Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flotation Experiments
2.3. Particle-Bubble Stability Study
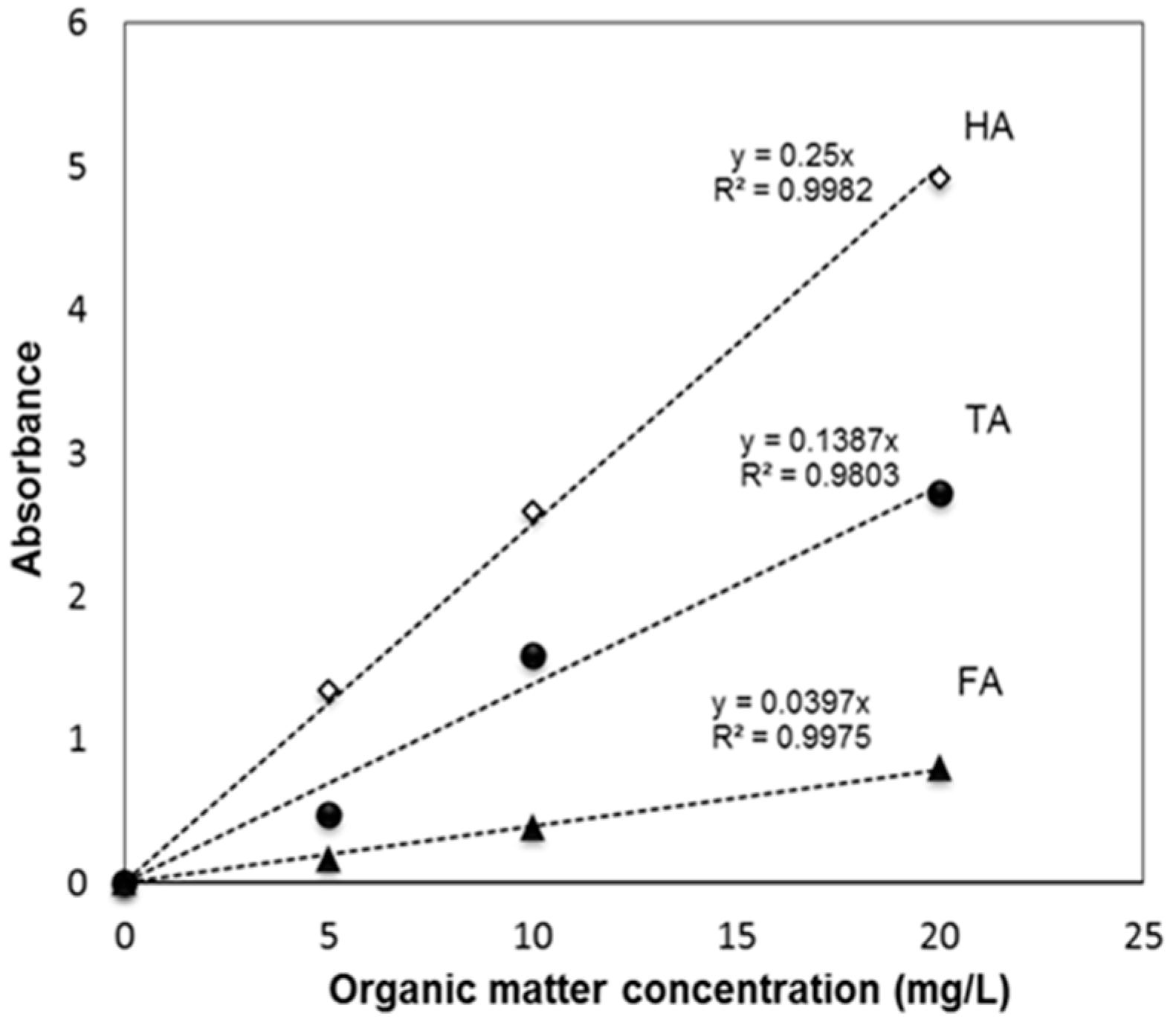
2.4. OM Adsorption Experiments
2.5. XRD Analysis
2.6. XPS Analysis
3. Results
3.1. Flotation of Molybdenite and Chalcopyrite in Single Mineral Experiments
3.2. Effect of Organic Materials on the Flotation of Copper and Molybdenum in a Sulphide Cu–Mo Ore
3.3. Adsorption of Organic Materials on Molybdenite and Chalcopyrite
3.4. Critical Amplitude, Detachment Force and Contact Angle
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Thurman, E.M. Organic Geochemistry of Natural Waters; Springer: Berlin, Germany, 1985. [Google Scholar]
- Manahan, S.E. Environmental Chemistry; Taylor & Francis Group: New York, NY, USA, 2010. [Google Scholar]
- Davis, J.A. Adsorption of natural dissolved organic matter at the oxide/water interface. Geochim. Cosmochim. Acta 1982, 46, 2381–2393. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Use of organic polymers in the flotation of polymetallic ores: A review. Miner. Eng. 1999, 12, 341–354. [Google Scholar] [CrossRef]
- Rao, S.; Finch, J. A review of water re-use in flotation. Miner. Eng. 1989, 2, 65–85. [Google Scholar] [CrossRef]
- Muzenda, E. An investigation into the effect of water quality on flotation performance. World Acad. Sci. Eng. Technol. 2010, 4, 237–241. [Google Scholar]
- Levay, G.; Smart, R.; Skinner, W. The impact of water quality on flotation performance. J. S. Afr. Inst. Min. Metall. 2001, 101, 69–75. [Google Scholar]
- Hughes, C. Enhanced Flotation Reagents for Beneficiation of Phosphate Ores. U.S. Patent 5962828 A, 21 November 2000. [Google Scholar]
- Liu, G.; Lu, Y.; Zhong, H.; Cao, Z.; Xu, Z. A novel approach for preferential flotation recovery of molybdenite from a porphyry copper-molybdenum ore. Miner. Eng. 2012, 36–38, 37–44. [Google Scholar] [CrossRef]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part I. Adsortion studies. Miner. Eng. 2007, 20, 600–608. [Google Scholar] [CrossRef]
- Herrera-Urbina, R. Recent developments and advances in formulations and applications of chemical reagents used in froth flotation. Miner. Process. Extr. Metall. Rev. 2003, 24, 139–182. [Google Scholar] [CrossRef]
- Hoover, M. Water chemistry effects in the flotation of sulphide ores a review and discussion for molybdenite. In Complex Sulphide Ores; Institute of Mining and Metal: London, UK, 1980; pp. 100–112. [Google Scholar]
- Lai, R.W.M.; Stone, L.C.; Rimmasch, B.E. Effect of humus organics on the flotation recovery of molybdenite. Int. J. Miner. Process. 1984, 12, 163–172. [Google Scholar] [CrossRef]
