The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Zeta Potential Measurements
2.4. Infrared Spectroscopic Measurements
2.5. X-ray Photoelectron Spectroscopy Measurements
3. Results and Discussion
3.1. Flotation of Kyanite and Sillimanite
3.2. Chemical Speciation of Sodium Oleate
3.3. FTIR Analysis
3.4. Zeta Potential Analysis
3.5. XPS Analysis
3.5.1. Surface Species of Kyanite and Sillimanite
3.5.2. Sodium Oleate Adsorption on Mineral Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmidt, M.W.; Poli, S.; Comodi, P.; Zanazzi, P.F. High pressure behavior of kyanite: Decomposition of kyanite into stishovite and corundum. Am. Mineral. 1997, 82, 460–466. [Google Scholar] [CrossRef]
- Harben, P.W. The Industrial Minerals Handbook; Warwick Printing: Warwick, UK, 1995. [Google Scholar]
- Aryal, S.; Rulis, P.; Ching, W.Y. Density functional calculation of the electronic structure and optical properties of aluminosilicate polymorphs (Al2SiO5). Am. Mineral. 2008, 93, 114–123. [Google Scholar] [CrossRef]
- Whitney, D.L. Coexisting andalusite, kyanite and sillimanite: Sequential formation of three Al2SiO5 polymorphs during progressive metamorphism near the triple point, Sivrihisar, Turkey. Am. Mineral. 2002, 87, 405–416. [Google Scholar] [CrossRef]
- Kerrick, D.M. The Al2SiO5 Polymorphs; The Mineralogical Society of America: Washington, D.C., USA, 1990. [Google Scholar]
- Klein, C.; Hurlbut, C.S.; Dana, J.D. Manual of Mineralogy, 20th ed.; Wiley: New York, NY, USA, 1993. [Google Scholar]
- Chinner, G.A. The significance of the aluminum silicates in metamorphism. Earth Sci. Rev. 1966, 2, 111–126. [Google Scholar] [CrossRef]
- Hietanen, A. Kyanite, andalusite, and sillimanite in the schist in Boehls Butte quadrangle, Idaho. Am. Mineral. 1956, 41, 1–27. [Google Scholar]
- Fuerstenau, M.C. Flotation: A.M. Gaudin Memorial Volume; American Institute of Mining, Metallurgical, and Petroleum Engineers: New York, NY, USA, 1976. [Google Scholar]
- White, A.J.R.; Compston, W.; Kleeman, A.W. The Palmer Granite—A study of a granite within a regional metamorphic environment. J. Petrol. 1967, 8, 29–50. [Google Scholar] [CrossRef]
- Yang, H.; Hazen, R.M.; Finger, L.W.; Prewitt, C.T.; Downs, R.T. Compressibility and crystal structure of sillimanite, Al2SiO5 at high pressure. Phys. Chem. Miner. 1997, 25, 39–47. [Google Scholar] [CrossRef]
- Yang, H.X.; Downs, R.T.; Finger, L.W.; Hazen, R.M.; Prewitt, C.T. Compressibility and crystal structure of kyanite, Al2SiO5, at high pressure. Am. Mineral. 1997, 82, 467–474. [Google Scholar] [CrossRef]
- Ohuchi, F.S.; Ghose, S.; Engelhard, M.H.; Baer, D.R. Chemical bonding and electronic structures of the Al2SiO5 polymorphs, andalusite, sillimanite, and kyanite: X-ray photoelectron- and electron energy loss spectroscopy studies. Am. Mineral. 2006, 91, 740–746. [Google Scholar] [CrossRef]
- Burnham, C.W. Refinement of the crystal structure of sillimanite. Z. Krist. Cryst. Mater. 1963, 118, 127–148. [Google Scholar] [CrossRef]
- McMichael, B. Aluminosilicate minerals. Refractories steel the Show. Ind. Miner. 1990, 277, 27–43. [Google Scholar]
- Skoog, A.J.; Moore, R.E. Refractory of the past for the future: Mullite and its use as a bonding phase. Am. Ceram. Soc. Bull. 1988, 67, 1180–1185. [Google Scholar]
- Guo, H.; Ye, F.B.; Li, W.F.; Song, X.Z.; Xie, G.F. Preparation and characterization of foamed microporous mullite ceramics based on kyanite. Ceram. Int. 2015, 41, 14645–14651. [Google Scholar] [CrossRef]
- Xu, L.F.; Xi, X.A.; Zhu, W.L.; Shui, A.Z.; Dai, W.B. Investigation on the influence factors for preparing mullite-whister-structured porous ceramic. J. Alloy. Compd. 2015, 649, 739–745. [Google Scholar] [CrossRef]
- Brandao, E.R.G.; Mendes, S.L.C. Kyanite from Minas Gerais, Brasil: Characterization for use inceramic. In Innovations in Mineral and Coal Processing, Proceedings of the 7th International Mineral Processing Symposium, Istanbul, Turkey, 15–17 September 1998.
