.; McPhie, J.; Ewart, A. Tectonic evolution of the northern New England Fold Belt: The Permian–Triassic Hunter–Bowen event. In Tectonics and Metallogenesis of the New England Orogen; Ashley, P.M., Flood, P.G., Eds.; Geological Society of Australia: Sydney, Australia, 1997; pp. 52–65. [Google Scholar]
- Corral, I.; Chang, Z.; Dirks, P.H.G.M.; Sahlström, F.; Behnsen, H.; Hall, C.; Creaser, R. The Capsize prospect (NE Queensland, Australia): Geology, geochemistry and geochronology of a multipulse rhyolite-driven porphyry and associated lithocap. (unpublished; manuscript in preparation).
- Sahlström, F.; Blake, K.; Corral, I.; Chang, Z. Hyperspectral cathodoluminescence study of indium-bearing sphalerite from the Mt Carlton high-sulphidation epithermal deposit, Queensland, Australia. Eur. J. Mineral. 2017, in press. [Google Scholar] [CrossRef]
- Ohta, E. Occurrence and chemistry of indium-containing minerals from the Toyoha mine, Hokkaido, Japan. Min. Geol. 1989, 39, 355–371. [Google Scholar] [CrossRef]
- Sinclair, W.; Kooiman, G.; Martin, D.; Kjarsgaard, I. Geology, geochemistry and mineralogy of indium resources at Mount Pleasant, New Brunswick, Canada. Ore Geol. Rev. 2006, 28, 123–145. [Google Scholar] [CrossRef]
- Murillo, F.; Sanjinés, O.; Barrera, L.; Jiménez, N.; Lizeca, J.; Flores, O.; Hoffstra, A.; Hardyman, R.; Nash, T. Geología, alteración, y mineralización del depósito mineral tipo sulfato-ácido de Laurani, Altiplano Norte de Bolivia. In Investigaciones de Metales Preciosos en el Complejo Volcánico Neógeno-Cuaternario de los Andes Centrales; Servicio Geológico de Bolivia: La Paz, Bolivia, 1993; pp. 123–130. [Google Scholar]
- Losada-Calderón, A.; McPhail, D. Porphyry and high-sulfidation epithermal mineralization in the Nevados del Famatina mining district, Argentina. SEG Spec. Publ. 1996, 5, 91–118. [Google Scholar]
- Perelló, J. Geology, porphyry Cu-Au, and epithermal Cu-Au-Ag mineralization of the Tombulilato district, North Sulawesi, Indonesia. J. Geochem. Explor. 1994, 50, 221–256. [Google Scholar] [CrossRef]
- Chambefort, I. The Cu-Au Chelopech Deposit, Panagyurishte District, Bulgaria: Volcanic Setting, Hydrothermal Evolution and Tectonic Overprint of a Late Cretaceous High-Sulfidation Epithermal Deposit. Ph.D. Thesis, University of Geneva, Geneva, Switzerland, 2005. [Google Scholar]
- Corral, I.; Cardellach, E.; Corbella, M.; Canals, À.; Gómez-Gras, D.; Griera, A.; Cosca, M.A. Cerro Quema (Azuero Peninsula, Panama): Geology, alteration, mineralization, and geochronology of a volcanic dome-hosted high-sulfidation Au-Cu deposit. Econ. Geol. 2016, 111, 287–310. [Google Scholar] [CrossRef]
- Gustafson, L.B.; Hunt, J.P. The porphyry copper deposit at El Salvador, Chile. Econ. Geol. 1975, 70, 857–912. [Google Scholar] [CrossRef]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A. Determination of reference values for NIST SRM 610–617 glasses following ISO guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical characterisation of the USGS reference glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G using EPMA, ID-TIMS, ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 2005, 29, 285–302. [Google Scholar] [CrossRef]
- Wilson, S.; Ridley, W.; Koenig, A. Development of sulfide calibration standards for the laser ablation inductively-coupled plasma mass spectrometry technique. J. Anal. At. Spectrom. 2002, 17, 406–409. [Google Scholar] [CrossRef]
