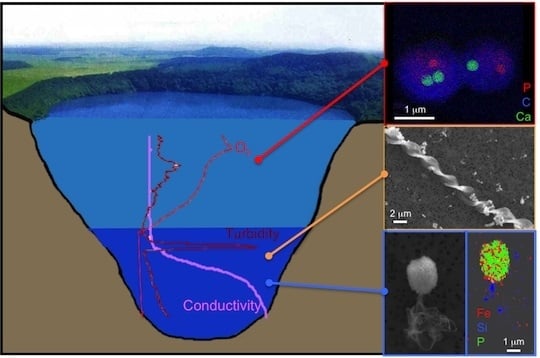
Mineralogical Diversity in Lake Pavin: Connections with Water Column Chemistry and Biomineralization Processes
Abstract
:1. Introduction
- (1)
- (2)
- Nitrate-dependent Fe(II)-oxidizing bacteria may also be present in the water column of meromictic lakes, as suggested by electron and X-ray microscopy analyses of Lake Pavin samples [20]. This metabolism has been shown to promote Fe-oxyhydroxide and Fe-phosphate precipitation, leading to bacterial cell encrustation [21,22,23].
- (3)
- (4)
2. Experimental Section
2.1. Geochemistry of Lake Pavin
2.2. Chemical Analyses
2.3. Electron Microscopy
2.3.1. Sample Preparation
2.3.2. Scanning Electron Microscopy
2.3.3. Transmission Electron Microscopy
3. Results and Discussion
3.1. Mineral Phases in the Superficial Zone of the Lake (0–30-m Depth)
3.2. Transition from Mn- to Fe-Mineral Formation (50–60-m Depth)
3.3. Fe Biomineralization across and below the Oxycline
3.4. Microorganisms: Mineral Interactions down to the Deep Monimolimnion (60–90-m Depth)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Ehrlich, H. Geomicrobiology: Its significance for geology. Earth Sci. Rev. 1998, 45, 45–60. [Google Scholar] [CrossRef]
- Nealson, K.H.; Stahl, D.A. Microorganisms and biogeochemical cycles: What can we learn from layered microbial communities? In Geomicrobiology: Interactions between Microbes and Minerals; Banfield, J.F., Nealson, K.H., Eds.; Mineralogical Society of America: Chantilly, VA, USA, 1997; Volume 35, pp. 5–34. [Google Scholar]
- Benzerara, K.; Miot, J.; Morin, G.; Ona-Nguema, G.; Skouri-Panet, F.; Férard, C. Significance, mechanisms and environmental implications of microbial biomineralization. C. R. Geosci. 2011, 343, 160–167. [Google Scholar] [CrossRef]
- Busigny, V.; Planavsky, N.J.; Jézéquel, D.; Crowe, S.; Louvat, P.; Moureau, J.; Viollier, E.; Lyons, T.W. Iron isotopes in an Archean ocean analogue. Geochim. Cosmochim. Acta 2014, 133, 443–462. [Google Scholar] [CrossRef]
- Crowe, S.A.; Døssing, L.N.; Beukes, N.J.; Bau, M.; Kruger, S.J.; Frei, R.; Canfield, D.E. Atmospheric oxygenation three billion years ago. Nature 2013, 501, 535–538. [Google Scholar] [CrossRef] [PubMed]
- Reinhard, C.T.; Planavsky, N.J.; Robbins, L.J.; Partin, C.A.; Gill, B.C.; Lalonde, S.V.; Bekker, A.; Konhauser, K.O.; Lyons, T.W. Proterozoic ocean redox and biogeochemical stasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5357–5362. [Google Scholar] [CrossRef] [PubMed]
- Canfield, D.E.; Poulton, S.W.; Knoll, A.H.; Narbonne, G.M.; Ross, G.; Goldberg, T.; Strauss, H. Ferruginous conditions dominated later neoproterozoic deep-water chemistry. Science 2008, 321, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Czaja, A.D.; Van Kranendonk, M.J.; Beard, B.L.; Roden, E.E.; Johnson, C.M. An anoxic, Fe(II)-rich, U-poor ocean 3.46 billion years ago. Geochim. Cosmochim. Acta 2013, 120, 65–79. [Google Scholar] [CrossRef]
- Planavsky, N.J.; McGoldrick, P.; Scott, C.T.; Li, C.; Reinhard, C.T.; Kelly, A.E.; Chu, X.; Bekker, A.; Love, G.D.; Lyons, T.W. Widespread iron-rich conditions in the mid-Proterozoic ocean. Nature 2011, 477, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Poulton, S.W.; Canfield, D.E. Ferruginous conditions: A dominant feature of the ocean through Earth’s history. Elements 2011, 7, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Busigny, V.; Lebeau, O.; Ader, M.; Krapež, B.; Bekker, A. Nitrogen cycle in the Late Archean ferruginous ocean. Chem. Geol. 2013, 362, 115–130. [Google Scholar] [CrossRef]
