1. Introduction
The production and economic benefit of every metal extraction process is directly related to the rate of the chemical reactions that take part in it. In hydrometallurgical processes, working temperatures are lower than those used in pyrometallurgical processes, which implies lower reaction rates and that the limitations found in these processes are mainly kinetic. Because of this, it is of particular importance to know the kinetic models and the mechanisms involved in the leaching process, which explain how the dissolution reaction occurs for extraction of Li.
Lithium is considered a strategic metal whose use has significantly expanded in the last years. Lithium compounds are used in the preparation of lubricants, in the manufacture of glass and special alloys, and also in the pharmaceutical industry, in the production of drugs used in psychiatry. One of the most important uses of Li and its compounds is in the manufacture of energy storage cells for electronic devices and hybrid or electric cars [
1,
2,
3].
Lithium is found in many minerals due to its high chemical reactivity. However, there are few minerals that have been used for the production of lithium compounds. The most important lithium mineral is spodumene, which generally is accompanied by quartz, feldspar, and mica [
2,
4]. Spodumene occurs naturally in α phase, with a monoclinic structure of the pyroxene type. This structure is resistant to the attack of chemical agents, either gaseous or liquid. Spodumene transforms into its β-phase through calcination at 1373 K; this phase is much more reactive and less resistant to ordinary chemical agents. The most common industrial processes for the extraction of lithium from spodumene are acid and alkaline digestion, and the ion exchange method. The products obtained through these methods are lithium carbonate, lithium hydroxide, and lithium chloride, respectively. Acid digestion is carried out with concentrated sulfuric acid at temperatures higher than 523 K, whereas alkaline digestion is carried out with CaCO
3 at 1313 K. In the process of ion exchange, β-spodumene is heated with organic salts of sodium and potassium at 673 K [
2,
4]. The sulfuric acid process has become the main method for the production of lithium carbonate from spodumene due to its high efficiency. However, these methods have intrinsic drawbacks, such as high levels of sulfate and heavy metal ions in the product, sophisticated process for recovering sodium sulfate, and high energy consumption [
3,
4]. Furthermore, in the sulfuric process, 0.95 tons of acid residue are generated for each ton of processed mineral; this indicates that only Li is recovered from β-spodumene, and Al and Si remain as waste.
There have been published relevant findings about the dissolution of β-spodumene in an autoclave at temperatures around 523 K with salts such as Na
2CO
3 and NaCl [
5]. Other recently used routes are the pyrometallurgical chlorination of β-spodumene with Cl
2(g) or by calcination with CaCl
2(s) at temperatures between 1173 and 1373 K [
6,
7].
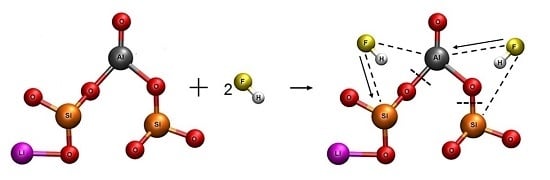
Rosales et al. investigated the leaching process of β-spodumene in hydrofluoric acid medium. They found that the optimal conditions to achieve a lithium extraction higher than 90% were: solid–liquid ratio, 1.82% (
w/
v); temperature, 348 K; HF concentration, 7% (
v/
v), and reaction time, 10 min. Furthermore, they proposed the following dissolution reaction [
7,
8]:
Then, Li, Al, and Si can be recovered as Li2CO3, Na3AlF6, and Na2SiF6, respectively. This process has the advantage that it can be performed at low leaching temperature (<348 K), low HF concentration, and it uses all the elemental components present in the mineral. The lithium recovery efficiency achieved in the process reaches 90%.
There have been published relevant findings about the dissolution kinetics of aluminosilicates (kaolins and feldspars) with HF. Kline and Fogler reported the probable mechanisms by which the dissolution reaction of some aluminosilicates takes place, proposing that the dissolution products are fluorosilicates and fluoroaluminates (SiF
m4−m and AlF
n3−n, where
m and
n: 1, 2, …, 6) of great stability. Moreover, these authors conclude that the rate of dissolution is controlled by the adsorption of HF molecules on the surface of the silicates. The dissolution was catalyzed by strong acids or salts, due to the adsorption of cations or protons over the hydroxyls of the surface of the structure [
9,
10]. Kumar et al. suggested that the dissolution of the aluminosilicates with HF is accompanied by the appearance of fluorinated acid complexes of Al and Si: H
3AlF
6 and H
2SiF
6, respectively [
11,
12].