- Van Achterbergh, E.; Ryan, C.; Jackson, S.; Griffin, W. GLITTER: data reduction software for LA-ICP-MS. Mineral. Assoc. Can. 2001, 29, 239–243. [Google Scholar]
- Rye, R.O.; Bethke, P.M.; Wasserman, M.D. The stable isotope geochemistry of acid sulfate alteration. Econ. Geol. 1992, 87, 225–262. [Google Scholar] [CrossRef]
- Rye, R.O. A review of the stable-isotope geochemistry of sulfate minerals in selected igneous environments and related hydrothermal systems. Chem. Geol. 2005, 215, 5–36. [Google Scholar] [CrossRef]
- Tananaev, I.; Kuznetsov, V.; Bolshakova, N. Basic gallium salts of the alunite type. Russ. J. Inorg. Chem. 1967, 12, 28–30. [Google Scholar]
- Jambor, J.L.; Owens, D.R.; Grice, J.D.; Feinglos, M.N. Gallobeudantite, PbGa3[(AsO4),(SO4)]2(OH)6, a new mineral species from Tsumeb, Namibia, and associated new gallium analogues of the alunite-jarosite family. Can. Mineral. 1996, 34, 1305–1315. [Google Scholar]
- Jambor, J.L. Nomenclature of the alunite supergroup. Can. Mineral. 1999, 37, 1323–1342. [Google Scholar]
- Dutrizac, J.; Chen, T. The behaviour of gallium during jarosite precipitation. Can. Metall. Q. 2000, 39, 1–14. [Google Scholar] [CrossRef]
- Weisbach, A. Argyrodit, ein neues silbererz. Neues Jahrbuch für Mineralogie Geologie und Palaontologie 1886, 2, 67–71. [Google Scholar]
- Paar, W.H.; Roberts, A.C.; Berlepsch, P.; Armbruster, T.; Topa, D.; Zagler, G. Putzite, (Cu4.7Ag3.3)∑8GeS6, a new mineral species from Capillitas, Catamarca, Argentina. Description and crystal structure. Can. Mineral. 2004, 42, 1757–1769. [Google Scholar] [CrossRef]
- De Ronde, C.E.; Massoth, G.J.; Butterfield, D.A.; Christenson, B.W.; Ishibashi, J.; Ditchburn, R.G.; Hannington, M.D.; Brathwaite, R.L.; Lupton, J.E.; Kamenetsky, V.S. Submarine hydrothermal activity and gold-rich mineralization at Brothers Volcano, Kermadec Arc, New Zealand. Mineralium Deposita 2011, 46, 541–584. [Google Scholar] [CrossRef]
- Gonzalez, A. Geology and Genesis of the Lepanto Copper Deposit, Mankayan, Mountain Province, Philippines. Ph.D. Thesis, Stanford University, Stanford, CA, USA, 1959. [Google Scholar]
- Deyell, C.; Hedenquist, J. Trace element geochemistry of enargite in the Mankayan district, Philippines. Econ. Geol. 2011, 106, 1465–1478. [Google Scholar] [CrossRef]
- Arribas, A.; Cunningham, C.G.; Rytuba, J.J.; Rye, R.O.; Kelly, W.C.; Podwysocki, M.H.; McKee, E.H.; Tosdal, R.M. Geology, geochronology, fluid inclusions, and isotope geochemistry of the Rodalquilar gold alunite deposit, Spain. Econ. Geol. 1995, 90, 795–822. [Google Scholar] [CrossRef]
- Martín-Vivaldi, J.; Sierra, J.; Leal, J. Some aspects of the mineralization and wall-rock alteration in the Rodalquilar goldfield, SE Spain. Soc. Min. Geol. Jpn. Spec. Issue 1971, 2, 145–152. [Google Scholar]
- Reich, M.; Kesler, S.E.; Utsunomiya, S.; Palenik, C.S.; Chryssoulis, S.L.; Ewing, R.C. Solubility of gold in arsenian pyrite. Geochim. Cosmochim. Acta 2005, 69, 2781–2796. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Pring, A.; Skinner, W.; Shimizu, M.; Danyushevsky, L.; Saini-Eidukat, B.; Melcher, F. Trace and minor elements in sphalerite: A LA-ICPMS study. Geochim. Cosmochim. Acta 2009, 73, 4761–4791. [Google Scholar] [CrossRef]
- Johan, Z. Indium and germanium in the structure of sphalerite: An example of coupled substitution with copper. Mineral. Petrol. 1988, 39, 211–229. [Google Scholar] [CrossRef]