- Zhou, L.C.; Zhang, Y.M. Flotation separation of Xixia andalusite ore. Trans. Nonferrous Met. Soc. China 2011, 21, 1388–1394. [Google Scholar] [CrossRef]
- Bulut, G.; Yurtsever, C. Flotation behavior of Bitlis kyanite ore. Int. J. Miner. Process. 2004, 73, 29–36. [Google Scholar] [CrossRef]
- Prabhakar, S.; Raju, G.B.; Rao, S.S. Beneficiation of sillimanite by column flotation—A pilot scale study. Int. J. Miner. Process. 2006, 81, 159–165. [Google Scholar] [CrossRef]
- Metson, J.B. Charge compensation and binding energy referencing in XPS analysis. Surf. Interface Anal. 1999, 27, 1069–1072. [Google Scholar] [CrossRef]
- Shibata, J.; Fuerstenau, D.W. Flocculation and flotation characteristics of fine hematite with sodium oleate. Int. J. Miner. Process. 2003, 72, 25–32. [Google Scholar] [CrossRef]
- Wen, X.T.; Yang, J.X.; He, B.; Gu, Z.W. Preparation of monodisperse magnetite nanoparticles under mild conditions. Curr. Appl. Phys. 2008, 8, 535–541. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhang, J.; Wang, W.Q.; Deng, J.; Chen, B.Y.; Yan, W.; Xiong, S.Q.; Huang, Y.; Liu, J. Flotation behaviors of ilmenite, titanaugite, and forsterite using sodium oleate as the collector. Miner. Eng. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Roonasi, P.; Holmgren, A. A Fourier transform infrared (FTIR) and thermogravimetric analysis (TGA) study of oleate adsorbed on magnetite nano-particle surface. Appl. Surf. Sci. 2009, 255, 5891–5895. [Google Scholar] [CrossRef]
- Wen, L.; Liang, W.X.; Zhang, Z.G.; Huang, J.C. The Infrared Spectroscopy of Minerals; Chongqing University Press: Chongqing, China, 1989. (In Chinese) [Google Scholar]