- Farquhar, J. Atmospheric influence of Earth’s earliest sulfur cycle. Science 2000, 289, 756–758. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Beard, B.L.; Klein, C.; Beukes, N.J.; Roden, E.E. Iron isotopes constrain biologic and abiologic processes in banded iron formation genesis. Geochim. Cosmochim. Acta 2008, 72, 151–169. [Google Scholar] [CrossRef]
- Planavsky, N.; Rouxel, O.; Bekker, A.; Shapiro, R.; Fralick, P.; Knudsen, A. Iron-oxidizing microbial ecosystems thrived in late Paleoproterozoic redox-stratified oceans. Earth Planet. Sci. Lett. 2009, 286, 230–242. [Google Scholar] [CrossRef]
- Crowe, S.A.; Jones, C.; Katsev, S.; Magen, C.; O’Neill, A.H.; Sturm, A.; Canfield, D.E.; Haffner, G.D.; Mucci, A.; Sundby, B.; et al. Photoferrotrophs thrive in an Archean Ocean analogue. Proc. Natl. Acad. Sci. USA 2008, 105, 15938–15943. [Google Scholar] [CrossRef] [PubMed]
- Walter, X.A.; Picazo, A.; Miracle, M.R.; Vicente, E.; Camacho, A.; Aragno, M.; Zopfi, J. Phototrophic Fe(II)-oxidation in the chemocline of a ferruginous meromictic lake. Front. Microbiol. 2014, 5, 713. [Google Scholar] [CrossRef] [PubMed]
- Kappler, A.; Newman, D.K. Formation of Fe(III)-minerals by Fe(II)-oxidizing photoautotrophic bacteria. Geochim. Cosmochim. Acta 2004, 68, 1217–1226. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Obst, M.; Kappler, A.; Hegler, F.; Schadler, S.; Bouchez, C.; Guyot, F.; Morin, G. Extracellular iron biomineralization by photoautotrophic iron-oxidizing bacteria. Appl. Environ. Microbiol. 2009, 75, 5586–5591. [Google Scholar] [CrossRef] [PubMed]
- Cosmidis, J.; Benzerara, K.; Morin, G.; Busigny, V.; Lebeau, O.; Jézéquel, D.; Noël, V.; Dublet, G.; Othmane, G. Biomineralization of iron-phosphates in the water column of Lake Pavin (Massif Central, France). Geochim. Cosmochim. Acta 2014, 126, 78–96. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Morin, G.; Kappler, A.; Bernard, S.; Obst, M.; Férard, C.; Skouri-Panet, F.; Guigner, J.-M.; Posth, N.; et al. Iron biomineralization by anaerobic neutrophilic iron-oxidizing bacteria. Geochim. Cosmochim. Acta 2009, 73, 696–711. [Google Scholar] [CrossRef]
- Miot, J.; Benzerara, K.; Morin, G.; Bernard, S.; Beyssac, O.; Larquet, E.; Kappler, A.; Guyot, F. Transformation of vivianite by anaerobic nitrate-reducing iron-oxidizing bacteria. Geobiology 2009, 7, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Li, J.; Benzerara, K.; Sougrati, M.T.; Ona-Nguema, G.; Bernard, S.; Jumas, J.-C.; Guyot, F. Formation of single domain magnetite by green rust oxidation promoted by microbial anaerobic nitrate-dependent iron oxidation. Geochim. Cosmochim. Acta 2014, 139, 327–343. [Google Scholar] [CrossRef]
- Lehours, A.-C.; Batisson, I.; Guedon, A.; Mailhot, G.; Fonty, G. Diversity of culturable bacteria, from the Anaerobic Zone of the meromictic Lake Pavin, able to perform dissimilatory-iron reduction in different in vitro conditions. Geomicrobiol. J. 2009, 26, 212–223. [Google Scholar] [CrossRef]
