Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Growth Conditions
2.2. Preparation of Chalcopyrite Coupons
2.3. The Operation of the Atomic Force Microscope
2.4. Zeta Potential and Contact Angle Measurements
2.5. FTIR Measurements
2.6. Acid-Base Titration
2.7. Adhesion Test
3. Results and Discussion
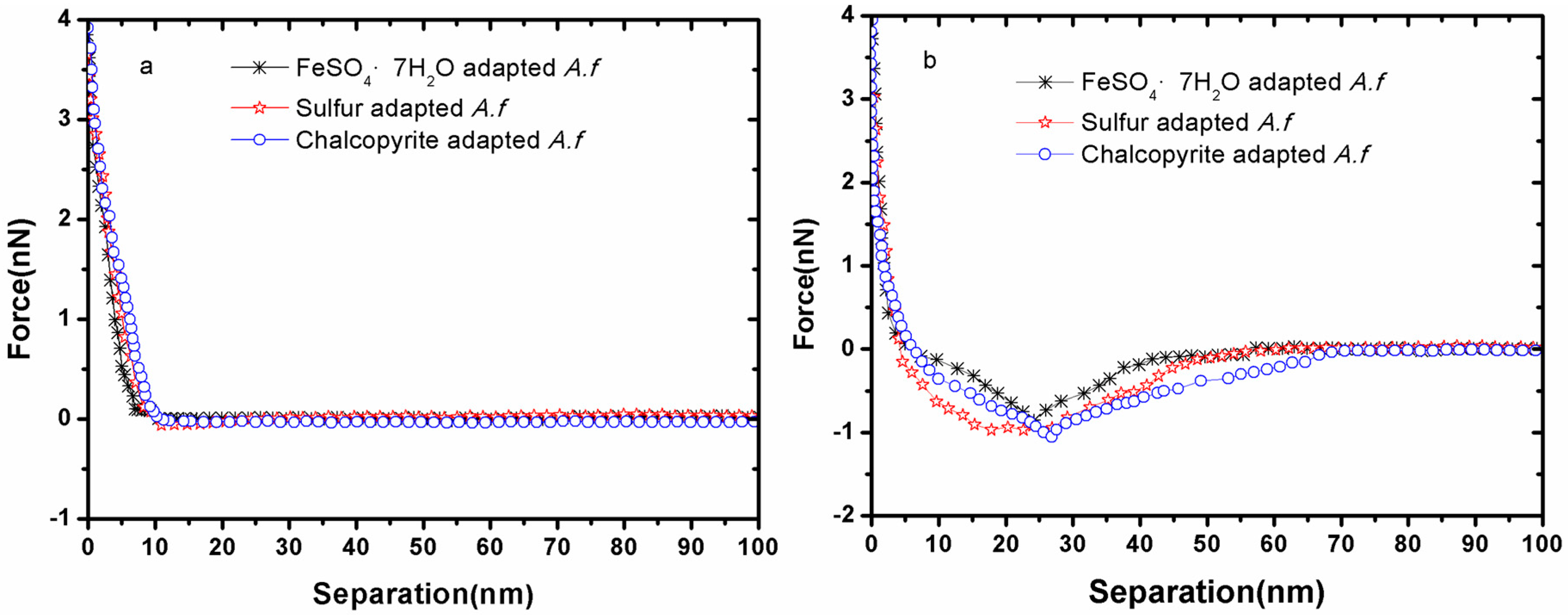
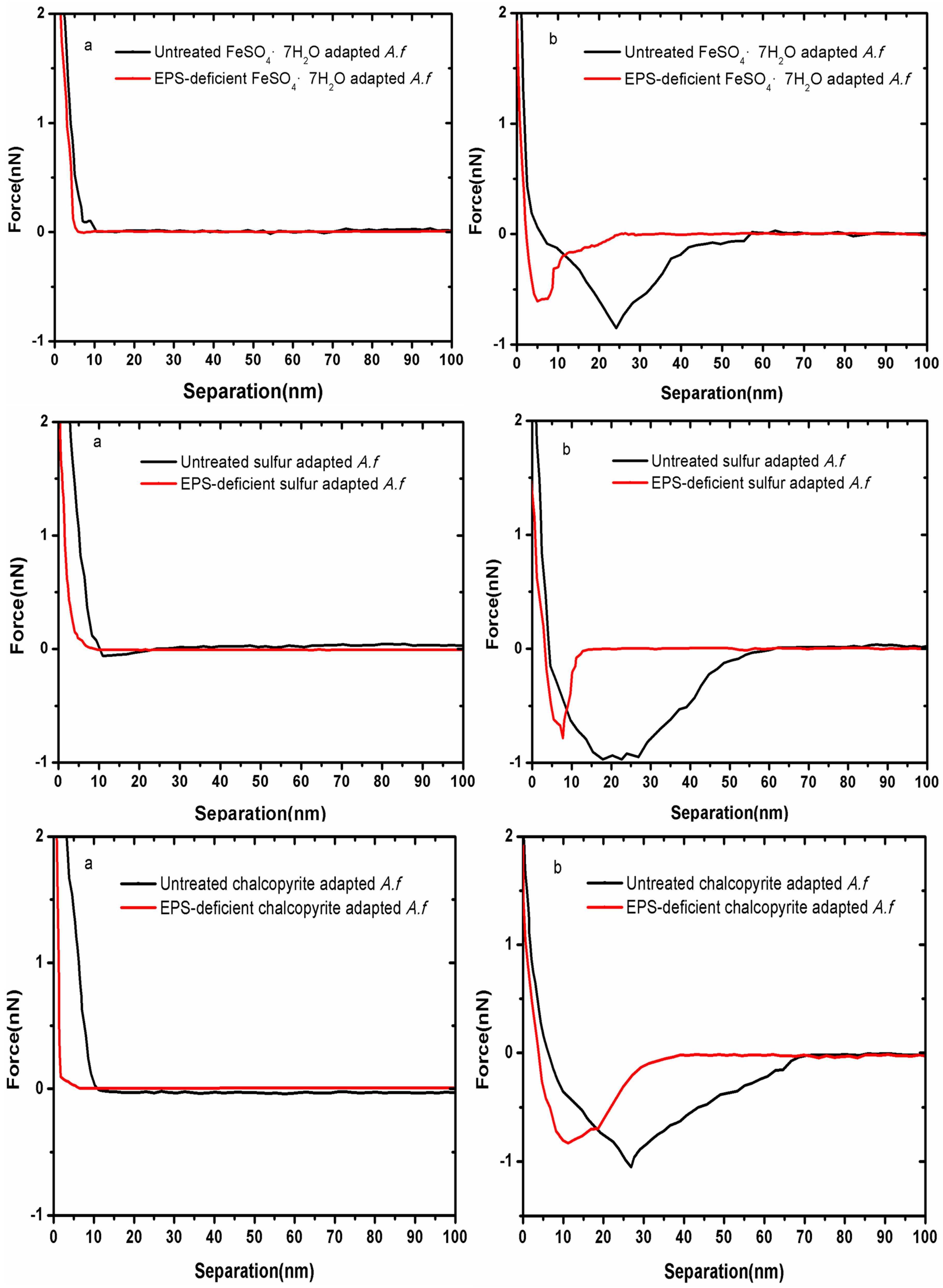
3.1. Forces between Different Cell Tips and Chalcopyrite before and after EPS Removal
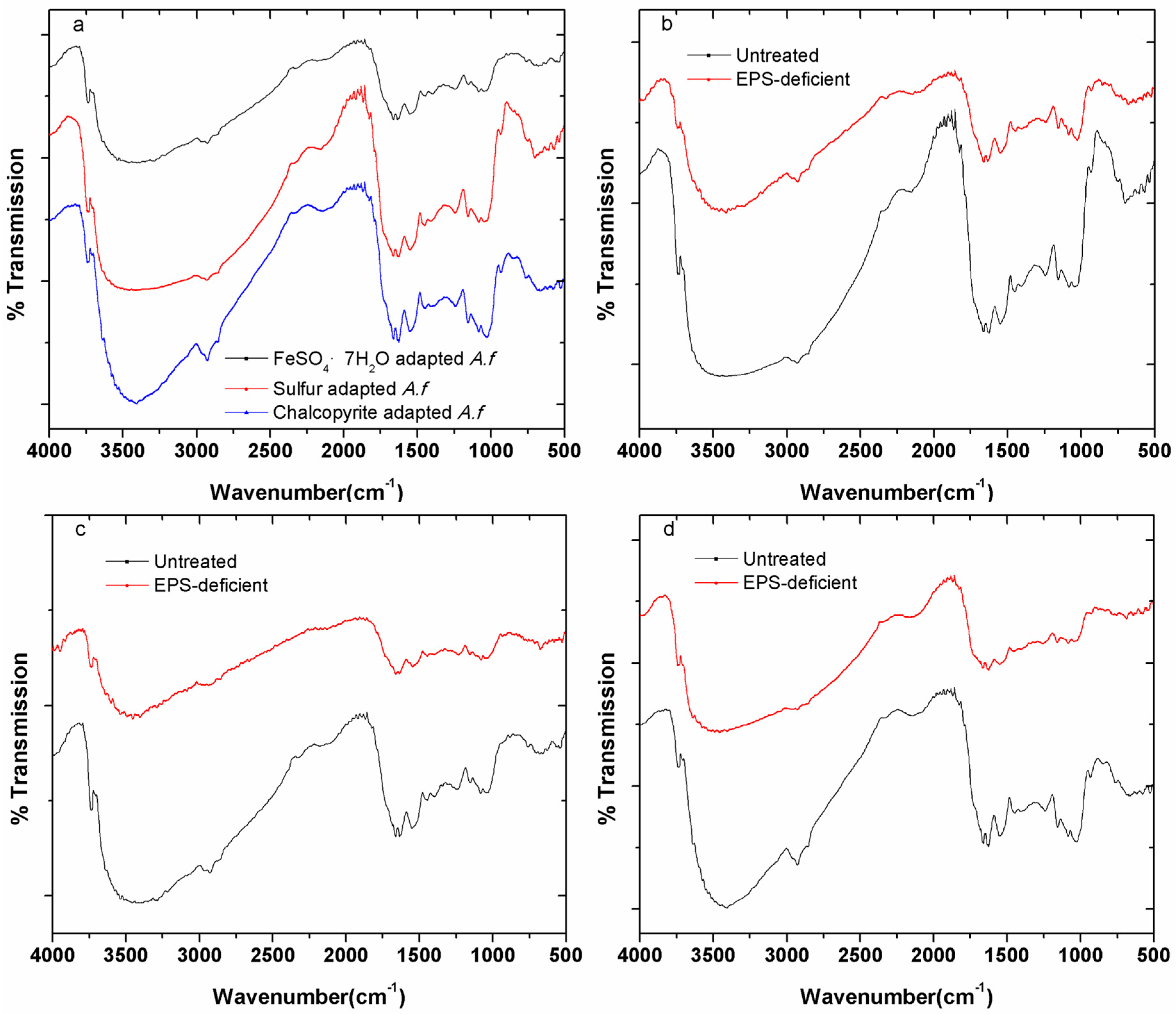
3.2. Fourier Transform Infrared (FTIR) Spectroscopy
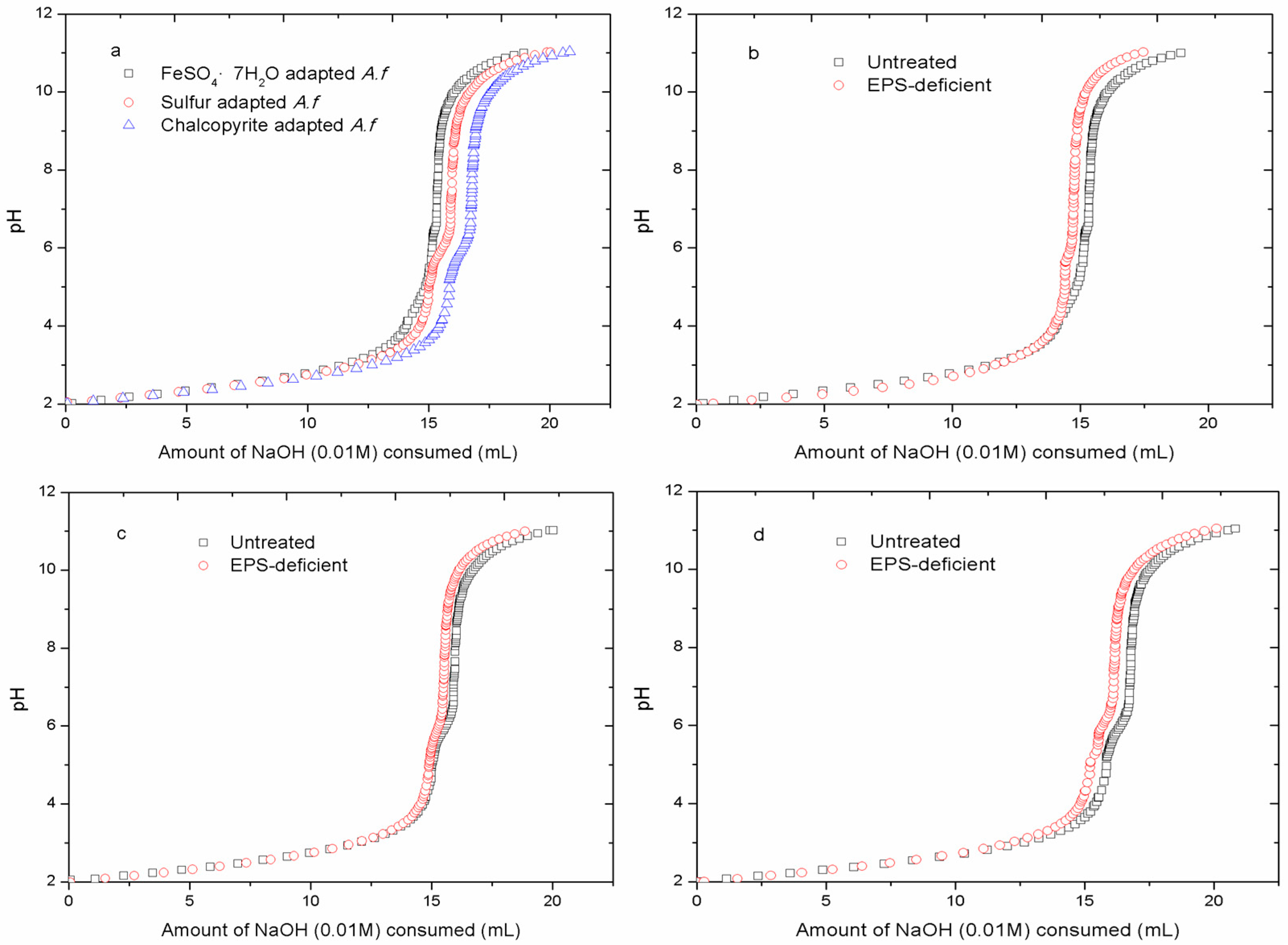
3.3. Acid-Base Titration
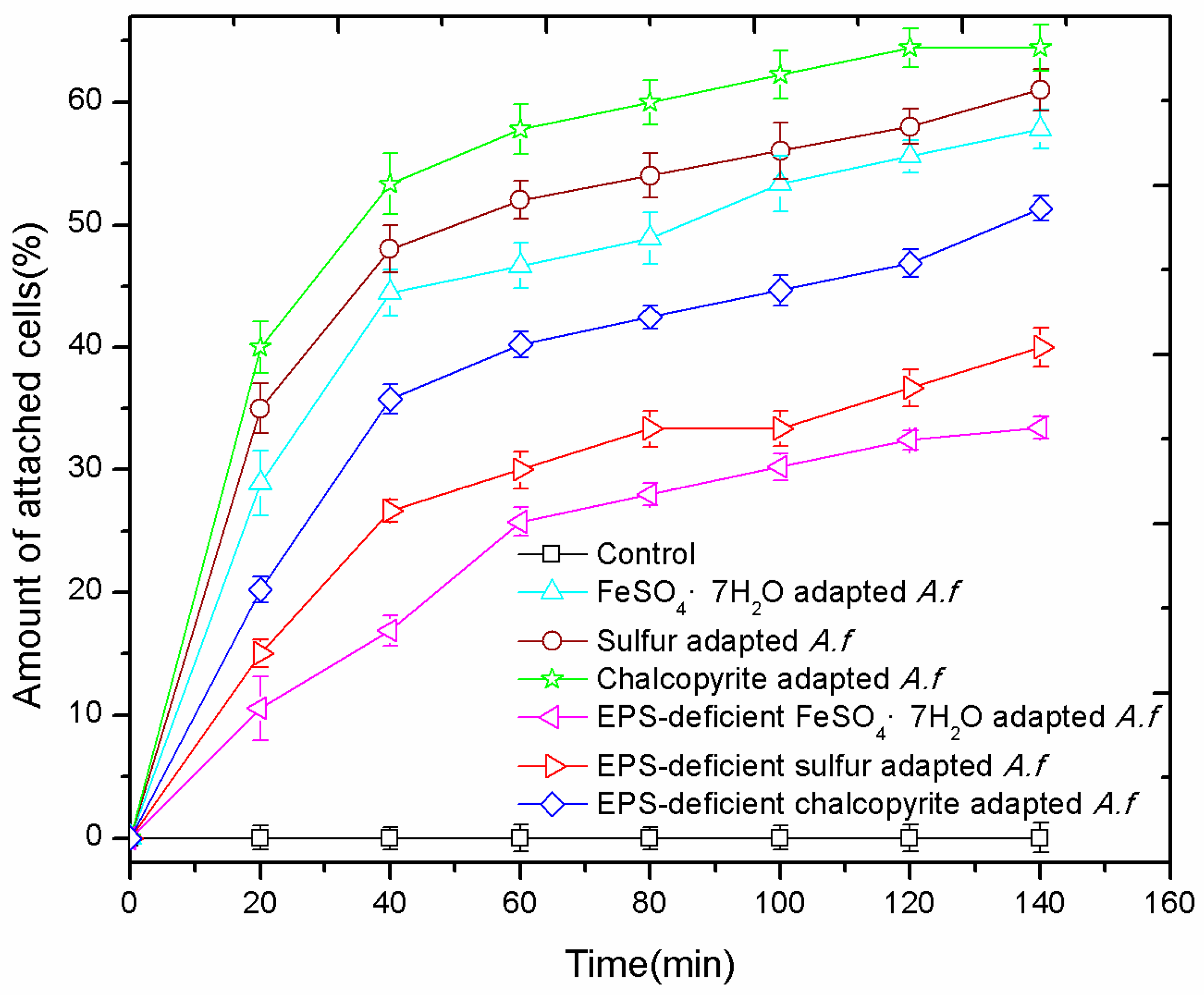
3.4. Adhesion Curves
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Africa, C.J.; van Hille, R.P.; Harrison, S.T. Attachment of Acidithiobacillus ferrooxidans and Leptospirillum ferriphilum cultured under varying conditions to pyrite, chalcopyrite, low-grade ore and quartz in a packed column reactor. Appl. Microbiol. Biotechnol. 2013, 97, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.N.; Chen, M. Early stage adsorption behaviour of Acidithiobacillus ferrooxidans on minerals I: An experimental approach. Hydrometallurgy 2012, 119–120, 87–94. [Google Scholar] [CrossRef]
- Diao, M.; Nguyen, T.A.H.; Taran, E.; Mahler, S.; Nguyen, A.V. Differences in adhesion of A. thiooxidans and A. ferrooxidans on chalcopyrite as revealed by atomic force microscopy with bacterial probes. Miner. Eng. 2014, 61, 9–15. [Google Scholar] [CrossRef]