- Tipping, E.; Cooke, D. The effects of adsorbed humic substances on the surface charge of goethite (α-FeOOH) in freshwaters. Geochim. Cosmochim. Acta 1982, 46, 75–80. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W. Multi-scale investigation of applying secondary effluent in sulfide flotation. In Water in Mineral Processing; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2012; pp. 279–290. [Google Scholar]
- Ranney, M.W. Copper sulfide ores. In Flotation Agents and Processes: Technology and Applications; Noyes Data Corp: Park Ridge, NJ, USA, 1980; pp. 28–33. [Google Scholar]
- Fisher, W.; Rudy, S. Municipal waste water utilization for froth flotation of copper and molybdenum sulfides. Soc. Min. Eng. AIME 1976, 264, 698–1702. [Google Scholar]
- Kelebek, S.; Yoruk, S.; Smith, G.W. Wetting behavior of molybdenite and talc in lignosulphonate/MIBC solutions and their separation by flotation. Sep. Sci. Technol. 2001, 36, 145–157. [Google Scholar] [CrossRef]
- Fuerstenau, D. Interfacial process in mineral/water systems. Pure Appl. Chem. 1970, 24, 135–164. [Google Scholar] [CrossRef]
- Liu, W.; Moran, C.; Vink, S. Flotation of Chalcopyrite in Water Containing Bacteria. In Water in Mineral Processing; Society for Mining, Metallurgy and Exploration: Englewood, CO, USA, 2012; pp. 165–173. [Google Scholar]
- Somasundaran, P. Encyclopedia of Surface and Colloid Science, 5th ed.; Taylor & Francis: New York, NY, USA, 2006; Volume 1. [Google Scholar]
- Espinoza-Gomez, R.; Finch, J.; Laplante, A. Effects of the type of water on the selective flotation of pyrochlore from Niobec. Colloids Surf. 1987, 26, 333–350. [Google Scholar] [CrossRef]
- Schumanm, R.; Levay, G.; Dunne, R.; Hart, S. Managing process water quality in base metal sulfide flotation. In Proceeding of Water in Mining; Scott, A., Mckee, D., Eds.; Australasian Institute of Mining and Metallurgy: Carlton, Australia, 2003. [Google Scholar]
- Santhiya, D.; Subramanian, S.; Natarajan, K.A. Surface chemical studies on sphalerite and salena using bacillus polymyxa II. Mechanisms of microbe-mineral interactions. J. Colloid Interface Sci. 2001, 235, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Bozo, L.; Higueras, P.; Godoy-Faundez, A.; Sobarzo, F.; Saez-Navarrete, C.; Vasquez-Bestagno, J.; Herrera-Urbina, R. Assessment of the floatability of chalcopyrite, molybdenite and pyrite using biosolids and their main components as collectors for greening the froth flotation of copper sulphide ores. Miner. Eng. 2014, 64, 38–43. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Herrera-Urbina, R.; Escudey, M.; Godoy-Faundez, A.; Sáez-Navarrete, C. Role of biosolids on hydrophobic properties of sulfide ores. Int. J. Miner. Process. 2011, 100, 124–129. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Herrera-Urbina, R.; Saez-Navarrete, C.; Otero, A.F.; Godoy-Faundez, A.; Ginocchio, R. Rougher flotation of copper sulphide ore using biosolids and humic acids. Miner. Eng. 2011, 24, 1603–1608. [Google Scholar] [CrossRef]