Therefore, the aim of this study was to present a kinetic study of the dissolution of β-spodumene with HF. This study explains the kinetics and the way in which the dissolution reaction occurs (nature, rate, and dependence of the reaction on variables such as time, temperature, and stirring speed). Along with the chemical kinetics, we determined a mathematical model that yields a good approximation of the way HF behaves when it reacts with the mineral.
2. Materials and Methods
The leaching agent was HF, 40%
w/
w, analytical grade. The mineral used was α-spodumene, extracted from the mine “Las Cuevas”, located in the department of San Martín, San Luis, Argentina. The α-spodumene sample was calcined from room temperature to 1373 K in an electric furnace with a heating rate of 10 K/min in order to transform it into its β-phase, which is more susceptible to acid dissolution [
1,
3]. Characterization of the ore was performed by X-ray fluorescence (XRF) on a Philips PW 1400 instrument (Philips, Amsterdam, The Netherlands) and by X-ray diffraction (XRD) on a Rigaku D-Max III C diffractometer (Rigaku, Osaka, Japan), operated at 35 kV and 30 mA. The Kα radiation of Cu and a filter of Ni, λ = 0.15418 nm were used. Morphological analysis was done by scanning electron microscope (SEM) in a LEO 1450 VP (Zeiss, Jena, Germany) which was equipped with an X-ray dispersive spectrometer EDAX, Genesis 2000, used to determine semi-quantitative composition of the residues obtained through the leaching of the minerals by electron probe microanalysis (EPMA).
