Microstructural Control on Perlite Expansibility and Geochemical Balance with a Novel Application of Isocon Analysis: An Example from Milos Island Perlite (Greece)
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Petrographic Characterisation
3.2. Perlite Expansion
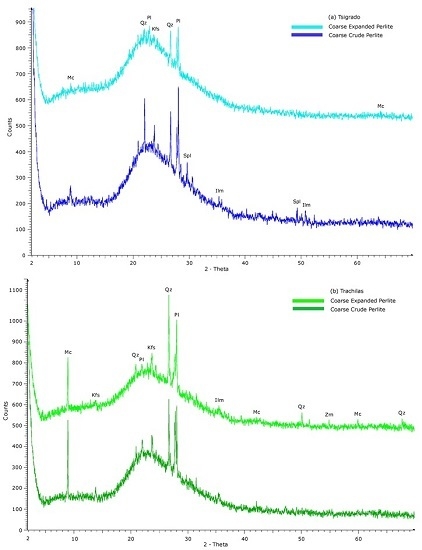
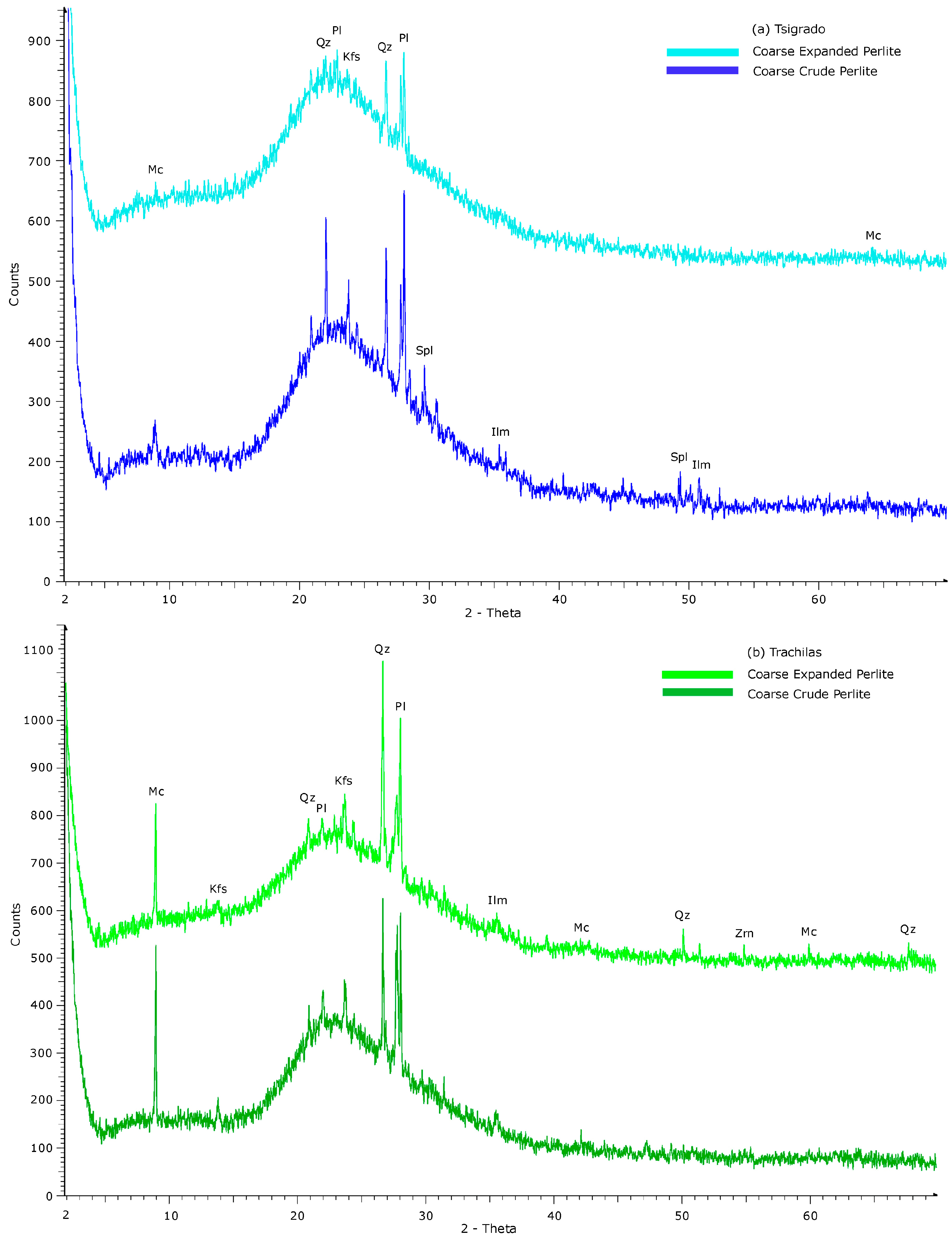
3.3. Interpretation of XRD Patterns
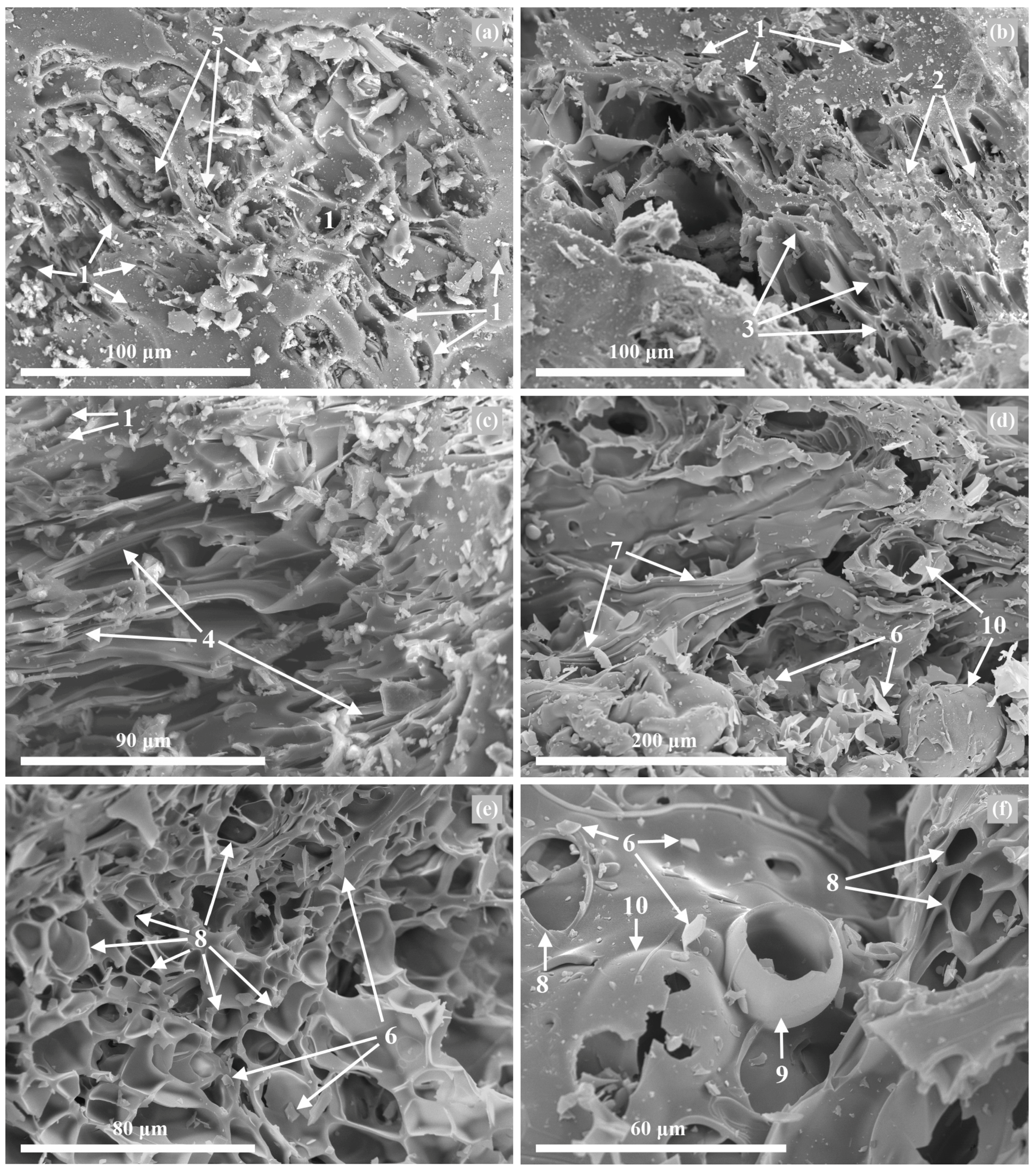
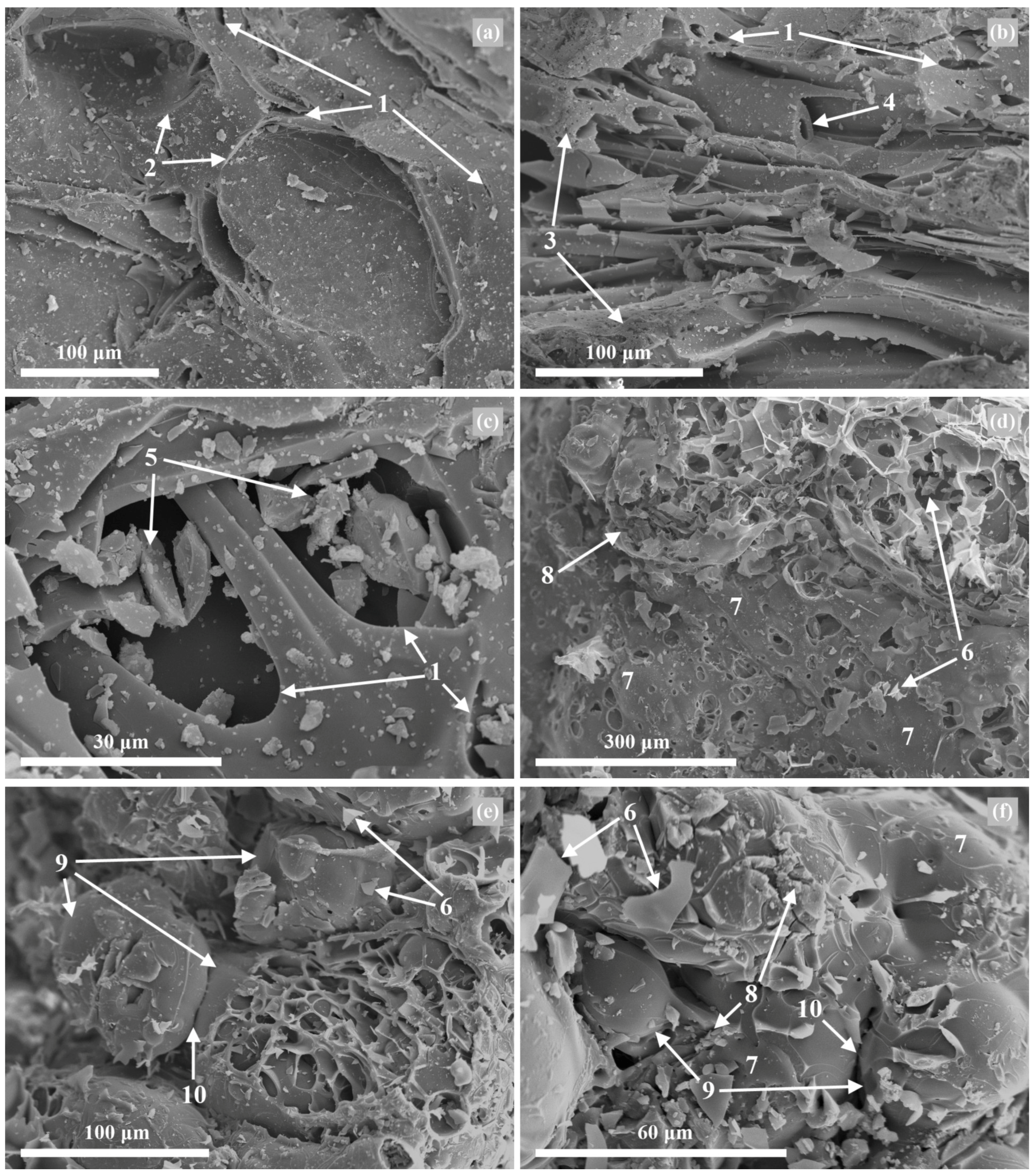
3.4. Morphological and Microstructural Properties
3.5. Whole-Rock Chemistry
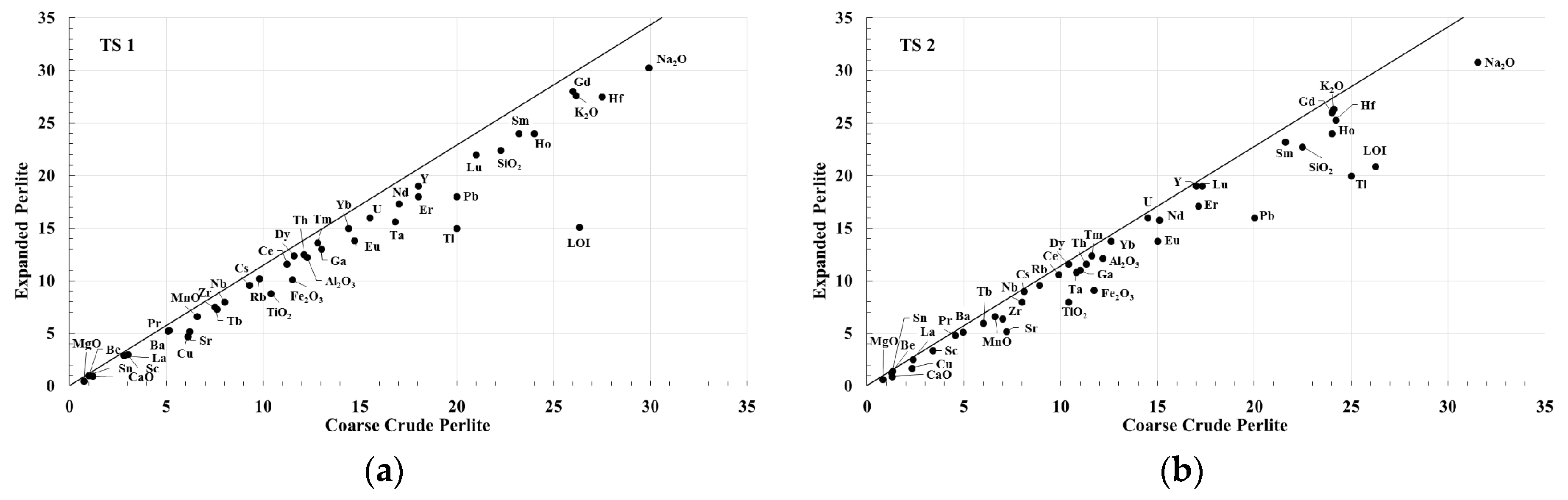
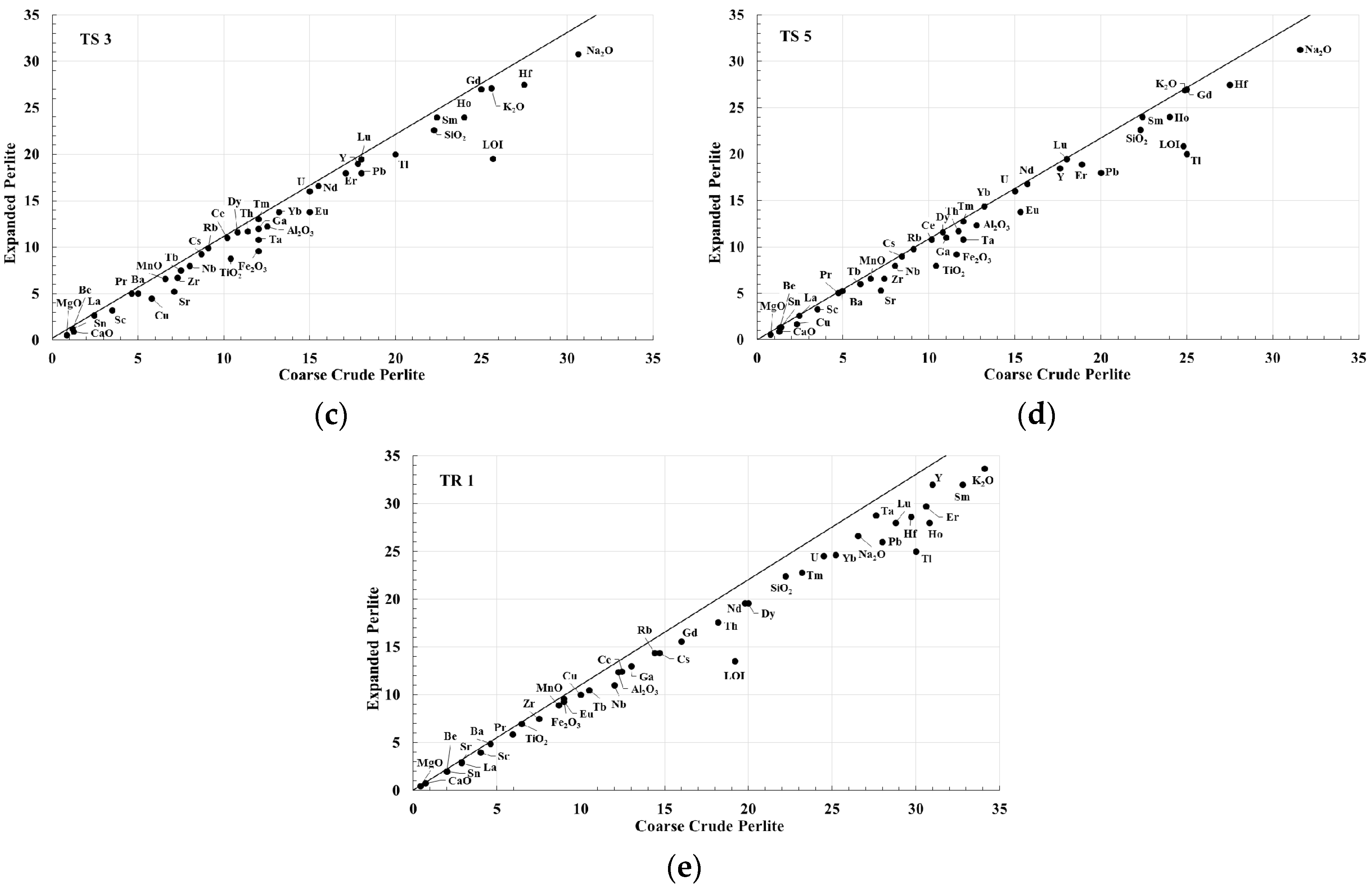
3.6. Isocon Approach
4. Discussion
4.1. Expansion of Perlite from Milos Island
4.2. Isocon Analysis of Expanded Perlite from Milos Island
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kogel, J.E.; Trivedi, N.C.; Barker, J.M.; Krukowski, S.T. Industrial Minerals and Rocks: Commodities, Markets and Uses, 7th ed.; Society for Mining, Metallurgy and Exploration Inc.: Littleton, CO, USA, 2006; p. 1548. [Google Scholar]
- Angelopoulos, P.M.; Gerogiorgis, D.I.; Paspaliaris, I. Mathematical modeling and process simulation of perlite grain expansion in a vertical electrical furnace. Appl. Math. Model. 2014, 38, 1799–1822. [Google Scholar] [CrossRef]