- Aiken, G.; McKnight, D.; Wershaw, R.; MacCarthy, P. Humic Substances in Soil, Sediment and Water; Wiley: New York, NY, USA, 1985. [Google Scholar]
- Kononova, M. Soil Organic Matter; Pergamon: Oxford, UK, 1966. [Google Scholar]
- Xu, D.; Ametov, I.; Grano, S. Detachment of coarse particles from oscillating bubbles-the effect of particle contact angle, shape and medium viscosity. Int. J. Miner. Process. 2011, 101, 50–57. [Google Scholar] [CrossRef]
- Holtham, P.N.; Cheng, T. Study of probability of detachment of particles from bubbles in flotation. Trans. Inst. Min. Metall. 1991, 100, C147–C153. [Google Scholar]
- Cheng, T.; Holthman, P. The particle detachment process in flotation. Miner. Eng. 1995, 8, 883–891. [Google Scholar] [CrossRef]
- Nutt, C.W. Froth flotation: The adhesion of solid particles to flat interfaces and bubbles. Chem. Eng. Sci. 1960, 12, 133–141. [Google Scholar] [CrossRef]
- Wagner, C.D. Photoelectron and auger energies and the auger parameters: A data set. In Practical Surface Analysis; Briggs, D., Seah, M.P., Eds.; Wiley: New York, NY, USA, 1994; pp. 595–634. [Google Scholar]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Effect of oxidation on the collectorless flotation of chalcopyrite. Int. J. Miner. Process. 1997, 49, 31–48. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Miner. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Lazghab, M.; Saleh, K.; Pezron, I.; Guigon, P.; Komunjer, L. Wettability assesment of finely divided solids. Powder Technol. 2005, 157, 79–91. [Google Scholar] [CrossRef]
- Subrahmanyam, T.V.; Prestidge, C.A.; Ralston, J. Contact angle and surface analysis studies of sphalerite particles. Miner. Eng. 1996, 9, 727–741. [Google Scholar] [CrossRef]
- Diggins, D.; Ralston, J. Particle wettability by equilibrium capillary pressure measurements. Coal Prep. 1993, 13, 1–19. [Google Scholar] [CrossRef]
- Diggins, D.; Fokkink, L.; Ralston, J. The wetting of angular quartz particles: Capillary pressure and contact angles. Colloids Surf. 1990, 44, 299–313. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Critical copper concentration in sphalerite flotation: Effect of temperature and collector. Int. J. Miner. Process. 2016, 146, 15–22. [Google Scholar] [CrossRef]
- Wang, W.; Fornasiero, D. Flotation of composite synthetic particles. In Proceedings of the 25th International Mineral Processing Congress, Brisbane, Australia, 6–10 September 2010; pp. 2503–2511.