Determination of lithium content in the ore was performed by atomic absorption spectroscopy (AAS) using a Varian SpectrAA 55 spectrometer (Palo Alto, CA, USA) with a hollow-cathode lamp (analytical error 1.5%). Previously, the sample (α-spodumene) was dissolved using a concentrated mixture of sulfuric acid and hydrofluoric acid according to the method of Brumbaugh and Fanus [
13]. The bulk composition of the ore is shown in
Table 1, as determined by AAS (Li and Na) and XRF (Si, Al, Fe, Ca, Mg, K, and Ti).
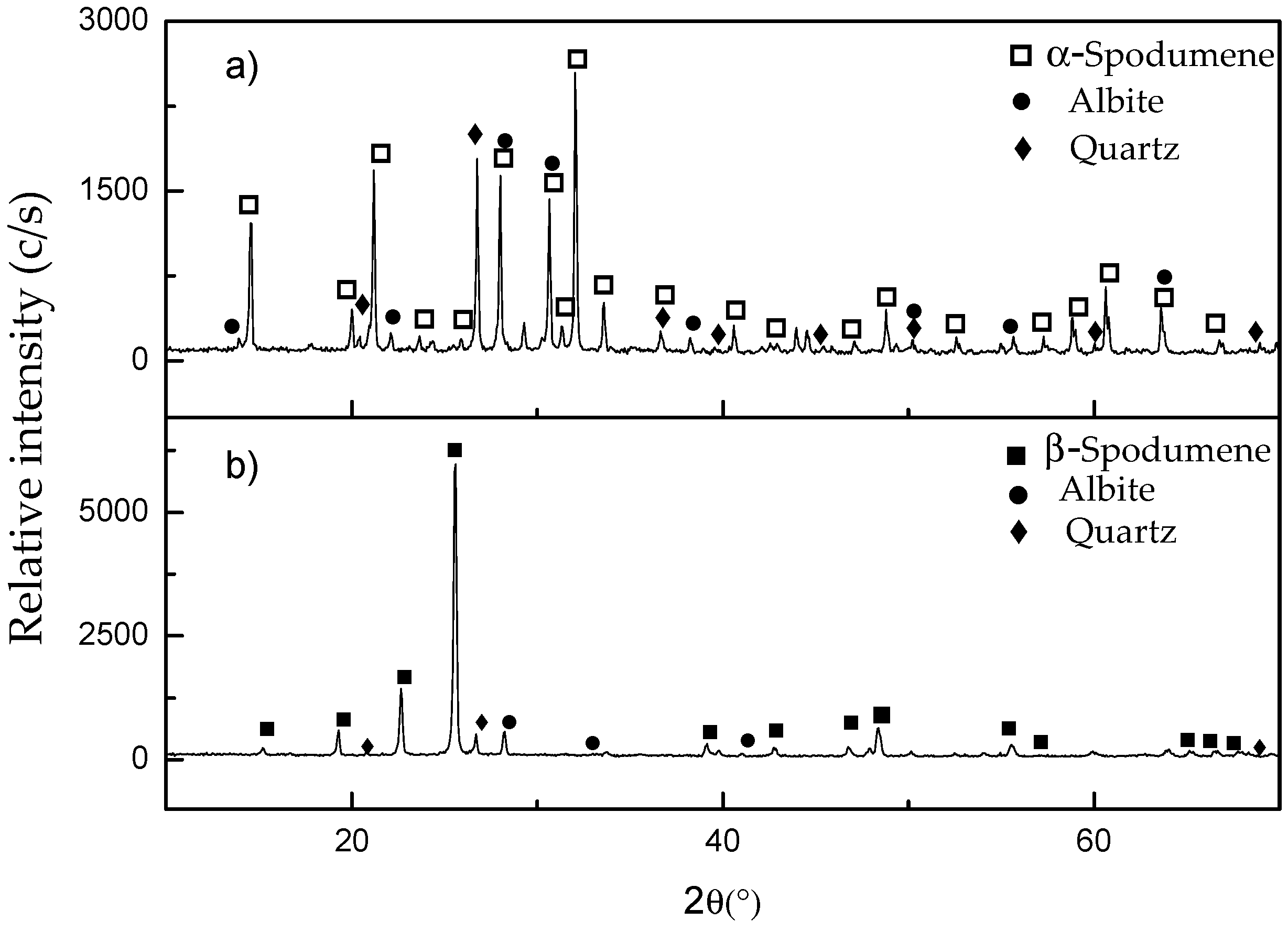
The results of the characterization of the ore by XRD are shown in
Figure 1.
In
Figure 1a, XRD patterns show that the sample is mainly composed of α-spodumene (Joint Committee on Powder Diffraction Standards—JCPDS 33-786), with the presence of albite (International Centre for Diffraction Data—ICDD 96-900-1631) and quartz (JCPDS 33-1161) as gangue. The diffractogram in
Figure 1b shows the appearance of β-spodumene (JCPDS 35-797) after thermal treatment of the ore. The α-phase is not detected, indicating that the transformation of α-phase to β-phase was complete. Albite and quartz were also detected. The quartz content in sample (b) (8%
w/
w) was determined by XRD, using the standard addition method, diffraction line 26.7 degrees of SiO
2 as standard [
8].
In
Figure 2 and
Figure 3, the results of the characterization of the mineral by SEM are shown.