- Kaufhold, S.; Reese, A.; Schwiebacher, W.; Dohrmann, R.; Grathoff, G.H.; Warr, L.N.; Halisch, M.; Müller, C.; Schwarz-Schampera, U.; Ufer, K. Porosity and distribution of water in perlite from the island of Milos, Greece. SpringerPlus 2014, 3, 598. [Google Scholar] [CrossRef] [PubMed]
- Fytikas, M.; Innocenti, F.; Kolios, N.; Manneti, P.; Mazzuoli, R.; Poli, G.; Rita, F.; Villari, L. Volcanology and petrology of volcanic products from the island of Milos and neighbouring islets. J. Volcanol. Geotherm. Res. 1986, 28, 297–317. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Piper, D.J.W. The South Aegean active volcanic arc: Relationships between magmatism and tectonics. Dev. Volcanol. 2007, 7, 113–133. [Google Scholar]
- International Centre for Diffraction Data. Powder Diffraction File; International Centre for Diffraction Data: Newtown Square, PA, USA, 2006. [Google Scholar]
- Krimm, S.; Tobolsky, A.V. Quantitative X-ray studies of order in amorphous and crystalline polymers. Quantitative X-ray determination of crystallinity in polyethylene. J. Polym. Sci. 1951, 7, 57–76. [Google Scholar] [CrossRef]