- Cook, N.J.; Sundblad, K.; Valkama, M.; Nygård, R.; Ciobanu, C.L.; Danyushevsky, L. Indium mineralisation in A-type granites in southeastern Finland: Insights into mineralogy and partitioning between coexisting minerals. Chem. Geol. 2011, 284, 62–73. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Brugger, J.; Etschmann, B.; Howard, D.L.; de Jonge, M.D.; Ryan, C.; Paterson, D. Determination of the oxidation state of Cu in substituted Cu-In-Fe-bearing sphalerite via μ-XANES spectroscopy. Am. Mineral. 2012, 97, 476–479. [Google Scholar] [CrossRef]
- Bonnet, J. Distribution et Contrôle Cristallographique des Éléments Ge, Ga et Cd dans les Sphalérites des Gisements de Type Mississippi Valley dans les Districts de Central et East Tennessee, USA. Ph.D. Thesis, University of Lorraine, Lorraine, France, 2014. [Google Scholar]
- Cook, N.J.; Etschmann, B.; Ciobanu, C.L.; Geraki, K.; Howard, D.L.; Williams, T.; Rae, N.; Pring, A.; Chen, G.; Johannessen, B. Distribution and substitution mechanism of Ge in a Ge-(Fe)-bearing sphalerite. Minerals 2015, 5, 117–132. [Google Scholar] [CrossRef]
- Belissont, R.; Muñoz, M.; Boiron, M.-C.; Luais, B.; Mathon, O. Distribution and oxidation state of Ge, Cu and Fe in sphalerite by μ-XRF and K-edge μ-XANES: Insights into Ge incorporation, partitioning and isotopic fractionation. Geochim. Cosmochim. Acta 2016, 177, 298–314. [Google Scholar] [CrossRef]
- George, L.; Cook, N.J.; Cristiana, L.; Wade, B.P. Trace and minor elements in galena: A reconnaissance LA-ICP-MS study. Am. Mineral. 2015, 100, 548–569. [Google Scholar] [CrossRef]
- Di Benedetto, F.; Pelo, S.D.; Caneschi, A.; Lattanzi, P. Chemical state of arsenic and copper in enargite: Evidences from EPR and X-ray absorption spectroscopies, and SQUID magnetometry. J. Mineral. Geochem. 2011, 188, 11–19. [Google Scholar] [CrossRef]
- Mills, S.J.; Kampf, A.R.; Raudsepp, M.; Christy, A. The crystal structure of Ga-rich plumbogummite from Tsumeb, Namibia. Mineral. Mag. 2009, 73, 837–845. [Google Scholar] [CrossRef]
- Dutrizac, J.; Jambor, J.; Chen, T. Host minerals for the gallium-germanium ores of the Apex Mine, Utah. Econ. Geol. 1986, 81, 946–950. [Google Scholar] [CrossRef]
- Bernstein, L.R. Geology and mineralogy of the Apex germanium-gallium mine, Washington County, Utah. USGS Bull. 1986, 1577, 1–9. [Google Scholar]
- Schulte, R.F.; Foley, N.K. Compilation of gallium resource data for bauxite deposits. In USGS Open-File Report 2013-1272; US Geological Survey: Reston, VA, USA, 2014. [Google Scholar] [CrossRef]
- Rytuba, J.J.; John, D.A.; Foster, A.; Ludington, S.D.; Kotlyar, B. Chapter C: Hydrothermal enrichment of gallium in zones of advanced argillic alteration—Examples from the Paradise Peak and McDermitt ore deposits, Nevada. In Contributions to Industrial Minerals Research—US Geological Survey Bulletin nr. 2209; Bliss, J.D., Moyle, P.R., Long, K.R., Eds.; US Geological Survey: Reston, VA, USA, 2003. [Google Scholar]
- Cunningham, C.G.; Rye, R.O.; Steven, T.A.; Mehnert, H.H. Origins and exploration significance of replacement and vein-type alunite deposits in the Marysvale volcanic field, west central Utah. Econ. Geol. 1984, 79, 50–71. [Google Scholar] [CrossRef]
- Bove, D.J.; Rye, R.O.; Hon, K. Evolution of the Red Mountain alunite deposit, Lake City, Colorado. In US Geological Survey Open-File Reports 90-235; US Geological Survey: Reston, VA, USA, 1990. Available online: https://pubs.er.usgs.gov/publication/ofr90235 (accessed on 19 August 2017).