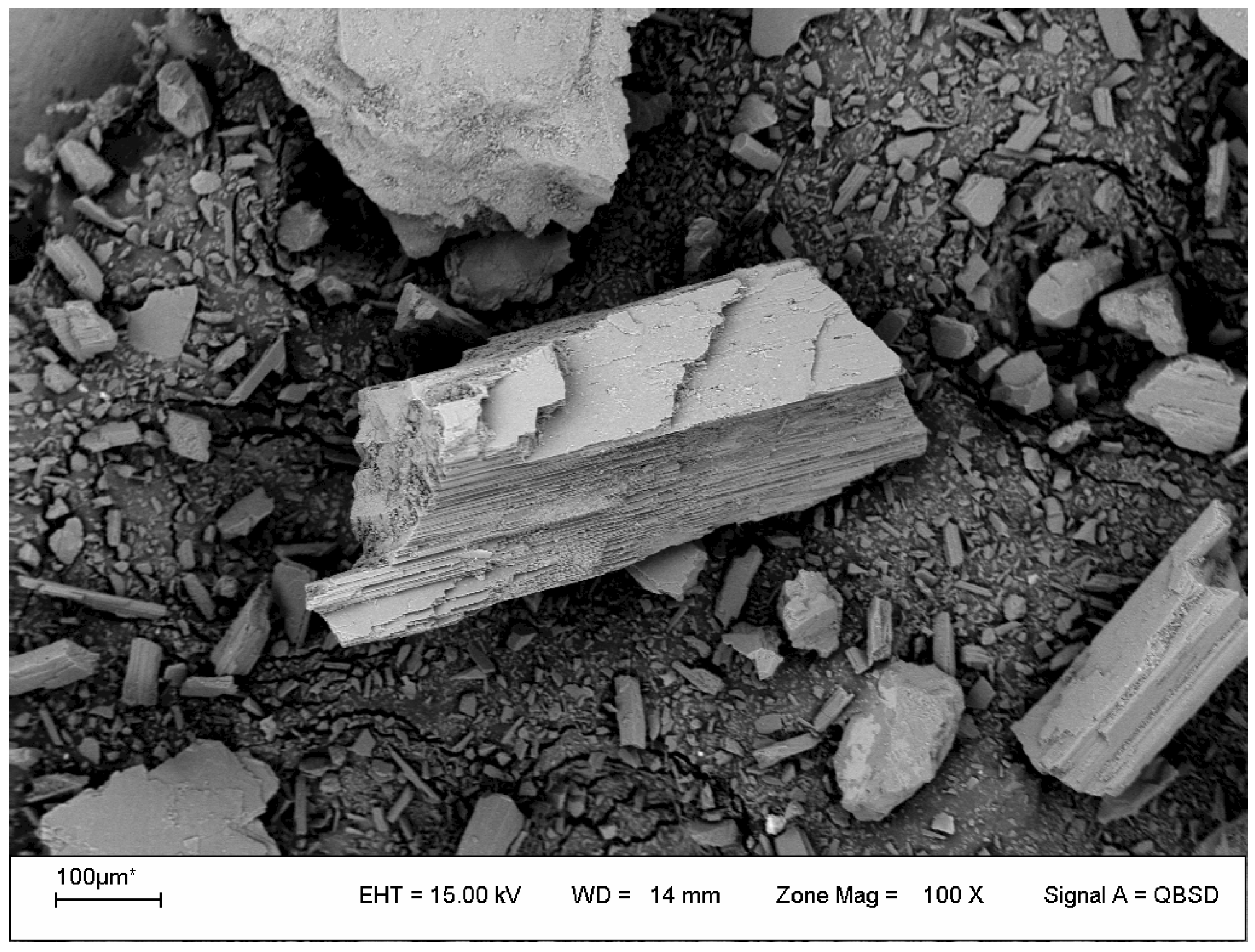
In
Figure 2, the micrograph corresponding to the mineral in its α phase is presented. It can be observed that the particles of the mineral without thermal treatment exhibit an irregular shape and a prismatic structure with a flattened elongated habit, which agrees with that reported in the bibliography [
2,
14].
Figure 3 shows the results of the SEM characterization of the mineral with thermal treatment.
In these micrographs, it can be observed that β-spodumene particles are different from the α-spodumene in their morphology, since no prismatic structures are observed in the β-phase particles. When the α-structure has transformed into the new β-spodumene structure, the increasing stress due to the volumetric expansion leads to the breakdown of the original particles, and therefore to a particle size reduction. This is caused by the thermal treatment of the sample [
14].
The specific surface area of the β-spodumene was determined by Brunauer–Emmett–Teller (BET) with N2 adsorption at 77 K in a Micromeritics Gemini V (Micromeritics, Norcross, GA, USA). The BET analysis showed a low specific area (1.41 m2/g), indicating a low porosity of β-spodumene grains.
Experimental Equipment and Procedure
The experimental tests were performed in a closed 500 mL PVC vessel equipped with magnetic stirring and temperature control systems.
To perform each test, 3 g of ore and 300 mL of distilled water were placed into the reactor. This mixture was heated with stirring until the work temperature was reached. Then, 12 mL of HF (leaching agent) was added, and at that moment, reaction time began to be measured. Once the experiment was finished, the solid was filtered, dried at 348 K, and then weighed. All the experiments were performed in triplicate.
The course of the dissolution reaction was followed by Equation (2):
where
X is the conversion,
mi is the initial mass of the mineral and
mf is the final mass of the residue after the leaching.