- Yee, N.; Fein, J.B.; Daughney, C.J. Experimental study of the pH, ionic strength, and reversibility behavior of bacteria–mineral adsorption. Geochim. Cosmochim. Acta 2000, 64, 609–617. [Google Scholar] [CrossRef]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of extracellular polymeric substances from Thiobacillus ferrooxidans for bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Usher, K.M.; Shaw, J.A.; Kaksonen, A.H.; Saunders, M. Elemental analysis of extracellular polymeric substances and granules in chalcopyrite bioleaching microbes. Hydrometallurgy 2010, 104, 376–381. [Google Scholar] [CrossRef]
- Wei, X.; Fang, L.C.; Cai, P.; Huang, Q.Y.; Chen, H.; Liang, W.; Rong, X.M. Influence of extracellular polymeric substances (EPS) on Cd adsorption by bacteria. Environ. Pollut. 2011, 159, 1369–1374. [Google Scholar] [CrossRef] [PubMed]
- Devasia, P.; Natarajan, K.A.; Sathyanarayana, D.N.; Rao, G.R. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces. Appl. Environ. Microbiol. 1993, 59, 4051–4055. [Google Scholar] [PubMed]
- Sharma, P. Surface characterization of Acidithiobacillus ferrooxidans cells grown under different conditions. Hydrometallurgy 2003, 71, 285–292. [Google Scholar] [CrossRef]
