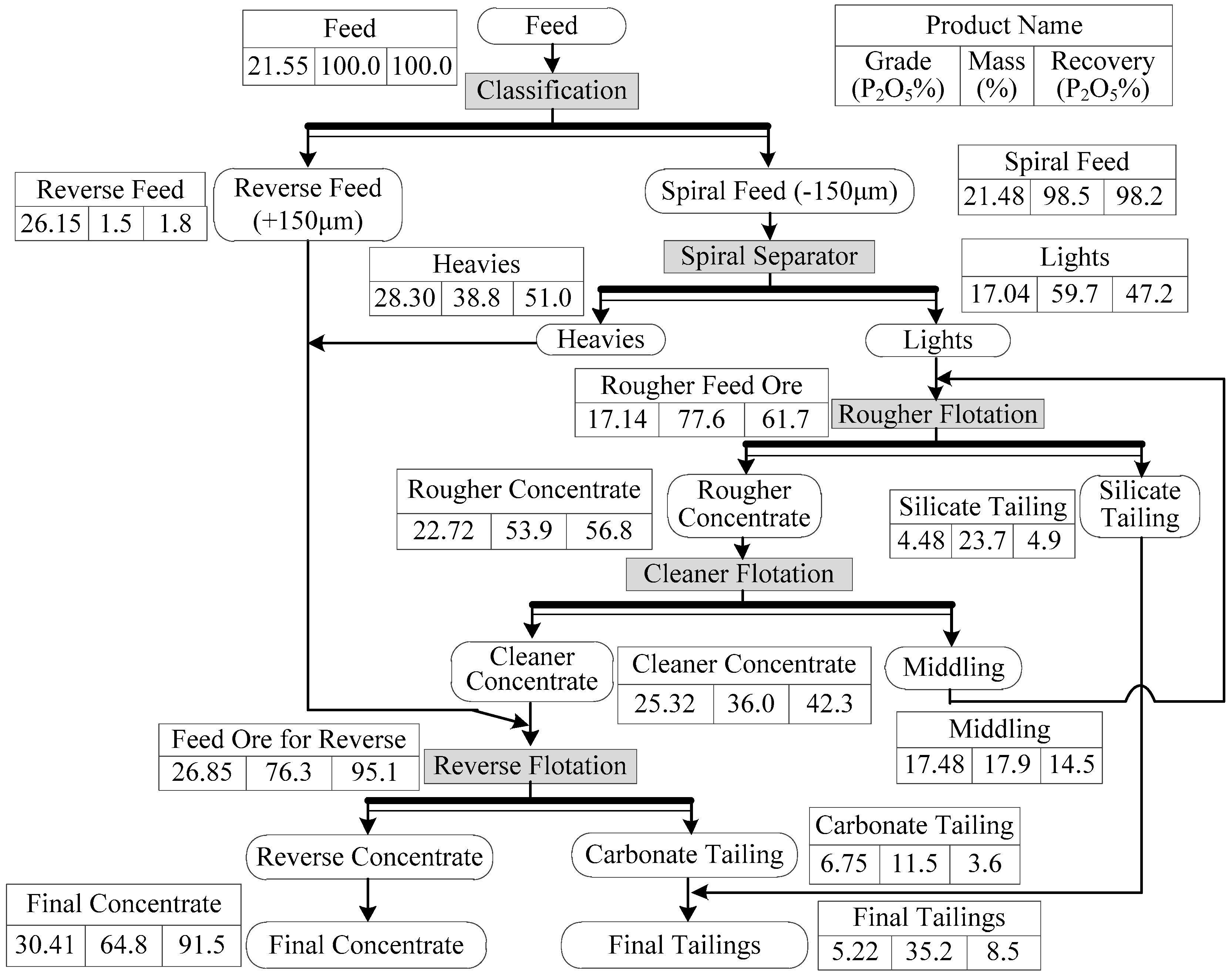
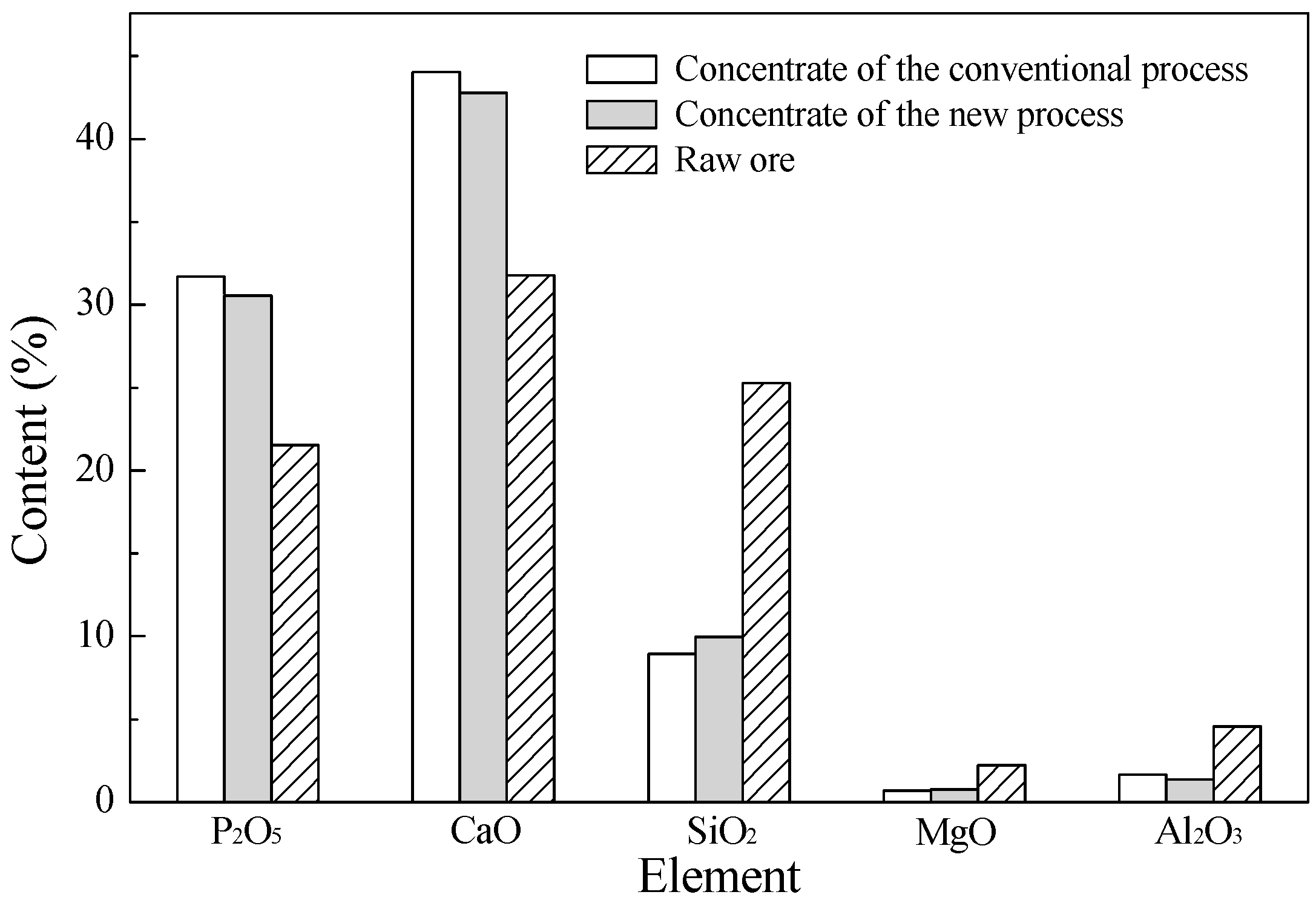
2.1. Materials
The sedimentary phosphate ore sample used in this work was collected from Yihua Group Co., Ltd., Yichang, China. The results of chemical composition analysis and mineral composition are shown in
Table 1 and
Table 2. Around 200 kg of representative ore samples was crushed to below 3 mm with a two-stage jaw crusher and a one-stage roll crusher. The materials were then well mixed and divided into 1 kg samples for separation studies. The chemical analysis was performed with the Xios advanced X-ray fluorescence (XRF) analyzer, the results are tabulated in
Table 1.