4. Conclusions
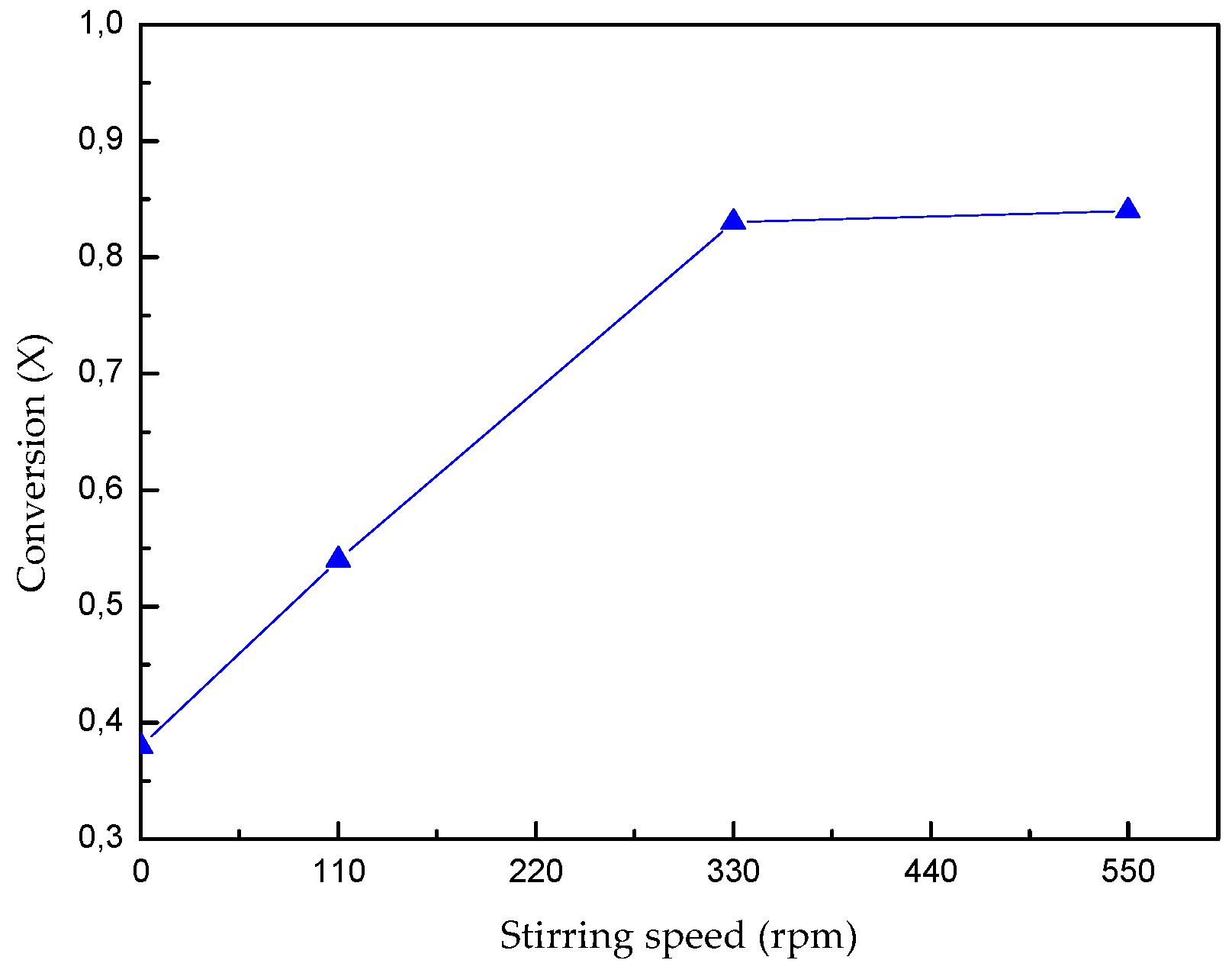
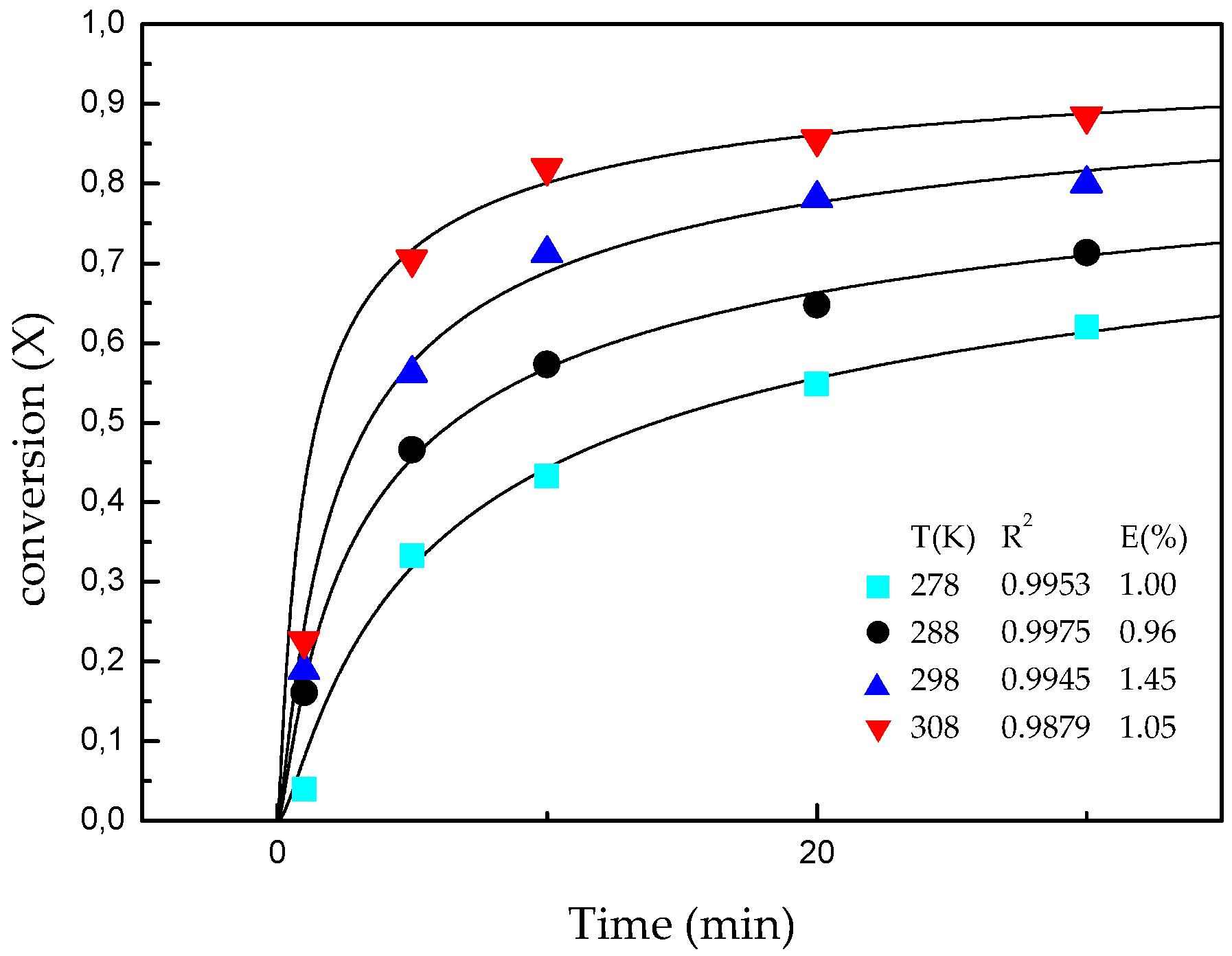
The extraction of lithium through the leaching of β-spodumene with HF is highly dependent on the temperature and reaction time. The stirring speed does not have an important effect on the dissolution of the mineral above 330 rpm. The maximum dissolution of the mineral (88%) is reached in 30 min with 4% v/v HF acid at 308 K with a stirring speed of 330 rpm.
The SEM characterization of the leaching residues indicates that β-spodumene undergoes an irregular located-type attack in preferential sites of the particle, where the reaction develops.