- Fredrickson, J.K.; Zachara, J.M.; Kennedy, D.W.; Dong, H.; Onstott, T.C.; Hinman, N.W.; Li, S. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim. Cosmochim. Acta 1998, 62, 3239–3257. [Google Scholar] [CrossRef]
- Kukkadapu, R.K.; Zachara, J.M.; Fredrickson, J.K.; Kennedy, D.W. Biotransformation of two-line silica-ferrihydrite by a dissimilatory Fe(III)-reducing bacterium: Formation of carbonate green rust in the presence of phosphate. Geochim. Cosmochim. Acta 2004, 68, 2799–2814. [Google Scholar] [CrossRef]
- Ona-Nguema, G.; Abdelmoula, M.; Jorand, F.; Benali, O.; Block, J.-C.; Génin, J.-M.R. Iron(II,III) hydroxycarbonate green rust formation and stabilization from lepidocrocite bioreduction. Environ. Sci. Technol. 2002, 36, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Zegeye, A.; Bonneville, S.; Benning, L.G.; Sturm, A.; Fowle, D.A.; Jones, C.; Canfield, D.E.; Ruby, C.; MacLean, L.C.; Nomosatryo, S.; et al. Green rust formation controls nutrient availability in a ferruginous water column. Geology 2012, 40, 599–602. [Google Scholar] [CrossRef]
- Zegeye, A.; Mustin, C.; Jorand, F. Bacterial and iron oxide aggregates mediate secondary iron mineral formation: Green rust versus magnetite: Iron oxide aggregation and green rust formation. Geobiology 2010, 8, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Etique, M.; Jorand, F.P.A.; Zegeye, A.; Grégoire, B.; Despas, C.; Ruby, C. Abiotic process for Fe(II) oxidation and green rust mineralization driven by a heterotrophic nitrate reducing bacteria (Klebsiella mobilis). Environ. Sci. Technol. 2014, 48, 3742–3751. [Google Scholar] [CrossRef] [PubMed]
- Pantke, C.; Obst, M.; Benzerara, K.; Morin, G.; Ona-Nguema, G.; Dippon, U.; Kappler, A. Green rust formation during Fe(II) oxidation by the nitrate-reducing Acidovorax sp. strain BoFeN1. Environ. Sci. Technol. 2012, 46, 1439–1446. [Google Scholar] [CrossRef] [PubMed]
- Biderre-Petit, C.; Boucher, D.; Kuever, J.; Alberic, P.; Jézéquel, D.; Chebance, B.; Borrel, G.; Fonty, G.; Peyret, P. Identification of sulfur-cycle prokaryotes in a low-sulfate lake (Lake Pavin) using aprA and 16S rRNA gene markers. Microb. Ecol. 2011, 61, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Bura-Nakić, E.; Viollier, E.; Ciglenečki, I. Electrochemical and colorimetric measurements show the dominant role of FeS in a permanently anoxic lake. Environ. Sci. Technol. 2013, 47, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Bura-Nakić, E.; Viollier, E.; Jézéquel, D.; Thiam, A.; Ciglenečki, I. Reduced sulfur and iron species in anoxic water column of meromictic crater Lake Pavin (Massif Central, France). Chem. Geol. 2009, 266, 311–317. [Google Scholar] [CrossRef]
- Donald, R.; Southam, G. Low temperature anaerobic bacterial diagenesis of ferrous monosulfide to pyrite. Geochim. Cosmochim. Acta 1999, 63, 2019–2023. [Google Scholar] [CrossRef]
- Chan, C.S.; Fakra, S.C.; Emerson, D.; Fleming, E.J.; Edwards, K.J. Lithotrophic iron-oxidizing bacteria produce organic stalks to control mineral growth: Implications for biosignature formation. ISME J. 2011, 5, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Labrenz, M.; Banfield, J.F. Sulfate-reducing bacteria-dominated biofilms that precipitate ZnS in a subsurface circumneutral-pH mine drainage system. Microb. Ecol. 2004, 47, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Bazylinski, D.A.; Frankel, R.B.; Konhauser, K.O. Modes of biomineralization of magnetite by microbes. Geomicrobiol. J. 2007, 24, 465–475. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; et al. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed]