- Xia, L.X.; Liu, X.X.; Zeng, J.; Yin, C.; Gao, J.; Liu, J.S.; Qu, G.Z. Mechanism of enhanced bioleaching efficiency of Acidithiobacillus ferrooxidans after adaptation with chalcopyrite. Hydrometallurgy 2008, 92, 95–101. [Google Scholar] [CrossRef]
- Kinzler, K.; Gehrke, T.; Telegdi, J.; Sand, W. Bioleaching—A result of interfacial processes caused by extracellular polymeric substances (EPS). Hydrometallurgy 2003, 71, 83–88. [Google Scholar] [CrossRef]
- Zeng, W.M.; Qiu, G.Z.; Zhou, H.B.; Liu, X.D.; Chen, M.; Chao, W.L.; Zhang, C.G.; Peng, J.H. Characterization of extracellular polymeric substances extracted during the bioleaching of chalcopyrite concentrate. Hydrometallurgy 2010, 100, 177–180. [Google Scholar] [CrossRef]
- Sharma, P.K.; Rao, K.H. Adhesion of Paenibacillus polymyxa on chalcopyrite and pyrite: Surface thermodynamics and extended DLVO theory. Colloids Surf. B Biointerfaces 2003, 29, 21–38. [Google Scholar] [CrossRef]
- Thwala, J.M.; Li, M.; Wong, M.C.; Kang, S.; Hoek, E.M.; Mamba, B.B. Bacteria–polymeric membrane interactions: Atomic force microscopy and XDLVO predictions. Langmuir 2013, 29, 13773–13782. [Google Scholar] [CrossRef] [PubMed]
- Harimawan, A.; Zhong, S.P.; Lim, C.T.; Ting, Y.P. Adhesion of B. subtilis spores and vegetative cells onto stainless steel—DLVO theories and AFM spectroscopy. J. Colloid Interface Sci. 2013, 405, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Farahat, M.; Hirajima, T.; Sasaki, K. Adhesion of Ferroplasma