- Grant, J.A. The isocon diagram—A simple solution to Gresens’ equation for metasomatic alteration. Econ. Geol. 1986, 81, 1976–1982. [Google Scholar] [CrossRef]
- Grant, J.A. Isocon analysis: A brief review of the method and applications. Phys. Chem. Earth 2005, 30, 997–1004. [Google Scholar] [CrossRef]
- Gresens, R.L. Composition-volume relationship of metasomatism. Chem. Geol. 1967, 2, 47–65. [Google Scholar] [CrossRef]
- Roulia, M.; Chassapis, K.; Kapoutsis, J.A.; Kamitsos, E.I.; Savvidis, T. Influence of thermal treatment on the water release and the glassy structure of perlite. J. Mater. Sci. 2006, 41, 5870–5881. [Google Scholar] [CrossRef]
- Chatterjee, K.K. Uses of Industrial Minerals, Rocks and Freshwater; Nova Science Publishers: New York, NY, USA, 2009; p. 584. [Google Scholar]
- Sodeyama, K.; Sakka, Y.; Kamino, Y. Preparation of fine expanded perlite. J. Mater. Sci. 1999, 34, 2461–2468. [Google Scholar] [CrossRef]
- Rotella, M.; Simandl, G. Marilla perlite—Volcanic glass occurrence, British Columbia. In Geological Fieldwork 2002; Paper 2003-1; British Columbia Geological Survey: Vancouver, BC, Canada, 2003; pp. 165–173. [Google Scholar]