Optical microscopy and X-ray diffraction (XRD) studies were performed to define the main minerals and their interlocking characteristics. The results of optical mineralogy are showed in
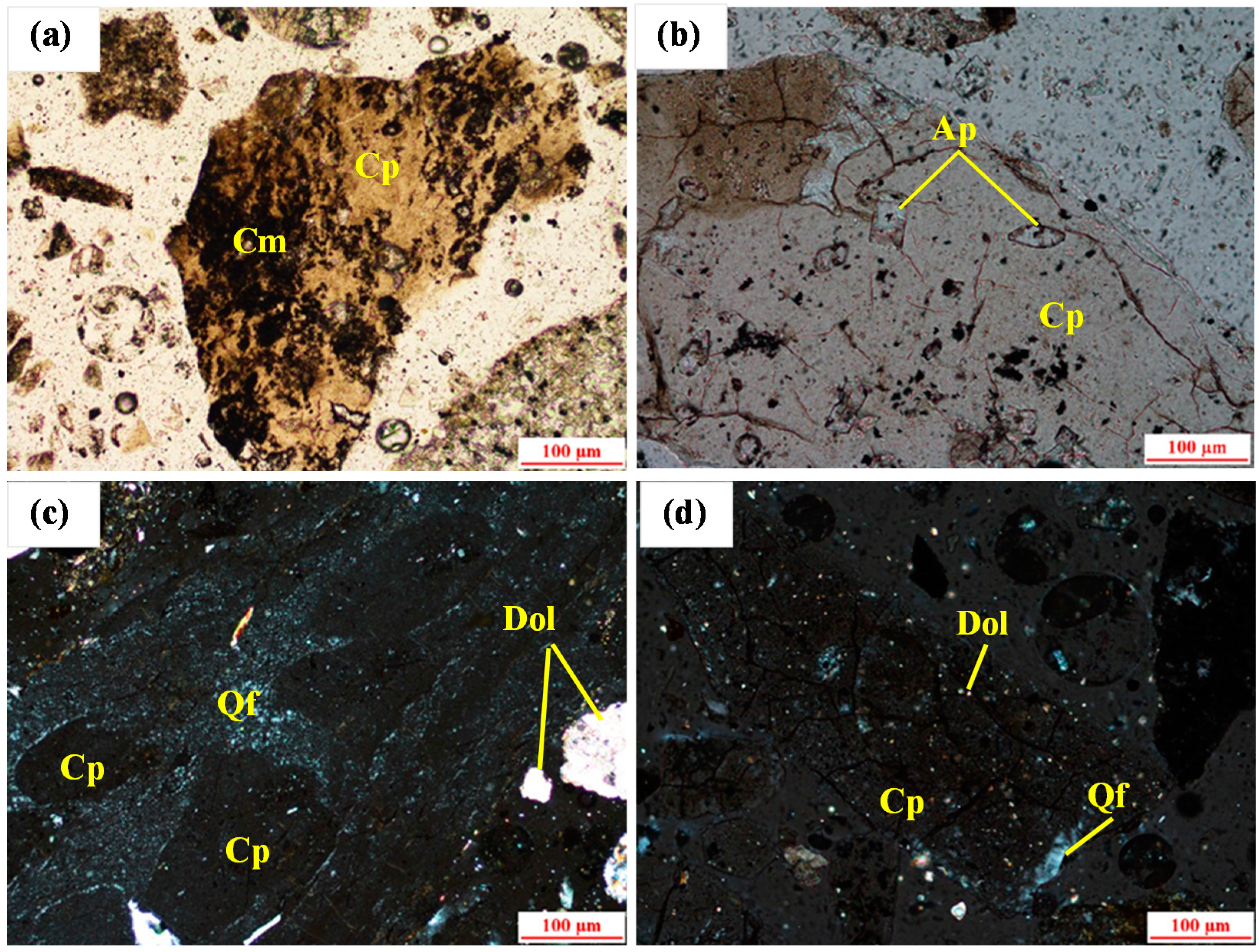
Figure 1. Collophanite grains are polygonal, plate-like, and are usually between 0.05 and 0.8 mm. Small apatite grains (0.01–0.05 mm) could be found in some collophanite grains (
Figure 1b). Some of the gangue minerals are occurs as endogangue in the collophanite grains as shown in
Figure 1a,c,d. The majority of gangue minerals are appear between the collophanite grains. These gangue minerals are clay minerals, the aggregate of quartz, feldspar, and dolomite. The grains of quartz and feldspar are small and difficult to distinguish between them. Their aggregate is between 0.03 and 0.1 mm, and is usually in the exogangue (
Figure 1d) and sporadically distributed in the collophanite grains (
Figure 1c). It can be seen from
Figure 1c that the dolomite minerals are hypidiomorphic granular structure, scattered, and grains size are usually between 0.03 and 0.1 mm. Dolomite as exogangue (
Figure 1c) and endogangue (
Figure 1d) exist between and in the collophanite grains. Clay mineral pellets are mainly composed of illite, kaolinite. They usually appear in the form of crystal stock, either or as endogangue in collophanite grains (
Figure 1a). In addition to this, there are slight iron minerals like pyrite (