- Couradeau, E.; Benzerara, K.; Gerard, E.; Moreira, D.; Bernard, S.; Brown, G.E.; Lopez-Garcia, P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, C.T.; Menguy, N.; Abreu, F.; Lins, U.; Posfai, M.; Prozorov, T.; Pignol, D.; Frankel, R.B.; Bazylinski, D.A. A cultured greigite-producing magnetotactic bacterium in a novel group of sulfate-reducing bacteria. Science 2011, 334, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Stief, P.; Behrens, S.; Kappler, A.; Schmidt, C. High spatial resolution of distribution and interconnections between Fe- and N-redox processes in profundal lake sediments: Microbial Fe and N redox cycling in lake sediments. Environ. Microbiol. 2014, 16, 3287–3303. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, K.U.; Loy, A.; Jakobsen, T.F.; Thomsen, T.R.; Wagner, M.; Ingvorsen, K. Diversity of sulfate-reducing bacteria from an extreme hypersaline sediment, Great Salt Lake (Utah): Diversity of SRB in Great Salt Lake. FEMS Microbiol. Ecol. 2007, 60, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Rees, G.N.; Baldwin, D.S.; Watson, G.O.; Hall, K.C. Sulfide formation in freshwater sediments, by sulfate-reducing microorganisms with diverse tolerance to salt. Sci. Total Environ. 2010, 409, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Michard, G.; Viollier, E.; Jézéquel, D.; Sarazin, G. Geochemical study of a crater lake: Pavin Lake, France—Identification, location and quantification of the chemical reactions in the lake. Chem. Geol. 1994, 115, 103–115. [Google Scholar] [CrossRef]
- Viollier, E.; Michard, G.; Jézéquel, D.; Pèpe, M.; Sarazin, G. Geochemical study of a crater lake: Lake Pavin, Puy de Dôme, France. Constraints afforded by the particulate matter distribution in the element cycling within the lake. Chem. Geol. 1997, 142, 225–241. [Google Scholar] [CrossRef]
- Podda, F.; Michard, G. Mesure colorimétrique de l’alcalinité. C. R. Acad. Sci. 1994, 319, 651–657. (In French) [Google Scholar]
- Lehours, A.-C.; Bardot, C.; Thenot, A.; Debroas, D.; Fonty, G. Anaerobic microbial communities in Lake Pavin, a unique meromictic lake in France. Appl. Environ. Microbiol. 2005, 71, 7389–7400. [Google Scholar] [CrossRef] [PubMed]
- Viollier, E.; Jézéquel, D.; Michard, G.; Pèpe, M.; Sarazin, G.; Alberic, P. Geochemical study of a crater lake (Pavin Lake, France): Trace-element behaviour in the monimolimnion. Chem. Geol. 1995, 125, 61–72. [Google Scholar] [CrossRef]
- Grami, B.; Rasconi, S.; Niquil, N.; Jobard, M.; Saint-Béat, B.; Sime-Ngando, T. Functional effects of parasites on food web properties during the spring diatom bloom in Lake Pavin: A linear inverse modeling analysis. PLoS ONE 2011, 6, e23273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasconi, S.; Grami, B.; Niquil, N.; Jobard, M.; Sime-Ngando, T. Parasitic chytrids sustain zooplankton growth during inedible algal bloom. Front. Microbiol. 2014, 5, 229. [Google Scholar] [CrossRef] [PubMed]