- Rath, R.K.; Subramanian, S.; Pradeep, T. Surface Chemical Studies on Pyrite in the Presence of Polysaccharide-Based Flotation Depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Fornasiero, D.; Ralston, J. Use of synthetic polymers for the depression of iron sulfide minerals in zinc and copper ores. In Proceeding of the 22nd International Mineral Processing Congress, Cape Town, South Africa, 28 September–3 October 2003; pp. 807–815.
- Santhiya, D.; Nandini, G.; Subramanian, S.; Natarajan, K.; Malghan, S. Effect of polymer molecular weight on the absorption of polyacrylic acid at the alumina-water interface. Colloids Surf. 1998, 133, 157–163. [Google Scholar] [CrossRef]
- Koopal, L. Colloid Chemistry in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Ochs, M.; Cosovic, B.; Stumm, W. Coordinative and hydrophobic interaction of humic substances with hydrophilic Al2O3 and hydrophobic mercury surfaces. Geochim. Cosmochim. Acta 1994, 58, 639–650. [Google Scholar] [CrossRef]
- Jardine, P.M.; McCarthy, J.F.; Weber, N.L. Mechanisms of dissolved organic carbon adsorption on soil. Soil Sci. Soc. Am. J. 1989, 53, 1378–1385. [Google Scholar] [CrossRef]
- Gu, B.; Schmitt, J.; Chen, Z.; Liang, L.; McCarthy, J. Adsorption and desorption of natural organic matter on iron oxide: Mechanisms and models. Environ. Sci. Technol. 1994, 28, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.M.; Zachara, J.M.; Smith, S.C.; Phillips, J.L.; Wietsma, T.W. Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ. Sci. Technol. 1994, 28, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.B.; Choppin, G.R. Kinetic of rare earth metal binding to aquatic humic acids. In Chemical Modelling of Aqueous System II; American Chemical Society: Washington, DC, USA, 1990; pp. 519–525. [Google Scholar]
- Chiou, C.T.; Malcolm, R.L.; Brinton, T.I.; Kile, D.E. Water solubility enhancement of some organic pollutants and pesticides by dissolved humic and fulvic acids. Environ. Sci. Technol. 1986, 20, 502–508. [Google Scholar] [CrossRef] [PubMed]
- White, W. Geochemistry; Wiley-Blackwell: Sussex, UK, 2013. [Google Scholar]
- Stevenson, F. Humus chemistry: Genesis, compositions, reactions. In Humus Chemistry; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Ansari, A. The Effect of Lignosulfonates on the Flotability of Molybdenite and Chalcopyrite. Ph.D. Thesis, University of British Columbia, Vancouver, BC, Canada, 2006. [Google Scholar]
- Ravishankar, S.; Khosla, N.K. Selective flocculation of iron oxide from its synthetic mixtures with clays: A comparison of polyacrylic acid and starch polymers. Int. J. Miner. Process. 1995, 43, 235–247. [Google Scholar] [CrossRef]
- Laskowski, J.; Liu, Q.; O’Connor, C.T. Current understanding of the mechanism of polysaccharide adsorption at the mineral/aqueous solution interface. Int. J. Miner. Process. 2007, 84, 59–68. [Google Scholar] [CrossRef]