- Sánchez, E.; Ibáñez, M.J.; García-Ten, J.; Quereda, M.F.; Hutchings, I.M.; Xu, Y.M. Porcelain tile microstructure: Implications for polished tile properties. J. Eur. Ceram. Soc. 2006, 26, 2533–2540. [Google Scholar]
- García-Ten, J.; Orts, M.J.; Saburit, A.; Silva, G. Thermal conductivity of traditional ceramics: Part II: Influence of mineralogical composition. Ceram. Int. 2010, 36, 2017–2024. [Google Scholar]
- Saisuttichai, D.; Manning, D.A.C. Geochemical characteristics and expansion properties of a highly potassic perlitic rhyolite from Lopburi, Thailand. Res. Geol. 2007, 57, 301–312. [Google Scholar] [CrossRef]
- Varuzhanyan, Av.A.; Varuzhanyan, Ar.A.; Varuzhanyan, H.A. A mechanism of perlite expansion. Inorg. Mater. 2006, 42, 1039–1045. [Google Scholar] [CrossRef]
- Green, T.H. Island arc and continent-building magmatism: A review of petrogenetic models based on experimental petrology and geochemistry. Tectonophysics 1980, 63, 367–385. [Google Scholar] [CrossRef]
- Best, M.G.; Christiansen, E.H. Igneous Petrology; Blackwell Science: Oxford, UK, 2001; p. 455. [Google Scholar]
- Belousova, E.; Griffin, W.; O’Reilly, S.Y.; Fisher, N. Igneous zircon: Trace element composition as an indicator of source rock type. Contrib. Mineral. Petrol. 2002, 143, 602–622. [Google Scholar] [CrossRef]