The model based on “nucleation and nuclei growth”, represented by Equation (3), best fits the experimental results of the mineral dissolution in HF media. Moreover, the characterization of the residues physically supports the hypothesis formulated in the kinetic model.
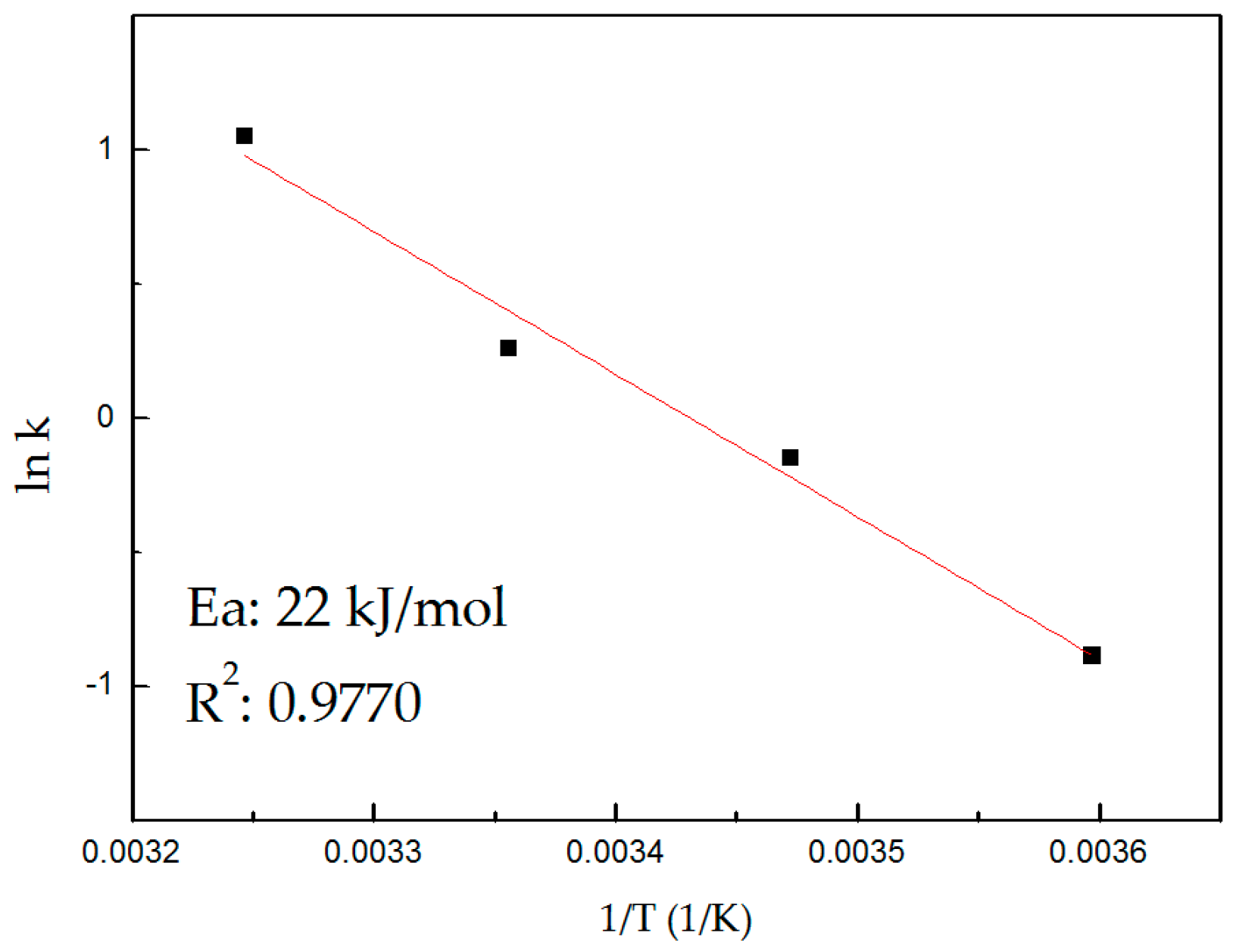
The determined apparent activation energy was 22 kJ/mol in the temperature range of 278–308 K. This Ea value would suggest that the leaching process is controlled by two stages, the formation of the reaction interface (diffusional control) and the chemical reaction (chemical control).