- Asahi Chemical Industry, Ltd. Method for the Recovery of Gallium from Alunite. U.S. Patent US3,168,372 A, 1965. [Google Scholar]
- Zijin Mining Group, Ltd. Method for Recycling Gallium in Alunite Concentrate. Patent CN102,517,461 A, 2012. [Google Scholar]
- Lombaard, A.; Günzel, A.; Innes, J.; Krüger, T. The Tsumeb lead-copper-zinc-silver deposit, south west Africa/Namibia. In Mineral Deposits of Southern Africa; Anhaeusser, C.R., Maske, S., Eds.; Geological Society of South Africa: Johannesburg, South Africa, 1986; pp. 1761–1787. [Google Scholar]
- Melcher, F.; Oberthür, T.; Rammlmair, D. Geochemical and mineralogical distribution of germanium in the Khusib Springs Cu–Zn–Pb–Ag sulfide deposit, Otavi Mountain Land, Namibia. Ore Geol. Rev. 2006, 28, 32–56. [Google Scholar] [CrossRef]
- Terziev, G. The conditions of germanium accumulation in hydrothermal mineralization (as illustrated by a Bulgarian copper-pyrite deposit). Geochem. Int. 1966, 3, 341–346. [Google Scholar]
- Terziev, G. Mineral composition and genesis of the Chelopech ore deposit. Geokhim. Mineral. Petrol. 1968, 17, 123–187. [Google Scholar]
- Kovalenker, V.; Tsonev, D.; Breskovska, V.; Malov, V.; Troneva, N. New data on the mineralogy of the massive-sulphide deposits in the Central Srednogorie of Bulgaria. In Metasomatism, Mineralogy and Genesis of Gold and Silver Deposits in Volcanic Series; Nauka: Moscow, Russia, 1986; pp. 91–110. [Google Scholar]
- Petrunov, R. Ore mineral parageneses and zoning in the deposit of Chelopech. Geokhim. Mineral. Petrol. 1995, 30, 89–98. [Google Scholar]
- Bonev, I.K.; Kerestedjian, T.; Atanassova, R.; Andrew, C.J. Morphogenesis and composition of native gold in the Chelopech volcanic-hosted Au–Cu epithermal deposit, Srednogorie zone, Bulgaria. Mineralium Deposita 2002, 37, 614–629. [Google Scholar] [CrossRef]
- Bogdanov, K.; Tsonev, D.; Popov, K. Mineral assemblages and genesis of the Cu-Au epithermal deposits in the southern part of the Panaguyrishte Ore District, Bulgaria. Bull. Geol. Soc. Greece 2004, 36, 406–415. [Google Scholar]
- Tămaş, C.G.; Bailly, L.; Ghergari, L.; O’Connor, G.; Minuţ, A. New occurrences of tellurides and argyrodite in Roşia Montană, Apuseni Mountains, Romania, and their metallogenic significance. Can. Mineral. 2006, 44, 367–383. [Google Scholar] [CrossRef]
- Putz, H.; Paar, W.H.; Topa, D. A contribution to the knowledge of the mineralization at Mina Capillitas, Catamarca. Rev. Asoc. Geol. Argent. 2009, 64, 514–524. [Google Scholar]
- Putz, H.; Paar, W.H.; Sureda, R.; Roberts, A. Germanium mineralization at Capillitas, Catamarca Province, Argentina. In Proceedings of the 18th General Meeting of the IMA, Edinburgh, Scotland, 1–6 September 2002. [Google Scholar]
- Paar, W.H.; Putz, H. Germanium associated with epithermal mineralization: Examples from Bolivia and Argentina. In Mineral Deposit Research: Meeting the Global Challenge, Proceedings of the 8th Biennial SGA Meeting, Beijing, China, 18-21 August 2005; Mao, J., Bierlein, F.P., Eds.; Society for Geology Applied to Mineral Deposits: Geneva, Switzerland, 2005; pp. 48–51. [Google Scholar]
- Putz, H.; Paar, W.H.; Topa, D.; Makovicky, E.; Roberts, A.C. Catamarcaite, Cu6GeWS8, a new germanium sulfide mineral species from Capillitas, Catamarca, Argentina: Description, paragenesis and crystal structure. Can. Mineral. 2006, 44, 1481–1497. [Google Scholar] [CrossRef]