Figure 1e), magnetite (
Figure 1f) and hematite created small grains (0.01–0.03 mm) which sporadically distribute in collophanite grains, quartz and feldspar aggregates, and clay minerals aggregates.
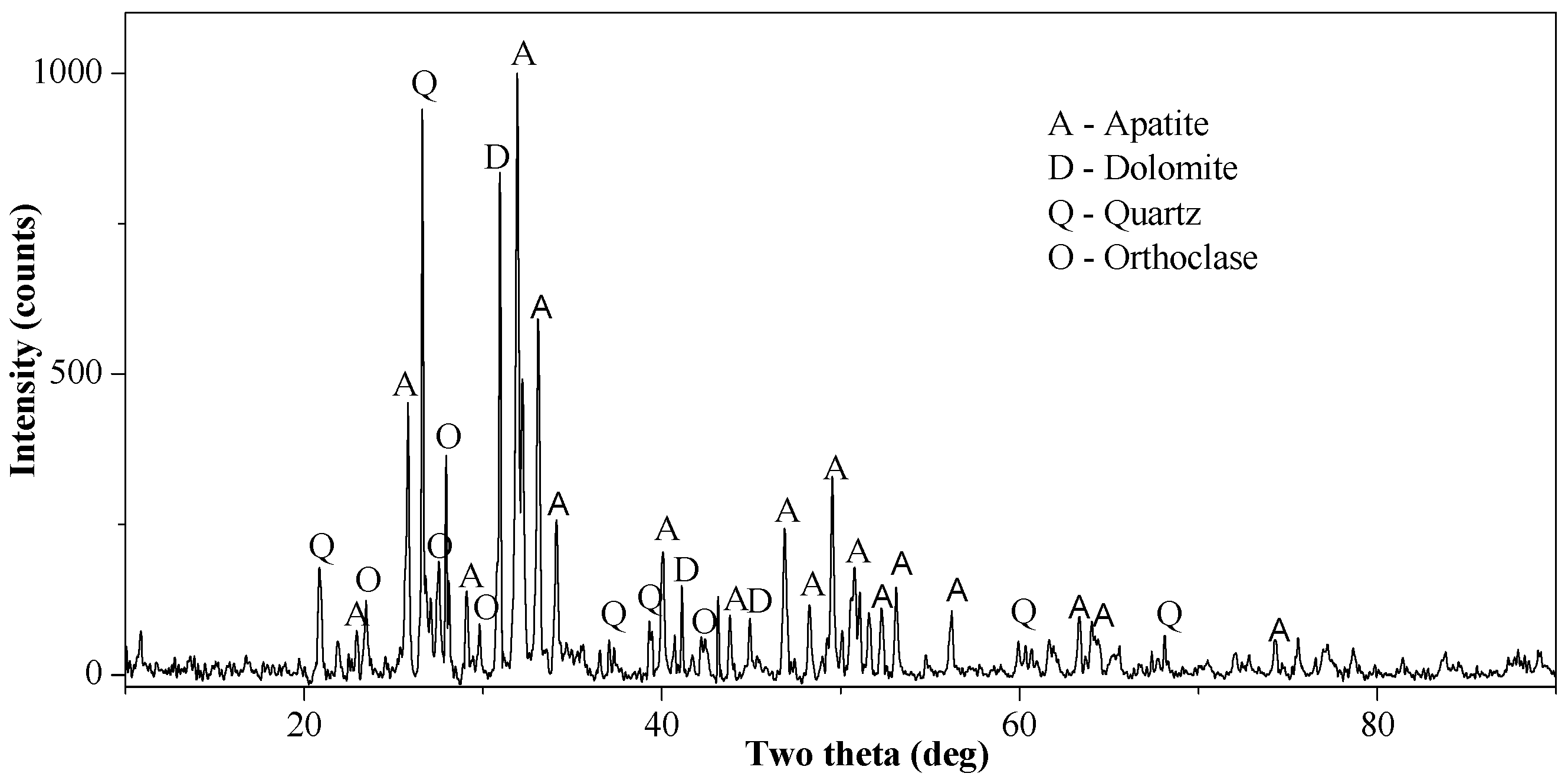
The results of optical mineralogy and the XRD analysis (
Figure 2) revealed the major phases of minerals which to be apatite, quartz, dolomite, clay minerals, and pyrite. The mineral composition of the sample was obtained by the comprehensive analysis of the chemical composition, optical microscopy and XRD. The results are shown in
Table 2. The density of the main minerals is also tabulated in
Table 2 [
22].
The samples were firstly wet ground in a HLXMQ-Φ240 × 90 laboratory ball mill from Wuhan Exploring Machinery Factory (Wuhan, China) at 50 wt % solids, until a particle size distribution of 70% passing 74 μm was achieved. The ground product was then subjected to characterization studies and separation studies. The particle size fraction analysis which was carried out using a Mastersizer 2000 laser particle characterization system (Malvern Instruments Ltd., Malvern, UK), showing that the specific surface of the ground product was 0.78 m2/g, and the d10, d50, and d90 (diameter at the cumulative undersize of 10%, 50%, and 90%) was 2, 51 and 115 μm, respectively. The liberation of collophanite was analyzed. To start with, a representative sample of particles weighing approximately 