- Ragon, M.; Benzerara, K.; Moreira, D.; Tavera, R.; López-García, P. 16S rDNA-based analysis reveals cosmopolitan occurrence but limited diversity of two cyanobacterial lineages with contrasted patterns of intracellular carbonate mineralization. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Tebo, B.M.; Johnson, H.A.; McCarthy, J.K.; Templeton, A.S. Geomicrobiology of manganese(II) oxidation. Trends Microbiol. 2005, 13, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; McCarthy, J.K.; Tebo, B.M. Elimination of manganese(II,III) oxidation in pseudomonas putida GB-1 by a double knockout of two putative multicopper oxidase genes. Appl. Environ. Microbiol. 2013, 79, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Geszvain, K.; Butterfield, C.; Davis, R.E.; Madison, A.S.; Lee, S.; Parker, D.L.; Soldatova, A.; Spiro, T.G.; Luther, G.W.; Tebo, B.M. The molecular biogeochemistry of manganese(II) oxidation. Biochem. Soc. Trans. 2012, 40, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Learman, D.R.; Voelker, B.M.; Vazquez-Rodriguez, A.I.; Hansel, C.M. Formation of manganese oxides by bacterially generated superoxide. Nat. Geosci. 2011, 4, 95–98. [Google Scholar] [CrossRef]
- Keim, C.N.; Nalini, H.A.; de Lena, J.C. Manganese oxide biominerals from freshwater environments in Quadrilatero Ferrifero, Minas Gerais, Brazil. Geomicrobiol. J. 2015, 32, 549–559. [Google Scholar] [CrossRef]
- Chubar, N.; Avramut, C.; Visser, T. Formation of manganese phosphate and manganese carbonate during long-term sorption of Mn2+ by viable Shewanella putrefaciens: Effects of contact time and temperature. Environ. Sci. Process. Impacts 2015, 17, 780–790. [Google Scholar] [CrossRef] [PubMed]
- Friedl, G.; Wehrli, B.; Manceau, A. Solid phases in the cycling of manganese in eutrophic lakes: New insights from EXAFS spectroscopy. Geochim. Cosmochim. Acta 1997, 61, 275–290. [Google Scholar] [CrossRef]
- Jones, C.; Crowe, S.A.; Sturm, A.; Leslie, K.L.; MacLean, L.C.W.; Katsev, S.; Henny, C.; Fowle, D.A.; Canfield, D.E. Biogeochemistry of manganese in ferruginous Lake Matano, Indonesia. Biogeosciences 2011, 8, 2977–2991. [Google Scholar] [CrossRef] [Green Version]
- Carrias, J.-F.; Thouvenot, A.; Amblard, C.; Sime-Ngando, T. Dynamics and growth estimates of planktonic protists during early spring in Lake Pavin, France. Aquat. Microb. Ecol. 2001, 24, 163–174. [Google Scholar] [CrossRef]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Buffle, J.; De Vitre, R.R.; Perret, D.; Leppard, G.G. Physico-chemical characteristics of a colloidal iron phosphate species formed at the oxic-anoxic interface of a eutrophic lake. Geochim. Cosmochim. Acta 1989, 53, 399–408. [Google Scholar] [CrossRef]
- Lienemann, C.P.; Monnerat, M.; Dominik, J.; Perret, D. Identification of stoichiometric iron-phosphorus colloids produced in a eutrophic lake. Aquat. Sci. 1999, 61, 133–149. [Google Scholar] [CrossRef]
- Voegelin, A.; Senn, A.-C.; Kaegi, R.; Hug, S.J.; Mangold, S. Dynamic Fe-precipitate formation induced by Fe(II) oxidation in aerated phosphate-containing water. Geochim. Cosmochim. Acta 2013, 117, 216–231. [Google Scholar] [CrossRef]
- Senn, A.-C.; Kaegi, R.; Hug, S.J.; Hering, J.G.; Mangold, S.; Voegelin, A. Composition and structure of Fe(III)-precipitates formed by Fe(II) oxidation in water at near-neutral pH: Interdependent effects of phosphate, silicate and Ca. Geochim. Cosmochim. Acta 2015, 162, 220–246. [Google Scholar] [CrossRef]