- Badalov, S.T.; Radinovich, A.V. The geochemistry of indium and thallium in the Karamazar ore region (the Uzbek and Tadzhik republics). Geochem. Int. 1966, 3, 1095–1101. [Google Scholar]
- Heinrichs, H.; Schulz-Dobrick, B.; Wedepohl, K.H. Terrestrial geochemistry of Cd, Bi, Tl, Pb, Zn and Rb. Geochim. Cosmochim. Acta 1980, 44, 1519–1533. [Google Scholar] [CrossRef]
- Albarède, F. Volatile accretion history of the terrestrial planets and dynamic implications. Nature 2009, 461, 1227–1233. [Google Scholar]
| Samples | Mass (g) | Total Mass Loss | ||||
|---|---|---|---|---|---|---|
| CCP | EP | Drops | (g) | wt % | ||
| Tsigrado | TS1 | 40.00 | 35.50 | 0.95 | 4.50 | 11.25 |
| TS2 | 40.00 | 35.52 | 0.93 | 4.48 | 11.20 | |
| TS3 | 40.00 | 36.24 | 1.40 | 3.76 | 9.40 | |
| TS4 | 40.00 | 35.30 | 1.20 | 4.70 | 11.75 | |
| TS5 | 40.00 | 36.67 | 1.23 | 3.33 | 8.33 | |
| TS6 | 40.00 | 36.35 | 1.33 | 3.65 | 9.13 | |
| Trachilas | TR1 | 40.00 | 36.20 | 0.05 | 3.80 | 9.50 |
| TR2 | 40.00 | 36.15 | 0.03 | 3.85 | 9.63 | |
| Major Elements wt % | Tsigrado | Trachilas | ||||||
|---|---|---|---|---|---|---|---|---|