- Rath, R.K.; Subramanian, S.; Sivanandam, V. Studies on the interaction of guar gum with chalcopyrite. Can. Metall. Q. 2001, 40, 1–12. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Escudey, M.; Vyhmeister, E.; Higueras, P.; Godoy-Faundez, A.; Salazar, J.; Valdes-Gonzalez, H.; Wolf-Sepulveda, G.; Herrera-Urbina, R. Adsorption of biosolids and their main components on chalcopyrite, molybdenite and pyrite: Zeta potential and FTIR spectroscopy studies. Miner. Eng. 2015, 78, 128–135. [Google Scholar] [CrossRef]
- Ewald, M.; Berger, P.; Visser, S. UV-Visible adsorption and fluorescence properties of fulvic acids of microbial origin as functions of their molecular weights. Geoderma 1988, 43, 11–20. [Google Scholar] [CrossRef]
- Ansari, A.; Pawlik, M. Floatability of chalcopyrite and molybdenite in the presence of lignosulfonates. Part II. Hallimond tube flotation. Miner. Eng. 2007, 20, 609–616. [Google Scholar] [CrossRef]




| Sample | Humic Acid (HA) | Tannic Acid (TA) | Fulvic Acid (FA) |
|---|---|---|---|
| MW (g/mol) | ~2000 | 1701 | 500–1000 |
| C (mg/L) | 592 | 510 | 340 |
| OM | % Recovery | ||||
|---|---|---|---|---|---|
| Chalcopyrite | Quartz | Kaolin | Kornelite | Pyrite | |
| N/O | 74 | 23 | 77 | 52 | 10 |
| HA | 64 | 28 | 99 | 78 | 20 |
| FA | 63 | 27 | 83 | 66 | 17 |
| Mineral | Condition | C | O | Cu | Mo | Fe | S | N |
|---|---|---|---|---|---|---|---|---|
| Chalcopyrite | No HA | 42 | 25 | 7 | - | 4 | 22 | 0 |
| With HA | 43 | 23 | 7 | - | 4 | 21 | 2 | |
| Molybdenite | No HA | 28 | 22 | - | 21 | - | 29 | 0 |
| With HA | 29 | 17 | - | 18 | - | 27 | 8 |
| Collector/OM g/t | Chalcopyrite | Molybdenite | ||||
|---|---|---|---|---|---|---|
| Amplitude, mm ± 0.02 mm | Fdetachment (N) | Contact Angle | Amplitude, mm ± 0.02 mm | Fdetachment (N) | Contact Angle | |
| SIPX, 0 | 0.56 | 8.17 × 10−6 | 36 | 3.48 | 5.12 × 10−5 | 99 |
| SIPX, 2 | 1.16 | 1.59 × 10−5 | 50 | - | - | - |
| SIPX, 2; HA, 10 | 1.16 | 1.59 × 10−5 | 50 | - | - | - |
| SIPX, 2; HA, 20 | 1.15 | 1.58 × 10−5 | 50 | - | - | - |
| HA, 10 | - | - | - | 2.02 | 3.02 × 10−5 | 72 |
| HA, 20 | - | - | - | 1.02 | 1.58 × 10−5 | 50 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinche-Gonzalez, M.; Fornasiero, D.; Zanin, M. Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water. Minerals 2016, 6, 105. https://doi.org/10.3390/min6040105
Sinche-Gonzalez M, Fornasiero D, Zanin M. Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water. Minerals. 2016; 6(4):105. https://doi.org/10.3390/min6040105
Chicago/Turabian StyleSinche-Gonzalez, Maria, Daniel Fornasiero, and Massimiliano Zanin. 2016. "Flotation of Chalcopyrite and Molybdenite in the Presence of Organics in Water" Minerals 6, no. 4: 105. https://doi.org/10.3390/min6040105