5 g was taken from +30 μm size fractions of the ground product. The −30 μm latter size fraction was not prepared for liberation analysis in a reflecting microscope, due to magnification restrictions, which makes it difficult to definitely distinguish mineral particles. The results showed that the liberation degree in +30 μm is 70.4%. The viscosity of slurry was measured by NDJ-5S viscometer (Hengping Scientific Instruments Ltd., Shanghai, China). The results showed that, while the solid content between 10% and 35%, the viscosity increased slowly from 1.168 to 1.176 mPa·s as the solid content increased. Surface tension measurement was conducted by the Du Noüy ring method. The results showed that the surface tension of the slurry was 63 mN/m at reverse flotation conditions, while 54 mN/m at direct flotation conditions.
Commercial water glass (WG) from Xiangxing Co. Ltd. (Loudi, China) of modulus 3.1 was used as the depressant for silicate minerals and dispersant, which had a purity of 97%. Na2CO3 from Xilong Chemical Co., Ltd. (Wuhan, China) with purity of 99% was used as a pH modifier in direct flotation. Sulfuric acid (SA) from Pingmei Group Co. Ltd. (Pingdingshan, China) was used as a pH modifier (98% purity) in reverse flotation. Commercial product CXY-P (a fatty acid mixture) from Chuxiang Phosphorus Technology Ltd. (Wuhan, China) was used as a collector.
The water used was the potable water in Wuhan, China.