- Lefèvre, C.T. Genomic insights into the early-diverging magnetotactic bacteria. Environ. Microbiol. 2016, 18, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Lefevre, C.T.; Bazylinski, D.A. Ecology, diversity, and evolution of magnetotactic bacteria. Microbiol. Mol. Biol. Rev. 2013, 77, 497–526. [Google Scholar] [CrossRef] [PubMed]
- Lehours, A.-C.; Evans, P.; Bardot, C.; Joblin, K.; Gerard, F. Phylogenetic diversity of archaea and bacteria in the anoxic zone of a meromictic lake (Lake Pavin, France). Appl. Environ. Microbiol. 2007, 73, 2016–2019. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Merrill Floyd, M. Enrichment and isolation of iron-oxidizing bacteria at neutral pH. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 2005; Volume 397, pp. 112–123. [Google Scholar]
- Chan, C.S. Microbial polysaccharides template assembly of nanocrystal fibers. Science 2004, 303, 1656–1658. [Google Scholar] [CrossRef] [PubMed]
- Comolli, L.R.; Luef, B.; Chan, C.S. High-resolution 2D and 3D cryo-TEM reveals structural adaptations of two stalk-forming bacteria to an Fe-oxidizing lifestyle: 3D cryo-TEM of Fe-oxidizing bacteria. Environ. Microbiol. 2011, 13, 2915–2929. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.J.; Langdon, A.E.; Martinez-Garcia, M.; Stepanauskas, R.; Poulton, N.J.; Masland, E.D.P.; Emerson, D. What’s new is old: Resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and FISH. PLoS ONE 2011, 6, e17769. [Google Scholar] [CrossRef] [PubMed]
- Seder-Colomina, M.; Morin, G.; Benzerara, K.; Ona-Nguema, G.; Pernelle, J.-J.; Esposito, G.; Van Hullebusch, E.D. Sphaerotilus natans, a neutrophilic iron-related sheath-forming bacterium: Perspectives for metal remediation strategies. Geomicrobiol. J. 2014, 31, 64–75. [Google Scholar] [CrossRef]
- Banfield, J.F. Aggregation-based crystal growth and microstructure development in natural iron oxyhydroxide biomineralization products. Science 2000, 289, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Melton, E.D.; Schmidt, C.; Kappler, A. Microbial iron(II) oxidation in littoral freshwater lake sediment: The potential for competition between phototrophic vs. nitrate-reducing iron(II)-oxidizers. Front. Microbiol. 2012, 3, 197. [Google Scholar] [CrossRef] [PubMed]
- Reguera, G.; Nevin, K.P.; Nicoll, J.S.; Covalla, S.F.; Woodard, T.L.; Lovley, D.R. Biofilm and nanowire production leads to increased current in geobacter sulfurreducens fuel cells. Appl. Environ. Microbiol. 2006, 72, 7345–7348. [Google Scholar] [CrossRef] [PubMed]
- Lehours, A.-C.; Rabiet, M.; Morel-Desrosiers, N.; Morel, J.-P.; Jouve, L.; Arbeille, B.; Mailhot, G.; Fonty, G. Ferric iron reduction by fermentative strain BS2 isolated from an iron-rich anoxic environment (Lake Pavin, France). Geomicrobiol. J. 2010, 27, 714–722. [Google Scholar] [CrossRef]
- Dohnalkova, A.C.; Marshall, M.J.; Arey, B.W.; Williams, K.H.; Buck, E.C.; Fredrickson, J.K. Imaging hydrated microbial extracellular polymers: Comparative analysis by electron microscopy. Appl. Environ. Microbiol. 2011, 77, 1254–1262. [Google Scholar] [CrossRef] [PubMed]
- Miot, J.; Benzerara, K.; Kappler, A. Investigating microbe-mineral interactions: Recent advances in X-ray and electron microscopy and redox-sensitive methods. Annu. Rev. Earth Planet. Sci. 2014, 42, 271–289. [Google Scholar] [CrossRef]