- Bindi, L.; Putz, H.; Paar, W.H.; Stanley, C. Omariniite, Cu8Fe2ZnGe2S12, the germanium-analogue of stannoidite, a new mineral species from Capillitas, Argentina. Mineral. Mag. 2017, 81, 1151–1159. [Google Scholar] [CrossRef]
- Maydagán, L.; Franchini, M.; Lentz, D.; Pons, J.; McFarlane, C. Sulfide composition and isotopic signature of the Altar Cu-Au deposit, Argentina: Constraints on the evolution of the porphyry-epithermal system. Can. Mineral. 2013, 51, 813–840. [Google Scholar] [CrossRef]
- Dekov, V.M.; Rouxel, O.; Kouzmanov, K.; Bindi, L.; Asael, D.; Fouquet, Y.; Etoubleau, J.; Burgaud, G.; Wälle, M. Enargite-luzonite hydrothermal vents in Manus Back-Arc Basin: Submarine analogues of high-sulfidation epithermal mineralization. Chem. Geol. 2016, 438, 36–57. [Google Scholar] [CrossRef]
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Dill, H.G.; Garrido, M.M.; Melcher, F.; Gomez, M.C.; Weber, B.; Luna, L.I.; Bahr, A. Sulfidic and non-sulfidic indium mineralization of the epithermal Au–Cu–Zn–Pb–Ag deposit San Roque (Provincia Rio Negro, SE Argentina)—With special reference to the “indium window” in zinc sulfide. Ore Geol. Rev. 2013, 51, 103–128. [Google Scholar] [CrossRef]
- Lopez, L.; Jovic, S.M.; Guido, D.M.; Vidal, C.P.; Páez, G.N.; Ruiz, R. Geochemical distribution and supergene behavior of indium at the Pingüino epithermal polymetallic vein system, Patagonia, Argentina. Ore Geol. Rev. 2015, 64, 747–755. [Google Scholar] [CrossRef]
- Wood, S.A.; Samson, I.M. The aqueous geochemistry of gallium, germanium, indium and scandium. Ore Geol. Rev. 2006, 28, 57–102. [Google Scholar] [CrossRef]
- Belissont, R.; Boiron, M.-C.; Luais, B.; Cathelineau, M. LA-ICP-MS analyses of minor and trace elements and bulk Ge isotopes in zoned Ge-rich sphalerites from the Noailhac–Saint-Salvy deposit (France): Insights into incorporation mechanisms and ore deposition processes. Geochim. Cosmochim. Acta 2014, 126, 518–540. [Google Scholar] [CrossRef]
- Belissont, R.; Boiron, M.C.; Luais, B.; Muchez, P.; de Oliveira, D.P.; Muñoz, M. Germanium distribution and isotopic study in sulphides from MVT-related and VMS-remobilised ore deposits. In Mineral Resources in a Sustainable World, Proceedings of the 13th SGA Biennial Meeting, Nancy, France, 24–27 August 2015; André-Meyer, A.S., Cathelineau, M., Muchez, P.H., Pirard, E., Sindern, S., Eds.; Society for Geology Applied to Mineral Deposits: Geneva, Switzerland, 2015; pp. 683–686. [Google Scholar]
- Driesner, T.; Pintea, I. Constraints on the conditions of wurtzite formation at the Agios Philippos Pb-Zn deposit, NE-Greece. Eur. J. Mineral. 1994, 6, 54. [Google Scholar]
- Skarpelis, N. Minor elements in the base metal part of an epithermal system: The Kirki (St. Philippe) Mine, Thrace, Northern Greece. In Proceedings of the 8th EUG Meeting, Strasbourg, France, 9–13 April 1995; p. 293. [Google Scholar]
- Melfos, V.; Voudouris, P. Geological, mineralogical and geochemical aspects for critical and rare metals in Greece. Minerals 2012, 2, 300–317. [Google Scholar] [CrossRef]