| TS1 | TS2 | TS3 | TS4 | TS5 | TS6 | TR1 | TR2 | |
| SiO2 | 74.21 | 74.93 | 74.12 | 75.46 | 74.29 | 75.59 | 74.08 | 75.73 |
| TiO2 | 0.13 | 0.13 | 0.13 | 0.14 | 0.13 | 0.14 | 0.08 | 0.14 |
| Al2O3 | 12.28 | 12.16 | 12.52 | 12.12 | 12.76 | 12.09 | 12.24 | 12.03 |
| Fe2O3 | 1.15 | 1.17 | 1.20 | 0.98 | 1.16 | 0.98 | 0.90 | 0.70 |
| MnO | 0.06 | 0.06 | 0.06 | - | 0.06 | - | 0.08 | - |
| MgO | 0.25 | 0.27 | 0.28 | 0.17 | 0.26 | 0.15 | 0.14 | 0.04 |
| CaO | 1.20 | 1.28 | 1.25 | 1.25 | 1.27 | 1.21 | 0.74 | 0.71 |
| Na2O | 3.74 | 3.94 | 3.83 | 4.07 | 3.95 | 4.10 | 3.32 | 3.52 |
| K2O | 3.27 | 3.01 | 3.20 | 3.11 | 3.11 | 3.15 | 4.67 | 4.68 |
| LOI | 3.29 | 3.28 | 3.21 | 2.71 | 3.10 | 2.60 | 2.40 | 2.87 |
| Total | 99.58 | 100.23 | 99.80 | 100.01 | 100.09 | 100.01 | 98.65 | 100.42 |
| Behaviour | Tsigrado | Trachilas |
|---|---|---|
| Elements lost in the expanded perlite in amounts higher than the total mass loss of the CCP | LOI, TiO2, Fe2O3, MgO, CaO, Sr, Zr, Eu, Cu, Ta, Tl, Pb | LOI, K2O, Th, La, Pr, Nd, Sm, Gd, Dy, Ho, Er, Tm, Yb, Lu, Nb, Cs, Hf, Tl, Pb |
| Elements lost in the expanded perlite in amounts similar to the total mass loss of the CCP | SiO2, Al2O3, MnO, Na2O, Tb, Ho, Er, Ga, Nb, Hf | SiO2, Al2O3, Na2O, Zr, Rb, Ce, Tb, Cu, Ga, U |