acidiphilum onto pyrite calculated from the extended DLVO theory using the van Oss–Good–Chaudhury approach. J. Colloid Interface Sci. 2010, 349, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Rijnaarts, H.H.M.; Norde, W.; Lyklema, J.; Zehnder, A.J.B. DLVO and steric contributions to bacterial deposition in media of different ionic strengths. Colloids Surf. B Biointerfaces 1999, 14, 179–195. [Google Scholar] [CrossRef]
- Zhu, J.; Li, Q.; Jiao, W.; Jiang, H.; Sand, W.; Xia, J.; Liu, X.; Qin, W.; Qiu, G.; Hu, Y.; et al. Adhesion forces between cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans or Leptospirillum ferrooxidans and chalcopyrite. Colloids Surf. B Biointerfaces 2012, 94, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.R.; Lovitt, R.W.; Wright, C.J. Direct quantification of Aspergillus niger spore adhesion to mica in air using an atomic force microscope. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 205–210. [Google Scholar] [CrossRef]
- Boonaert, C.J.P.; Dufrene, Y.F.; Derclaye, S.R.; Rouxhet, P.G. Adhesion of Lactococcus lactis to model substrata: Direct study of the interface. Colloids Surf. B Biointerfaces 2001, 22, 171–182. [Google Scholar] [CrossRef]