- Tămaş, C.G.; Grobety, B.; Bailly, L.; Bernhardt, H.-J.; Minuţ, A. Alburnite, Ag8GeTe2S4, a new mineral species from the Roşia Montana Au-Ag epithermal deposit, Apuseni Mountains, Romania. Am. Mineral. 2014, 99, 57–64. [Google Scholar] [CrossRef]
- Márquez-Zavalía, M.F.; Galliski, M.Á.; Drábek, M.; Vymazalová, A.; Watanabe, Y.; Murakami, H.; Bernhardt, H.-J. Ishiharaite, (Cu, Ga, Fe, In, Zn)S, a new mineral from the Capillitas mine, northwestern Argentina. Can. Mineral. 2014, 52, 969–980. [Google Scholar] [CrossRef]
- Carrillo-Rosúa, J.; Morales-Ruano, S.; Hach-Alí, P.F. Textural and chemical features of sphalerite from the Palai-Islica deposit (SE Spain): Implications for ore genesis and color. J. Mineral. Geochem. 2008, 185, 63–78. [Google Scholar] [CrossRef]
- Symonds, R.B.; Rose, W.I.; Reed, M.H.; Lichte, F.E.; Finnegan, D.L. Volatilization, transport and sublimation of metallic and non-metallic elements in high temperature gases at Merapi Volcano, Indonesia. Geochim. Cosmochim. Acta 1987, 51, 2083–2101. [Google Scholar] [CrossRef]
- Kavalieris, I. High Au, Ag, Mo, Pb, V and W content of fumarolic deposits at Merapi Volcano, central Java, Indonesia. J. Geochem. Explor. 1994, 50, 479–491. [Google Scholar] [CrossRef]
- Kovalenker, V.; Laputina, I.; Znamenskii, V.; Zotov, I. Indium mineralization of the Great Kuril Island arc. Geol. Ore Depos. 1993, 35, 547–552. [Google Scholar]
- Taran, Y.A.; Hedenquist, J.H.; Korzhinsky, M.; Tkachenko, S.; Shmulovich, K. Geochemistry of magmatic gases from Kudryavy Volcano, Iturup, Kuril Islands. Geochim. Cosmochim. Acta 1995, 59, 1749–1761. [Google Scholar] [CrossRef]
- De Ronde, C.E.; Hannington, M.; Stoffers, P.; Wright, I.; Ditchburn, R.; Reyes, A.; Baker, E.; Massoth, G.; Lupton, J.; Walker, S. Evolution of a submarine magmatic-hydrothermal system: Brothers Volcano, southern Kermadec arc, New Zealand. Econ. Geol. 2005, 100, 1097–1133. [Google Scholar] [CrossRef]
- Breiter, K.; Gardenová, N.; Kanický, V.; Vaculovič, T. Gallium and germanium geochemistry during magmatic fractionation and post-magmatic alteration in different types of granitoids: A case study from the Bohemian Massif (Czech Republic). Geol. Carpath. 2013, 64, 171–180. [Google Scholar] [CrossRef]
- Ohta, E. Common features and genesis of tin-polymetallic veins. Resour. Geol. 1995, 18, 187–195. [Google Scholar]
- Richards, J.P. The oxidation state, and sulfur and Cu contents of arc magmas: Implications for metallogeny. Lithos 2015, 233, 27–45. [Google Scholar] [CrossRef]
- Breger, I.A.; Schopf, J.M. Germanium and uranium in coalified wood from upper Devonian black shale. Geochim. Cosmochim. Acta 1955, 7, 287–293. [Google Scholar] [CrossRef]
- Shaw, D.M. The geochemistry of indium. Geochim. Cosmochim. Acta 1952, 2, 185–206. [Google Scholar] [CrossRef]

| Mineral | Class | Crystal System | Ideal Formula | Paragenesis | Contained Elements | Reference |
|---|---|---|---|---|---|---|
| Alunite | Sulfate | Trigonal | KAl3(SO4)2(OH)6 | 1A and 1B | Ga | this study |
| Argyrodite | Sulfide | Orthorhombic | Ag8GeS6 | 2A | Ge | this study |
| Enargite | Sulfosalt | Orthorhombic | Cu3AsS4 | 2A | Ge (In, Ga) | this study |
| Sphalerite | Sulfide | Isometric | ZnS | 2A and 2B | In, Ga, Ge | this study |
| Zn-In mineral | Sulfide | Uncharacterized | CuZn2InS4 | 2B | In, Ga | [42] |