| Elements lost in the expanded perlite in amounts less than the total mass loss of the CCP | K2O, Ba, Y, Rb, Th, La, Ce, Pr, Nd, Sm, Gd, Dy, Tm, Yb, Lu, Cs, U | TiO2, Fe2O3, MnO, MgO, CaO, Ba, Sr, Y, Eu, Ta |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsikouras, B.; Passa, K.-S.; Iliopoulos, I.; Katagas, C. Microstructural Control on Perlite Expansibility and Geochemical Balance with a Novel Application of Isocon Analysis: An Example from Milos Island Perlite (Greece). Minerals 2016, 6, 80. https://doi.org/10.3390/min6030080
Tsikouras B, Passa K-S, Iliopoulos I, Katagas C. Microstructural Control on Perlite Expansibility and Geochemical Balance with a Novel Application of Isocon Analysis: An Example from Milos Island Perlite (Greece). Minerals. 2016; 6(3):80. https://doi.org/10.3390/min6030080
Chicago/Turabian StyleTsikouras, Basilios, Kalliopi-Sofia Passa, Ioannis Iliopoulos, and Christos Katagas. 2016. "Microstructural Control on Perlite Expansibility and Geochemical Balance with a Novel Application of Isocon Analysis: An Example from Milos Island Perlite (Greece)" Minerals 6, no. 3: 80. https://doi.org/10.3390/min6030080