- Chandraprabha, M.N.; Somasundaran, P.; Natarajan, K.A. Modeling and analysis of nanoscale interaction forces between Acidithiobacillus ferrooxidans and AFM tip. Colloids Surf. B Biointerfaces 2010, 75, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Senechal, A.; Carrigan, S.D.; Tabrizian, M. Probing surface adhesion forces of Enterococcus faecalis to medical-grade polymers using atomic force microscopy. Langmuir 2004, 20, 4172–4177. [Google Scholar] [CrossRef] [PubMed]
- Sheng, X.; Ting, Y.P.; Pehkonen, S.O. Force measurements of bacterial adhesion on metals using a cell probe atomic force microscope. J. Colloid Interface Sci. 2007, 310, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wang, Q.; Zhou, S.; Li, Q.; Gan, M.; Jiang, H.; Qin, W.; Liu, X.; Hu, Y.; Qiu, G. Insights into the relation between adhesion force and chalcopyrite-bioleaching by Acidithiobacillus ferrooxidans. Colloids Surf. B Biointerfaces 2015, 126, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Diao, M.; Nguyen, T.A.; Taran, E.; Mahler, S.M.; Nguyen, A.V. Effect of energy source, salt concentration and loading force on colloidal interactions between Acidithiobacillus ferrooxidans cells and mineral surfaces. Colloids Surf. B Biointerfaces 2015, 132, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Tourney, J.; Ngwenya, B.T.; Mosselmans, J.W.F.; Tetley, L.; Cowie, G.L. The effect of extracellular polymers (EPS) on the proton adsorption characteristics of the thermophile Bacillus licheniformis S-86. Chem. Geol. 2008, 247, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.D.; Li, J.Y.; Jiang, J.; Hong, Z.N.; Xu, R.K. Adhesion