- Salje, E.; Werneke, C. The phase equilibrium between sillimanite and andalusite as determined from lattice vibrations. Contrib. Miner. Petrol. 1982, 79, 56–67. [Google Scholar] [CrossRef]
- Lovell, V.M.; Goold, L.A.; Finkelstein, N.P. Infrared studies of the adsorption of oleate species on calcium fluoride. Int. J. Miner. Process. 1974, 1, 183–192. [Google Scholar] [CrossRef]
- Smolik, J.J.; Harman, E.; Fuerstenau, D.W. Surface characteristics and flotation of aluminosilicate. Trans. AIME 1966, 235, 367–375. [Google Scholar]
- Chen, X.M.; Peng, Y.J.; Bradshaw, D. The effect of particle breakage mechanisms during regrinding on the subsequent cleaner flotation. Miner. Eng. 2014, 66–68, 157–164. [Google Scholar] [CrossRef]
- Peng, Y.J.; Wang, B.; Gerson, A. The effect of electrochemical potential on the activation of pyrite by copper and lead ions during grinding. Int. J. Miner. Process. 2012, 102–103, 141–149. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Onoda, G.Y.; Fuerstenau, D.W. Amine flotation of quartz in the presence of inorganic electrolytes. In Technical Papers 7th International Mineral Processing Congress; Gordon and Breach: New York, NY, USA, 1964; pp. 301–306. [Google Scholar]
- Robinson, M.; Pask, J.A.; Fuerstenau, D.W. Surface charge of alumina and magnesia in aqueous media. J. Am. Ceram. Soc. 1964, 47, 516–520. [Google Scholar] [CrossRef]
- Yumitori, S. Correlation of C1s chemical state intensities with the O1s intensity in the XPS analysis of anodically oxidized glass-like carbon samples. J. Mater. Sci. 2000, 35, 139–146. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.Q.; Liu, J.; Huang, Y.; Feng, Q.M.; Zhao, H. Fe (III) as an activator for the flotation of spodumene, albite, and quartz minerals. Miner. Eng. 2014, 61, 16–22. [Google Scholar]
- Takahagi, T.; Ishitani, A. XPS studies by use of the digital difference spectrum technique of functional groups on the surface of carbon fiber. Carbon 1984, 22, 43–46. [Google Scholar] [CrossRef]
- Jiang, Y.R.; Li, W.; Feng, R. Preparation and performance of 4-alkyl-4,4-bis(hydroxycarbamoyl) carboxylic acid for flotation separation of diaspore against aluminosilicates. Miner. Eng. 2011, 24, 1571–1579. [Google Scholar] [CrossRef]
- Sun, C.Y.; Yin, W.Z. Flotation Principles of Silicate Minerals; Science Press: Beijing, China, 2001. (In Chinese) [Google Scholar]
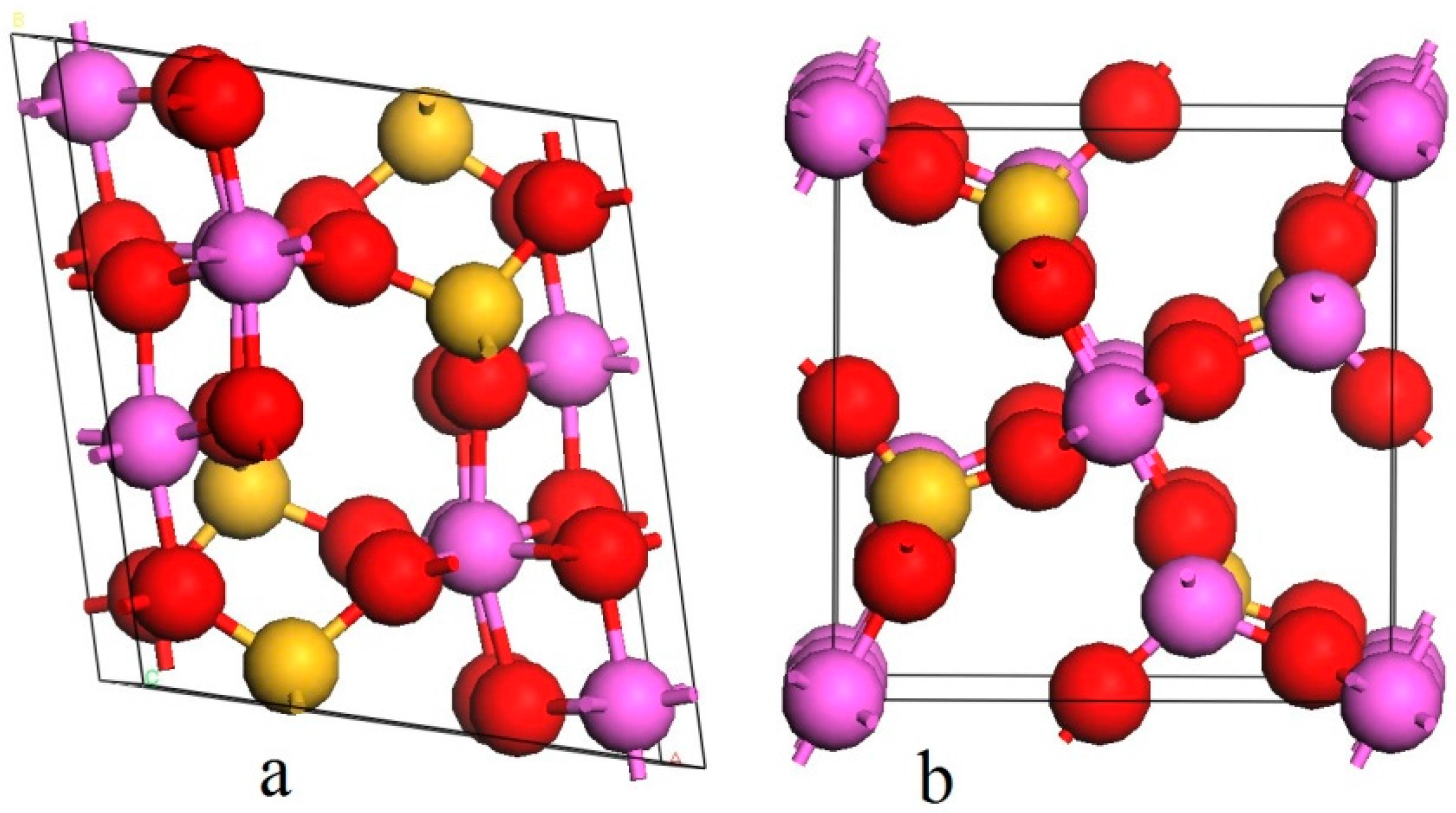
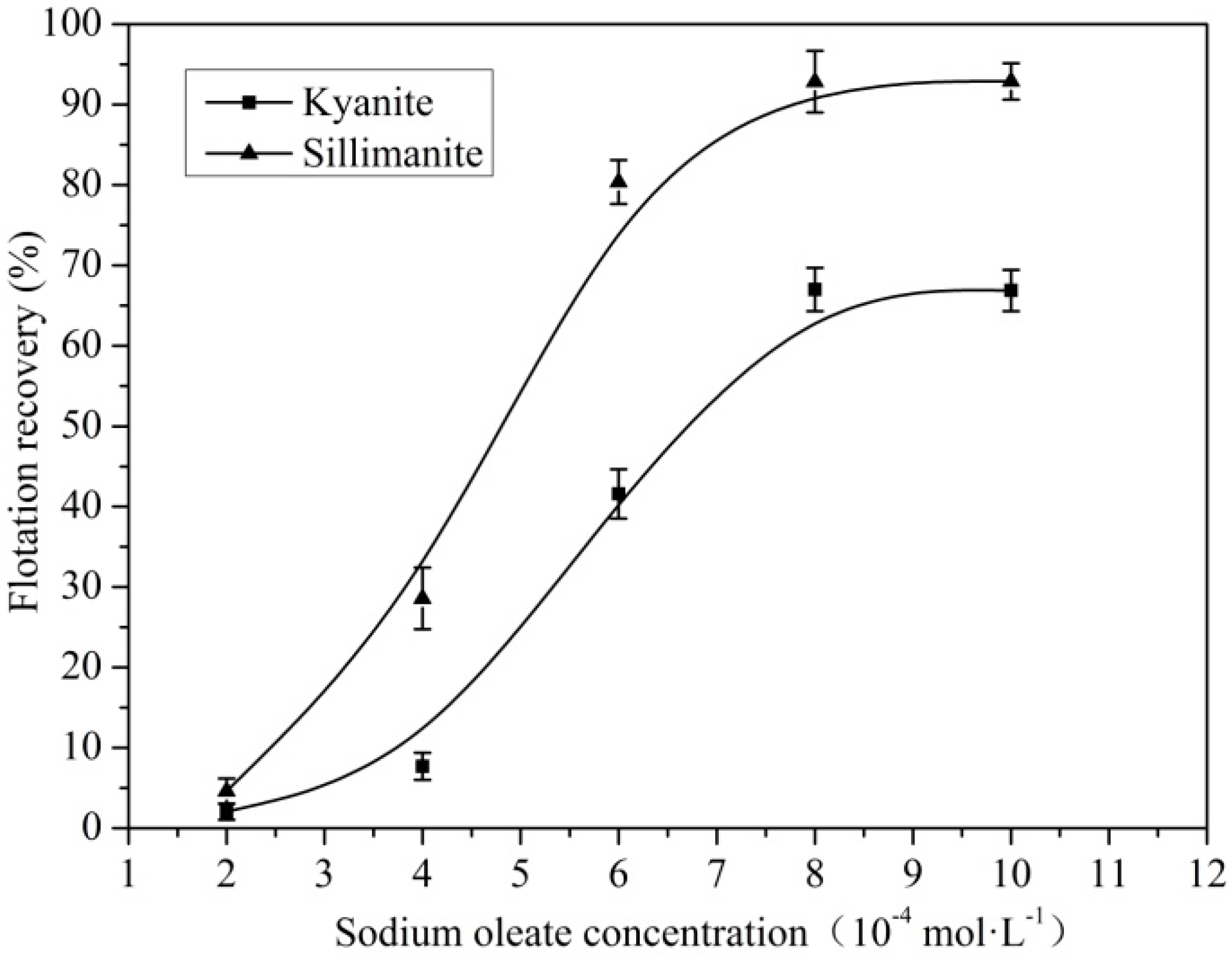
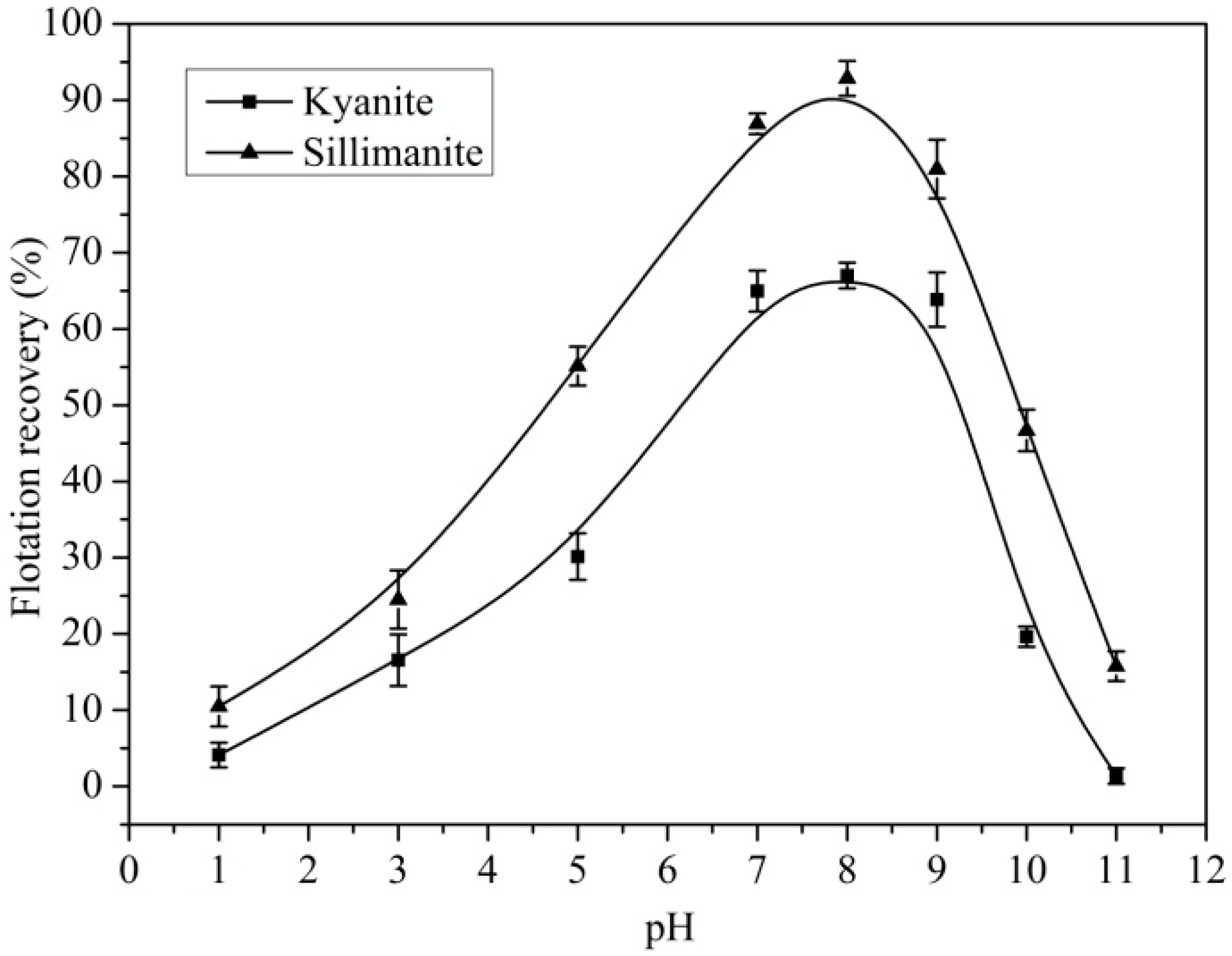
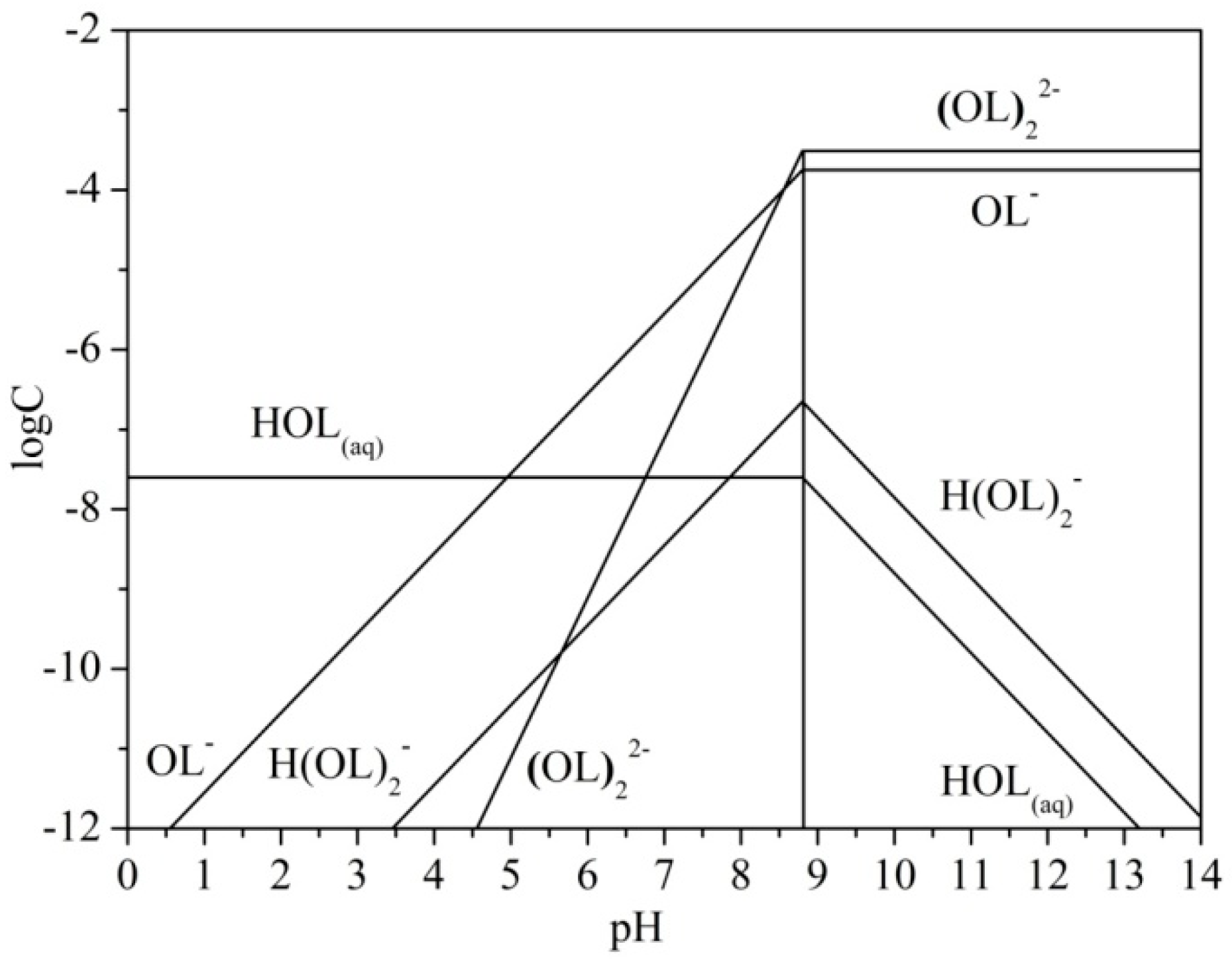

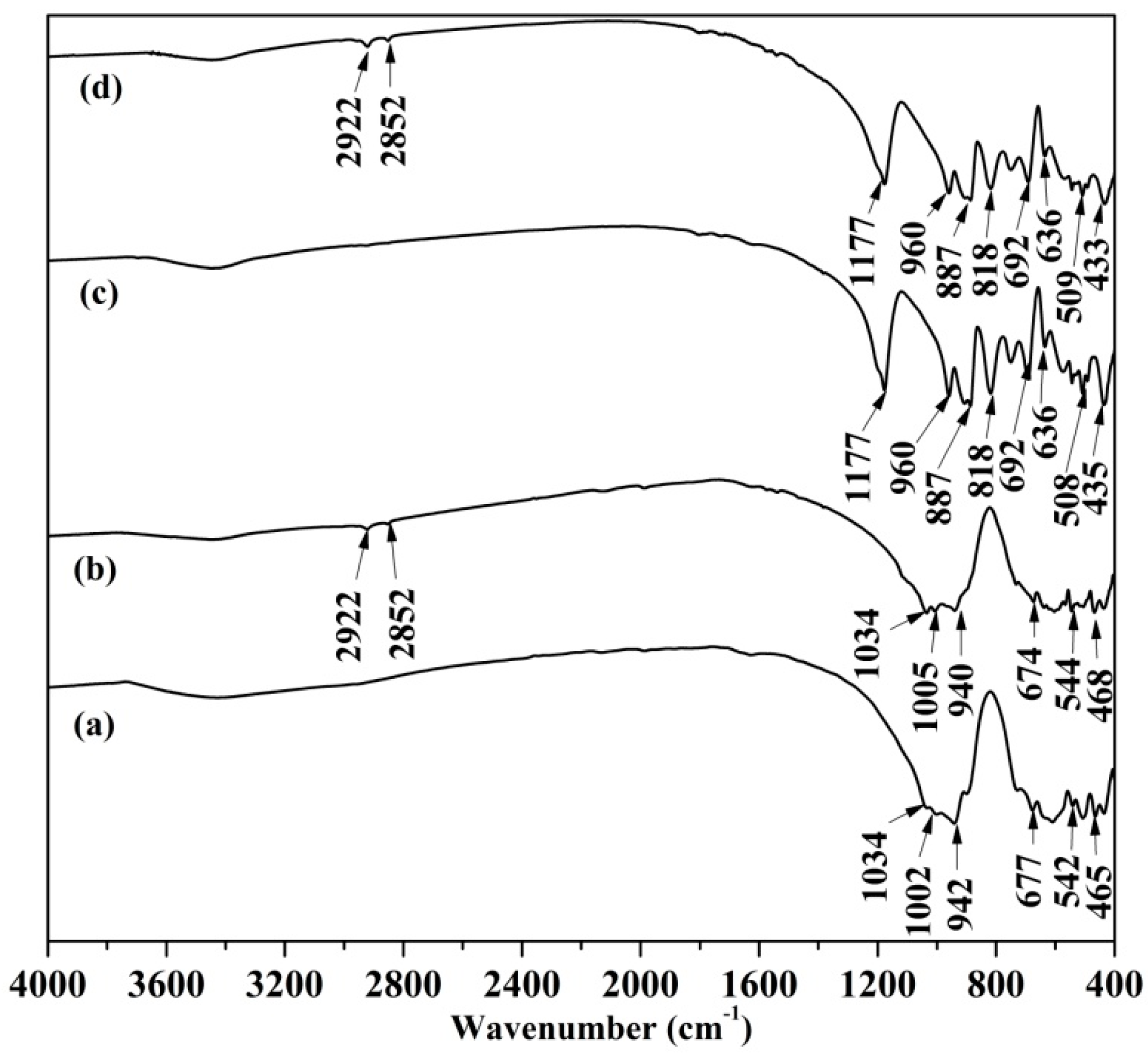

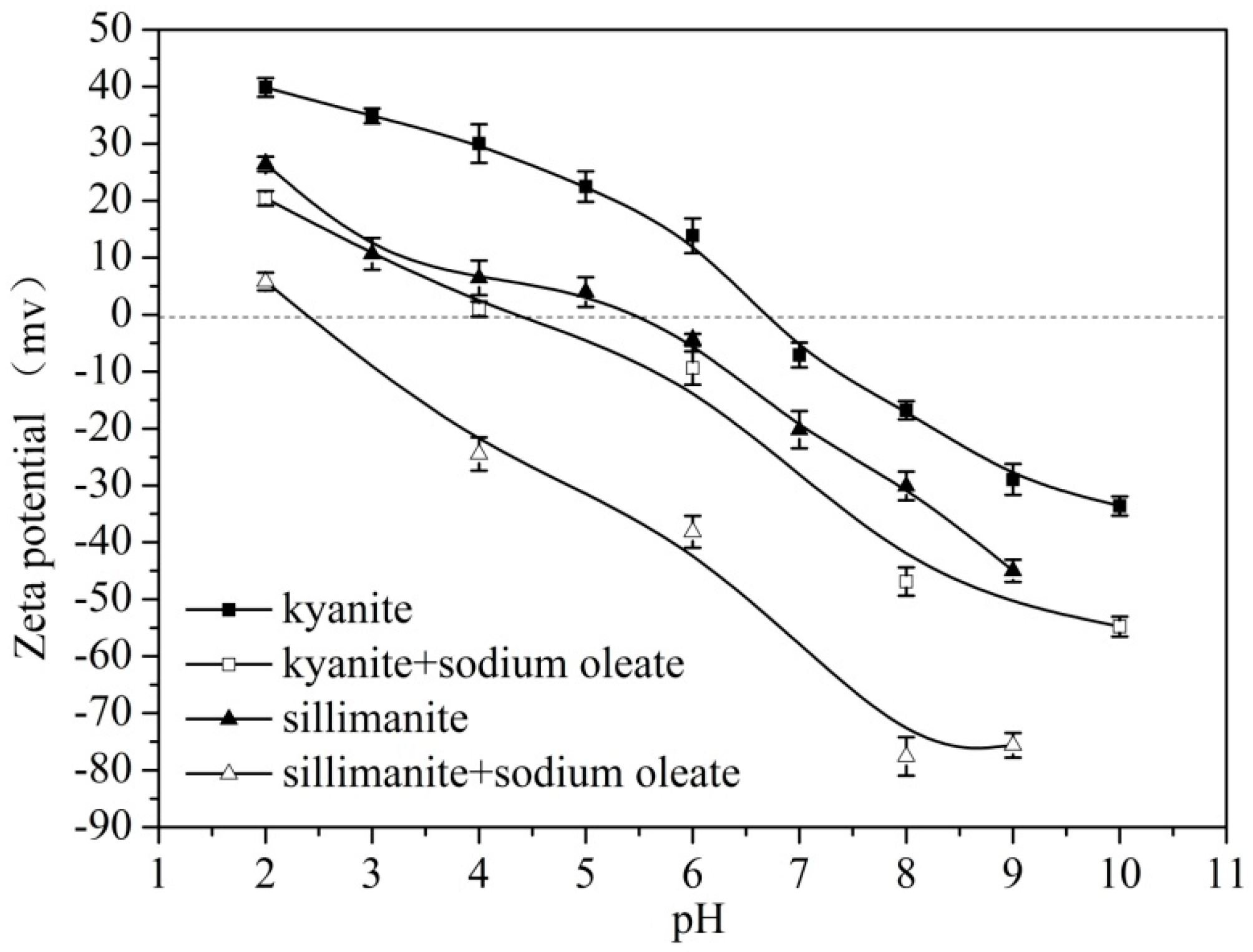
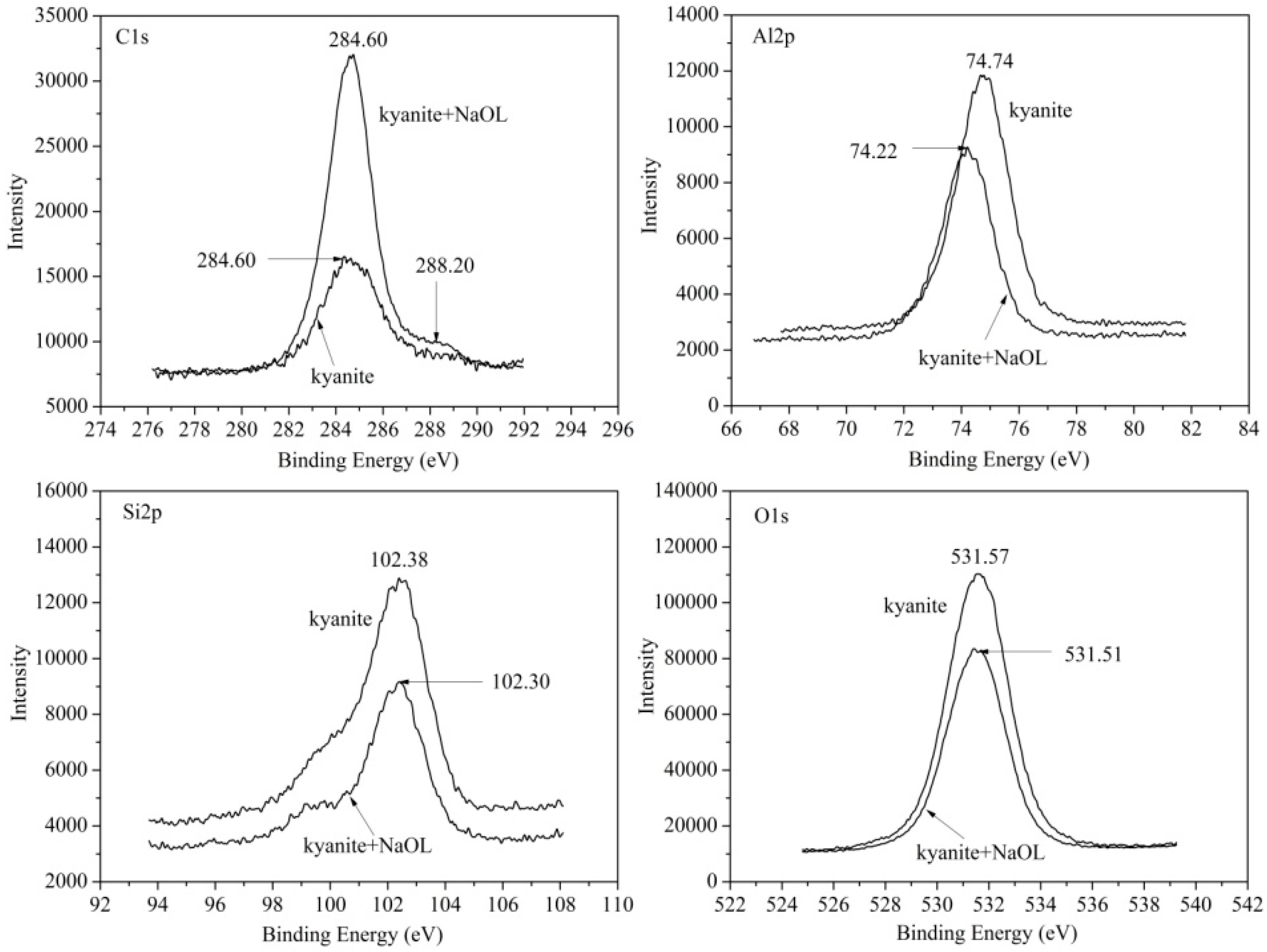

| Sample | Al2O3 | SiO2 | Fe2O3 | TiO2 | K2O | CaO | Ignition loss |
|---|---|---|---|---|---|---|---|
| Kyanite | 62.76 | 35.65 | 0.15 | - | 0.02 | 0.08 | 1.04 |
| Sillimanite | 60.56 | 38.22 | 0.41 | 0.12 | 0.02 | 0.43 | 0.24 |
| Sample | Element | Concentration (at%) |
|---|---|---|
| Kyanite | O | 60.47 |
| Si | 17.87 | |
| Al | 21.66 | |
| Sillimanite | O | 61.85 |
| Si | 19.49 | |
| Al | 18.66 |
| Sample | Concentrations (at%) | |||
|---|---|---|---|---|
| C1s | O1s | Si2p | Al2p | |
| Kyanite | 15.20 | 51.35 | 15.13 | 18.32 |
| Kyanite + sodium oleate | 33.96 | 38.52 | 13.20 | 14.32 |
| Sillimanite | 15.22 | 52.52 | 16.49 | 15.77 |
| Sillimanite + sodium oleate | 39.24 | 35.93 | 14.17 | 10.65 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Gao, H.; Ren, Z.; Chen, Z. The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector. Minerals 2016, 6, 90. https://doi.org/10.3390/min6030090
Jin J, Gao H, Ren Z, Chen Z. The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector. Minerals. 2016; 6(3):90. https://doi.org/10.3390/min6030090
Chicago/Turabian StyleJin, Junxun, Huimin Gao, Zijie Ren, and Zhijie Chen. 2016. "The Flotation of Kyanite and Sillimanite with Sodium Oleate as the Collector" Minerals 6, no. 3: 90. https://doi.org/10.3390/min6030090