| Dickite | Silicate | Monoclinic | Al2Si2O5(OH)4 | 3 | Ga | this study |
| Deposit | Type | Age | Tectonic Setting | Stratigraphic Column (excluding post-mineralization units) | Reference |
|---|---|---|---|---|---|
| Andesite-dacite pyroclastic rocks and porphyry intrusions (Imbanguila Dacite) | |||||
| Lepanto | HS | Quarternary | Island arc | Andesite volcaniclastic rocks (Balili Volcaniclastics), intruded by a quartz-diorite porphyry complex | [29] |
| (Philippines) | Basement of metamorphosed submarine basalt-andesite volcanic rocks (Lepanto metavolcanic rocks) | ||||
| Andesite-rhyolite lava and pyroclastic rocks (Laurani volcanic complex) | |||||
| Laurani | HS | Neogene | Continental arc | Red bed conglomerate, sandstone and siltstone (Chuquichambi Formation) | [45] |
| (Bolivia) | (foreland basin) | Basement comprising shale, siltstone and sandstone (Llalagua, Uncia and Catavi Formations) | |||
| Dacite-rhyodacite porphyritic rocks (Mogote Formation) | |||||
| La Mejicana | HS | Neogene | Continental arc | Continental sandstone and conglomerate (Agua Colorada and Patquía Formations) | [46] |
| (Argentina) | Basement of metamorphosed shale, sandstone, siltstone (Negro Peinado Formation), intruded by Ñuñarco granites | ||||
| Subaerial andesite-rhyolite lava and pyroclastic rocks (Motomboto Volcanics) | |||||
| Motomboto | HS | Quarternary | Island arc | Submarine basalt-andesite lava (Bilungala Volcanics) | [47] |
| (Indonesia) | Unknown basement | ||||
| Argillaceous limestone, calcarenite and sandstone interlayered with volcanic rocks (Chelopech Formation) | |||||
| Chelopech | HS | Cretaceous | Island arc | Coal-bearing conglomerate and sandstone, intruded by subvolcanic andesite intrusions | [48] |
| (Bulgaria) | Metamorphic basement comprising migmatitic gneiss, amfibolite, phillite | ||||
| Cerro Quema | HS | Paleogene | Island arc | Andesite-dacite volcanic rocks, biomicritic hemipelagic limestone, turbidite, shale (Río Quema Formation) | [49] |
| (Panama) | (forearc basin) | Basement comprising basalt and pillow basalt (Azuero Igneous Basement) | |||
| El Salvador | Porphyry | Paleogene | Continental arc | Rhyolite dome complex (Indio Muerto Rhyolite), intruded by rhyolite and granodiorite porphyry complexes | [50] |
| (Chile) | Basement comprising andesite lava and volcaniclastic rocks |
| Mineral | Location | Mineral Assemblage | n (Samples) | Ge (ppm) Mean (max) | Ga (ppm) Mean (max) | In (ppm) Mean (max) | Au (ppm) Mean (max) | Other Elements Mean >1000 ppm | Other Elements Mean >100 ppm |
|---|---|---|---|---|---|---|---|---|---|
| Samples from Mt Carlton | |||||||||
| Alunite | V2 pit | Stage 1A | 44 (11) | 1.2 (9.1) | 32 (109) | 0.1 (1.3) | 0.0 (0.2) | Ba | P, Ca, Fe, Sr, Pb |
| Alunite | V2 pit | Stage 1B | 21 (3) | 0.9 (3.7) | 72 (339) | 0.0 (0.2) | 0.0 (0.2) | P, Sr | Ca, Ba, Ce, Pb |
| Enargite | V2 pit | Stage 2A | 14 (2) | 86 (330) | 6.0 (37) | 14 (155) | 12 (62) | Sb, Bi | Fe, Zn, Se, Ag, Sn, Te |
| Enargite | A39 pit | Stage 2A | 20 (2) | 612 (2189) | 5.2 (35) | 7.7 (76) | 0.2 (1.4) | Sb | Fe, Zn, Se, Ag, Te, Pb, Bi |
| Barite | A39 pit | Stage 2A | 53 (4) | 0.3 (1.8) | 0.4 (1.4) | 0.0 (0.0) | 0.0 (0.0) | Pb, Sr | Ca, La, Ce |
| Pyrite | V2 pit | Stage 2A | 14 (2) | 3.5 (4.7) | 0.2 (0.4) | 0.1 (0.2) | 2.4 (11) | - | Co, Ni, Cu, Zn, As, Ag |