of Escherichia coli to nano-Fe/Al oxides and its effect on the surface chemical properties of Fe/Al oxides. Colloids Surf. B Biointerfaces 2013, 110, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Lower, S.K.; Hochella, M.F.; Beveridge, T.J. Bacterial recognition of mineral surfaces: Nanoscale interactions between Shewanella and α-FeOOH. Science 2001, 292, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Wan, D.S.; Huang, S.S.; Leng, F.F.; Yan, L.; Ni, Y.Q.; Li, H.Y. Type IV pili of Acidithiobacillus ferrooxidans are necessary for sliding, twitching motility, and adherence. Curr. Microbiol. 2010, 60, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Wang, F.; Li, J.; Sha, J.; Lu, X.; Han, X. Analysis of genes and proteins in Acidithiobacillus ferrooxidans during growth and attachment on pyrite under different conditions. Geomicrobiol. J. 2013, 30, 255–267. [Google Scholar] [CrossRef]
- Dufrene, Y.F. Sticky microbes: Forces in microbial cell adhesion. Trends Microbiol. 2015, 23, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Brown, S. Engineered iron oxide-adhesion mutants of the Escherichia coli phage lambda receptor. Proc. Natl. Acad. Sci. USA 1992, 89, 8651–8655. [Google Scholar] [CrossRef] [PubMed]
- Doyle, R.J. Contribution of the hydrophobic effect to microbial infection. Microbes Infect. 2000, 2, 391–400. [Google Scholar] [CrossRef]