- Maki, J.S. Bacterial intracellular sulfur globules: Structure and function. J. Mol. Microbiol. Biotechnol. 2013, 23, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Devaux, J. Contribution a l’etude limnologique du Lac Pavin (France) I: Facteurs abiotiques et phytoplancton. Hydrobiologia 1980, 68, 167–189. (In French) [Google Scholar] [CrossRef]
- Ghosh, W.; Dam, B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 2009, 33, 999–1043. [Google Scholar] [CrossRef] [PubMed]
- De Gusseme, B.; De Schryver, P.; De Cooman, M.; Verbeken, K.; Boeckx, P.; Verstraete, W.; Boon, N. Nitrate-reducing, sulfide-oxidizing bacteria as microbial oxidants for rapid biological sulfide removal: NR-SOB as microbial oxidants for biological sulfide removal. FEMS Microbiol. Ecol. 2009, 67, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Campbell, B.J.; Engel, A.S.; Porter, M.L.; Takai, K. The versatile ε-proteobacteria: Key players in sulphidic habitats. Nat. Rev. Microbiol. 2006, 4, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Vetriani, C.; Tran, H.V.; Kerkhof, L.J. Fingerprinting microbial assemblages from the oxic/anoxic chemocline of the Black Sea. Appl. Environ. Microbiol. 2003, 69, 6481–6488. [Google Scholar] [CrossRef] [PubMed]
- Bonny, S.M.; Jones, B. Experimental precipitation of barite (BaSO4) among streamers of sulfur-oxidizing bacteria. J. Sediment. Res. 2008, 78, 357–365. [Google Scholar] [CrossRef]
- Bonny, S.M.; Jones, B. Barite (BaSO4) biomineralization at Flybye Springs, a cold sulphur spring system in Canada’s Northwest Territories. Can. J. Earth Sci. 2007, 44, 835–856. [Google Scholar] [CrossRef]
- Senko, J.M.; Campbell, B.S.; Henriksen, J.R.; Elshahed, M.S.; Dewers, T.A.; Krumholz, L.R. Barite deposition resulting from phototrophic sulfide-oxidizing bacterial activity. Geochim. Cosmochim. Acta 2004, 68, 773–780. [Google Scholar] [CrossRef]
- Karnachuk, S.; Kurochkina, O.; Tuovin, O. Growth of sulfate-reducing bacteria with solid-phase electron acceptors. Appl. Microbiol. Biotechnol. 2002, 58, 482–486. [Google Scholar] [CrossRef] [PubMed]
- Francois, F.; Lombard, C.; Guigner, J.-M.; Soreau, P.; Brian-Jaisson, F.; Martino, G.; Vandervennet, M.; Garcia, D.; Molinier, A.-L.; Pignol, D.; et al. Isolation and characterization of environmental bacteria capable of extracellular biosorption of mercury. Appl. Environ. Microbiol. 2012, 78, 1097–1106. [Google Scholar] [CrossRef] [PubMed]
- Hellal, J.; Guédron, S.; Huguet, L.; Schäfer, J.; Laperche, V.; Joulian, C.; Lanceleur, L.; Burnol, A.; Ghestem, J.-P.; Garrido, F.; et al. Mercury mobilization and speciation linked to bacterial iron oxide and sulfate reduction: A column study to mimic reactive transfer in an anoxic aquifer. J. Contam. Hydrol. 2015, 180, 56–68. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kucharzyk, K.H.; Kim, B.; Deshusses, M.A.; Hsu-Kim, H. Net methylation of mercury in estuarine sediment microcosms amended with dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol. 2014, 48, 9133–9141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Kim, B.; Levard, C.; Reinsch, B.C.; Lowry, G.V.; Deshusses, M.A.; Hsu-Kim, H. Methylation of mercury by bacteria exposed to dissolved, nanoparticulate, and microparticulate mercuric sulfides. Environ. Sci. Technol. 2012, 46, 6950–6958. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.L.-T.; Morris, A.; Zhang, T.; Ticknor, J.; Levard, C.; Hsu-Kim, H. Precipitation of nanoscale mercuric sulfides in the presence of natural organic matter: Structural properties, aggregation, and biotransformation. Geochim. Cosmochim. Acta 2014, 133, 204–215. [Google Scholar] [CrossRef]