| Pyrite | V2 pit | Stage 2B | 14 (2) | 3.7 (4.2) | 0.1 (0.3) | 0.1 (0.8) | 56 (367) | As | Cu, Pb |
| Sphalerite | V2 pit | Stage 2A | 5 (1) | 77 (143) | 677 (1181) | 469 (571) | 10 (21) | Cu, Cd, Pb | As, Se, Ag, Sb, Te |
| Sphalerite | V2 pit | Stage 2B | 44 (4) | 206 (611) | 444 (2829) | 369 (2169) | 13 (75) | Cu, Cd, Pb | As, Ag, Sb, Hg |
| Sphalerite | A39 pit | Stage 2B | 10 (1) | 5.1 (8.5) | 260 (476) | 20 (40) | 0.5 (2.1) | Cd, Pb | Fe, Cu, As, Sb |
| Galena | V2 pit | Stage 2B | 10 (1) | 0.6 (0.9) | 0.0 (0.1) | 2.4 (12) | 1.1 (2.1) | Cu, Sb | Zn, Cd |
| Galena | A39 pit | Stage 2B | 7 (1) | 0.5 (0.8) | bd | 0.7 (1.2) | 0.4 (0.9) | Ag, Sb | - |
| Dickite | V2 pit | Stage 3 | 10 (1) | 3.1 (4.1) | 100 (109) | 0.1 (0.5) | 0.0 (0.0) | P, Sr | Ca, Fe, Ba, Pb |
| Dickite | A39 pit | Stage 3 | 30 (3) | 5.2 (9.1) | 86 (150) | 0.0 (0.2) | 0.0 (0.0) | - | - |
| Samples from other high-sulfidation epithermal and porphyry deposits | |||||||||
| Enargite | Lepanto | Eng, Py, Luz, Tn-Td, Cv | 15 (1) | 38 (57) | 0.2 (1.3) | 3.7 (8.0) | 1.3 (7.6) | Sb, Te | Fe, Se, Sn |
| Enargite | Laurani | Eng, Py, Luz, Tn-Td | 15 (1) | 12 (15) | 0.0 (0.3) | 9.1 (17) | 0.2 (1.4) | Sb, Sn | Zn, Cd, Te, Hg |
| Enargite | La Mejicana | Eng, Py, Luz | 16 (1) | 717 (2679) | 4.2 (15) | 5.2 (18) | 1.4 (10) | Sb | Fe, Zn, Sn, Bi |
| Enargite | Motomboto | Eng, Py | 15 (1) | 21 (50) | 0.4 (3.3) | 1.3 (1.8) | 0.2 (0.8) | Sb, Bi | Fe, Se |
| Enargite | Chelopech | Eng, Py, Luz, Cp, Tn-Td | 15 (1) | 101 (186) | 0.2 (0.6) | 1.1 (1.3) | 0.1 (0.4) | Sb | Fe, Bi |
| Enargite | Cerro Quema | Eng, Py, Luz, Cp, Cv | 10 (1) | 562 (1202) | 0.1 (0.6) | 1.4 (2.0) | 0.0 (0.3) | Sb, Fe | Hg |
| Enargite | El Salvador | Eng, Py, Tn-Td, Cp | 5 (1) | 12 (24) | 0.0 (0.1) | 1.3 (1.6) | 15 (72) | Sb | Sn, Te |
| n = 23 | Se | Ge | Ag | Te | S | As | Cu | Au | Sb | Bi | Pb | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (wt %) | 8.06 | 5.76 | 69.65 | 3.26 | 12.84 | 0.22 | 1.26 | 0.01 | 0.05 | 0.10 | 0.03 | 101.25 |
| Minimum | 4.34 | 5.19 | 68.56 | 0.38 | 11.66 | 0.03 | 0.59 | 0.00 | 0.00 | 0.00 | 0.00 | 99.94 |
| Maximum | 9.52 | 6.95 | 71.47 | 4.90 | 16.32 | 0.50 | 3.78 | 0.03 | 0.57 | 0.26 | 0.14 | 101.77 |
| Mean (apfu) | 1.21 | 0.93 | 7.59 | 0.30 | 4.69 | 0.03 | 0.23 | 0.00 | 0.00 | 0.01 | 0.00 | |
| Minimum | 0.61 | 0.86 | 7.03 | 0.03 | 4.36 | 0.00 | 0.11 | 0.00 | 0.00 | 0.00 | 0.00 | |
| Maximum | 1.44 | 1.06 | 7.80 | 0.46 | 5.62 | 0.08 | 0.67 | 0.00 | 0.05 | 0.01 | 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sahlström, F.; Arribas, A.; Dirks, P.; Corral, I.; Chang, Z. Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide. Minerals 2017, 7, 213. https://doi.org/10.3390/min7110213
Sahlström F, Arribas A, Dirks P, Corral I, Chang Z. Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide. Minerals. 2017; 7(11):213. https://doi.org/10.3390/min7110213
Chicago/Turabian StyleSahlström, Fredrik, Antonio Arribas, Paul Dirks, Isaac Corral, and Zhaoshan Chang. 2017. "Mineralogical Distribution of Germanium, Gallium and Indium at the Mt Carlton High-Sulfidation Epithermal Deposit, NE Australia, and Comparison with Similar Deposits Worldwide" Minerals 7, no. 11: 213. https://doi.org/10.3390/min7110213