- Rohwerder, T.; Gehrke, T.; Kinzler, K.; Sand, W. Bioleaching review (part A): Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation. Appl. Microbiol. Biotechnol. 2003, 63, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Flemming, H.C. FTIR-spectroscopy in microbial and material analysis. Int. Biodeterior. Biodegrad. 1998, 41, 1–11. [Google Scholar] [CrossRef]
- Dittrich, M.; Sibler, S. Cell surface groups of two picocyanobacteria strains studied by zeta potential investigations, potentiometric titration, and infrared spectroscopy. J. Colloid Interface Sci. 2005, 286, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Huang, Q.; Wei, X.; Liang, W.; Rong, X.; Chen, W.; Cai, P. Microcalorimetric and potentiometric titration studies on the adsorption of copper by extracellular polymeric substances (EPS), minerals and their composites. Bioresour. Technol. 2010, 101, 5774–5779. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.S.; Smith, D.S.; Warren, L.A.; Ferris, F.G. Characterizing heterogeneous bacterial surface functional groups using discrete affinity spectra for proton binding. Environ. Sci. Technol. Lett. 1999, 33, 4514–4521. [Google Scholar] [CrossRef]

| Adhesion Force to Chalcopyrite | nN |
|---|---|
| FeSO4·7H2O-grown A. ferrooxidans | 0.8513 ± 0.0746 |
| EPS-deficient FeSO4·7H2O-grown A. ferrooxidans | 0.6095 ± 0.0912 |
| Sulfur-grown A. ferrooxidans | 0.9677 ± 0.1070 |