- Li, J.; Benzerara, K.; Bernard, S.; Beyssac, O. The link between biomineralization and fossilization of bacteria: Insights from field and experimental studies. Chem. Geol. 2013, 359, 49–69. [Google Scholar] [CrossRef]







| Minerals | Proposed Chemical Formula | Association with Microorganisms | Morphologies | Approximate Depth Range of Occurrence |
|---|---|---|---|---|
| Silica | SiO2 | Diatoms | Frustules | All depths, with higher concentrations in the mixolimnion |
| Phyllosilicate | - | - | - | All depths |
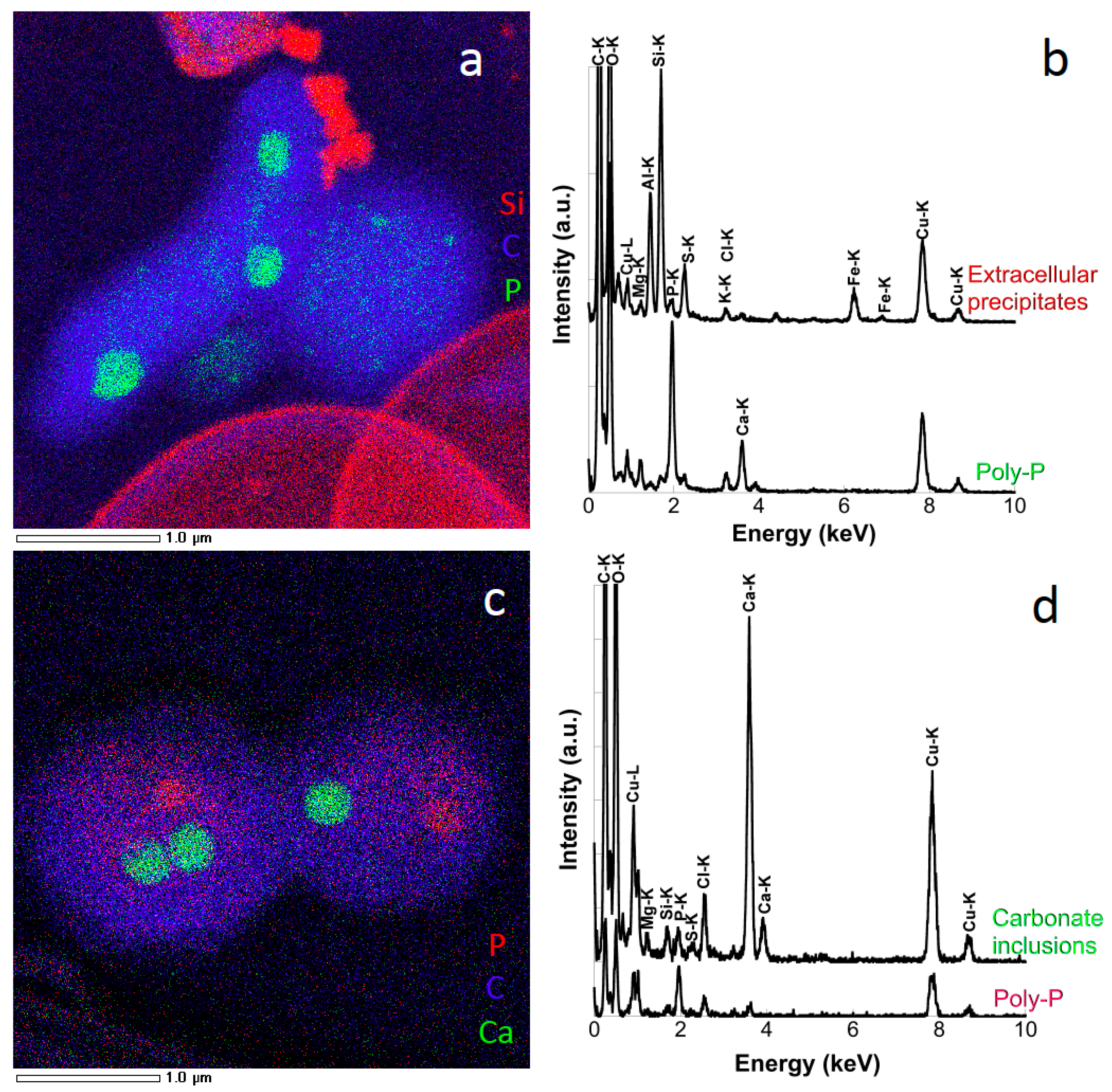
| Polyphosphate | H(n+2-2x-2y-z)KzMgyCaxPnO(3n+1) | Bacteria | Intracellular globules | All depths, with variations in amplitude depending on depth |
| Amorphous Ca-carbonate | CaCO3 | (Cyano)bacteria | Intracellular globules | 20 m |
| Mn-oxide | MnO2 | - | Star-like and 100–300-nm marbles | 52 m |
| Mn-phosphate | - | - | Star-like and 100–300-nm marbles | 52 m |
| Mn-Fe-phosphate | - | - | Star-like and 100–300-nm marbles | 55 m |
| Mn-phosphate | - | Peridinia | Noodle like | 52 m |
| Mn-Fe-phosphate | - | Peridinia | Noodle like | 55 m |
| Fe-phosphate | - | Peridinia | Noodle like | 58 m |
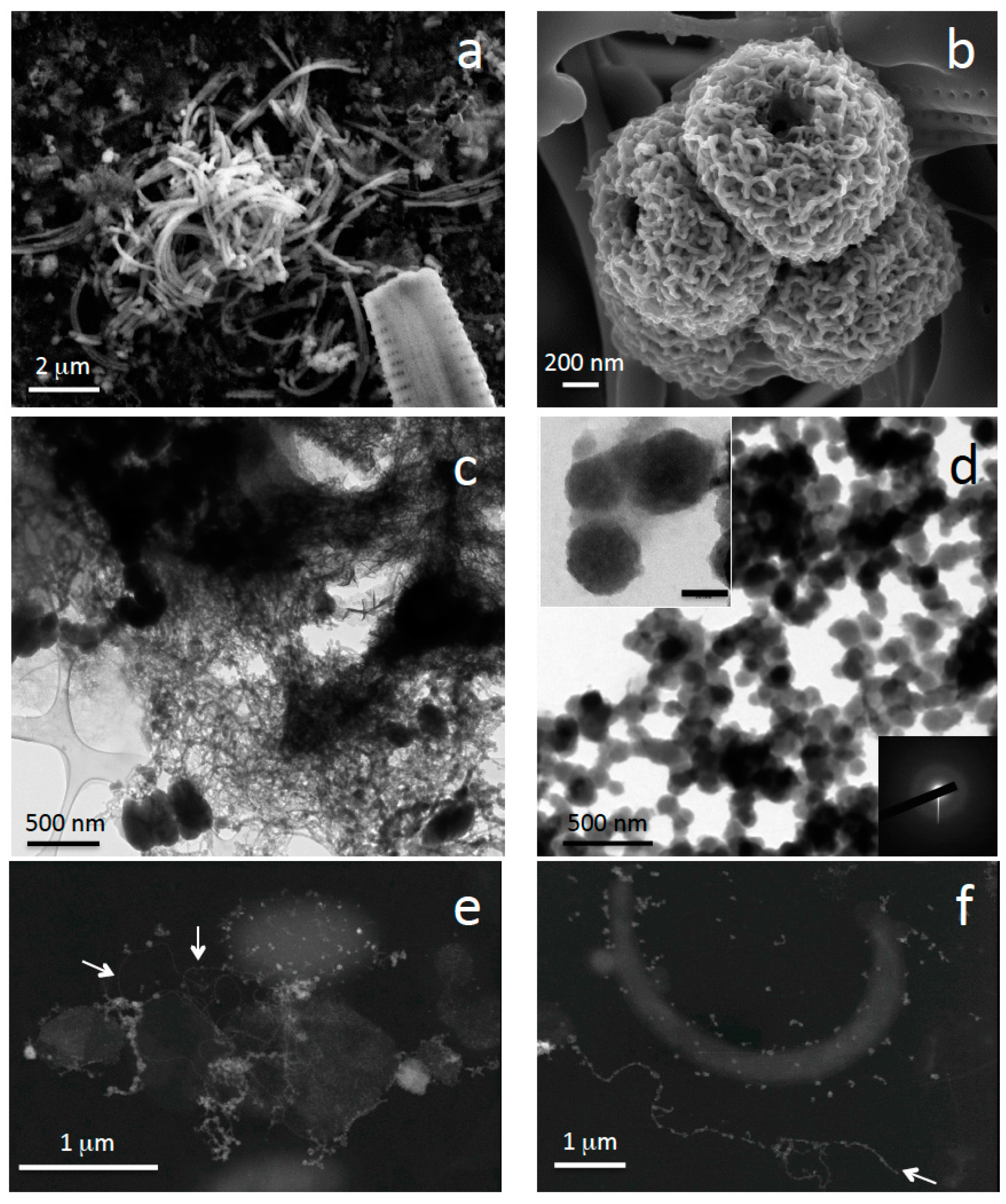
| Fe-phosphate | - | - | A few micrometer-long filaments, a few nm-long tangled filaments, 40–50-nm large spherical particles | Around the oxycline |
| Fe-phosphate | - | Bacteria (Fe(II)oxidizing nitrate-reducing bacteria?) | Cell surface encrustation | 60–80 m |
| Fe-phosphate | - | Gallionella ferruginea? | Stalks | 60–62 m |
| Fe-phosphate | - | Microbes | Cell surface-associated nanoparticles (3–5 nm) | 60–80 m |
| Fe-phosphate | - | - | 100–200-nm large particles | 60–90 m |
| Magnetite | Fe3O4 | Bacteria | Intracellular chains | Around the oxycline |
| Sulfur | S(°) | Sulfur-oxidizing bacteria | Intracellular globules | 62 m |
| Barium and sulfate bearing minerals | BaS or BaSO4 | - | Plurimicrometric | 67–90 m |
| Mercuric sulfide | HgS | Sulfate-reducing bacteria? | Nanoparticles (3–10 nm in diameter) | 52–80 m |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miot, J.; Jézéquel, D.; Benzerara, K.; Cordier, L.; Rivas-Lamelo, S.; Skouri-Panet, F.; Férard, C.; Poinsot, M.; Duprat, E. Mineralogical Diversity in Lake Pavin: Connections with Water Column Chemistry and Biomineralization Processes. Minerals 2016, 6, 24. https://doi.org/10.3390/min6020024
Miot J, Jézéquel D, Benzerara K, Cordier L, Rivas-Lamelo S, Skouri-Panet F, Férard C, Poinsot M, Duprat E. Mineralogical Diversity in Lake Pavin: Connections with Water Column Chemistry and Biomineralization Processes. Minerals. 2016; 6(2):24. https://doi.org/10.3390/min6020024
Chicago/Turabian StyleMiot, Jennyfer, Didier Jézéquel, Karim Benzerara, Laure Cordier, Sara Rivas-Lamelo, Fériel Skouri-Panet, Céline Férard, Mélanie Poinsot, and Elodie Duprat. 2016. "Mineralogical Diversity in Lake Pavin: Connections with Water Column Chemistry and Biomineralization Processes" Minerals 6, no. 2: 24. https://doi.org/10.3390/min6020024