| EPS-deficient sulfur-grown A. ferrooxidans | 0.7828 ± 0.1260 |
| Chalcopyrite-grown A. ferrooxidans | 1.0532 ± 0.1132 |
| EPS-deficient chalcopyrite-grown A. ferrooxidans | 0.8335 ± 0.0841 |
| Sample | Contact Angle (°) | Zeta Potential (mV) |
|---|---|---|
| FeSO4·7H2O-grown A. ferrooxidans | 22.1 ± 1.1 | 4.22 ± 0.52 |
| EPS-deficient FeSO4·7H2O-grown A. ferrooxidans | 17.6 ± 1.6 | 0.32 ± 0.21 |
| Sulfur-grown A. ferrooxidans | 28.3 ± 1.3 | 3.33 ± 0.62 |
| EPS-deficient sulfur-grown A. ferrooxidans | 19.1 ± 1.8 | −0.03 ± 0.43 |
| Chalcopyrite-grown A. ferrooxidans | 33.4 ± 1.8 | 4.63 ± 0.48 |
| EPS-deficient chalcopyrite-grown A. ferrooxidans | 20.3 ± 1.2 | 0.46 ± 0.35 |
| Chalcopyrite | 60.7 ± 6.2 | −52.6 ± 4.86 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Wang, Q.; Zhu, J.; Zhou, S.; Gan, M.; Jiang, H.; Sand, W. Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans. Minerals 2016, 6, 100. https://doi.org/10.3390/min6040100
Li Q, Wang Q, Zhu J, Zhou S, Gan M, Jiang H, Sand W. Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans. Minerals. 2016; 6(4):100. https://doi.org/10.3390/min6040100
Chicago/Turabian StyleLi, Qian, Qianfen Wang, Jianyu Zhu, Shuang Zhou, Min Gan, Hao Jiang, and Wolfgang Sand. 2016. "Effect of Extracellular Polymeric Substances on Surface Properties and Attachment Behavior of Acidithiobacillus ferrooxidans" Minerals 6, no. 4: 100. https://doi.org/10.3390/min6040100






