Direct Adherence of Fe(III) Particles onto Sheaths of Leptothrix sp. Strain OUMS1 in Culture
Abstract
:1. Introduction
2. Experimental Section
2.1. Strains, Medium and Culturing
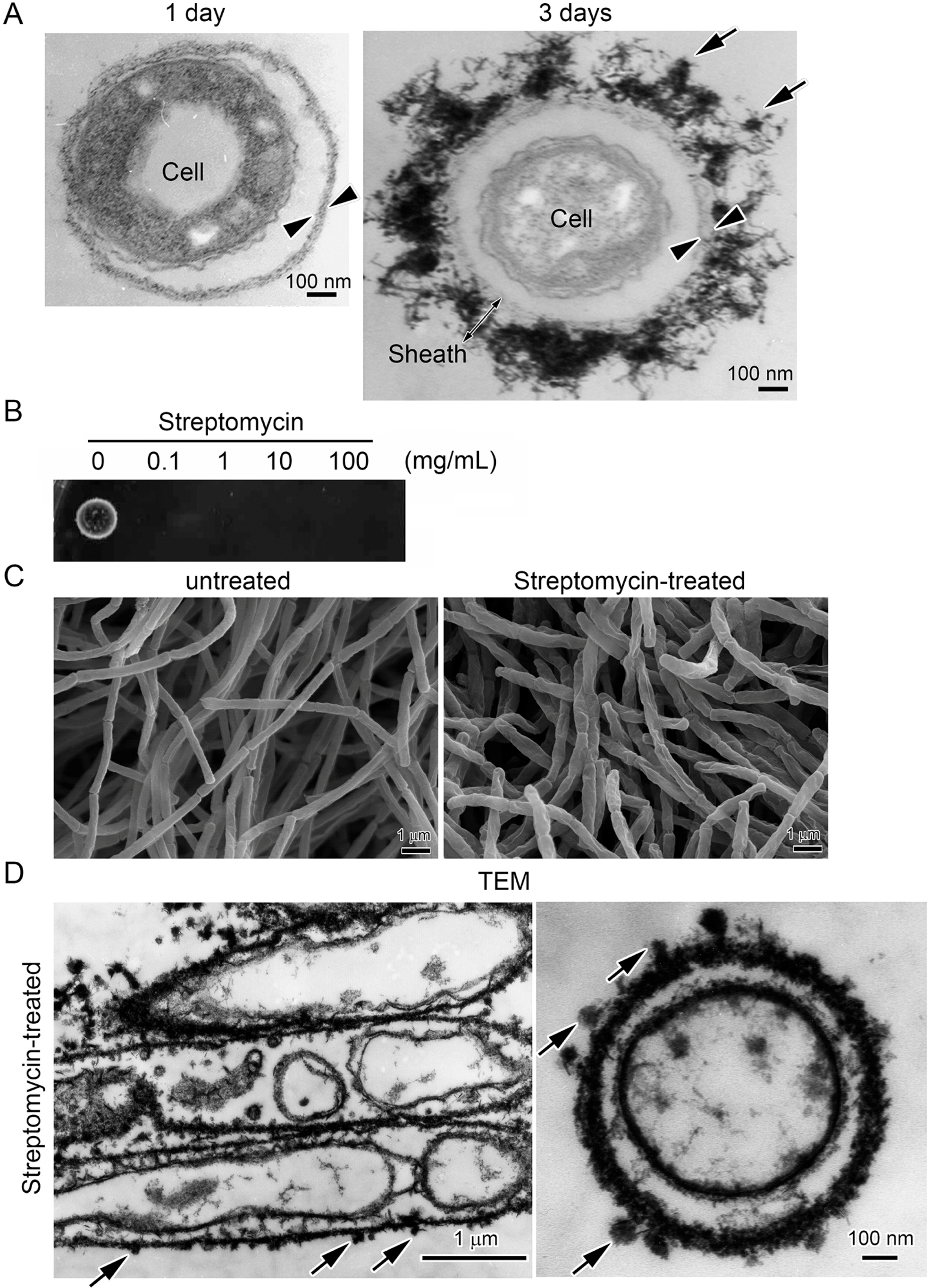
2.2. Streptomycin Treatment to Kill Cells within Sheaths
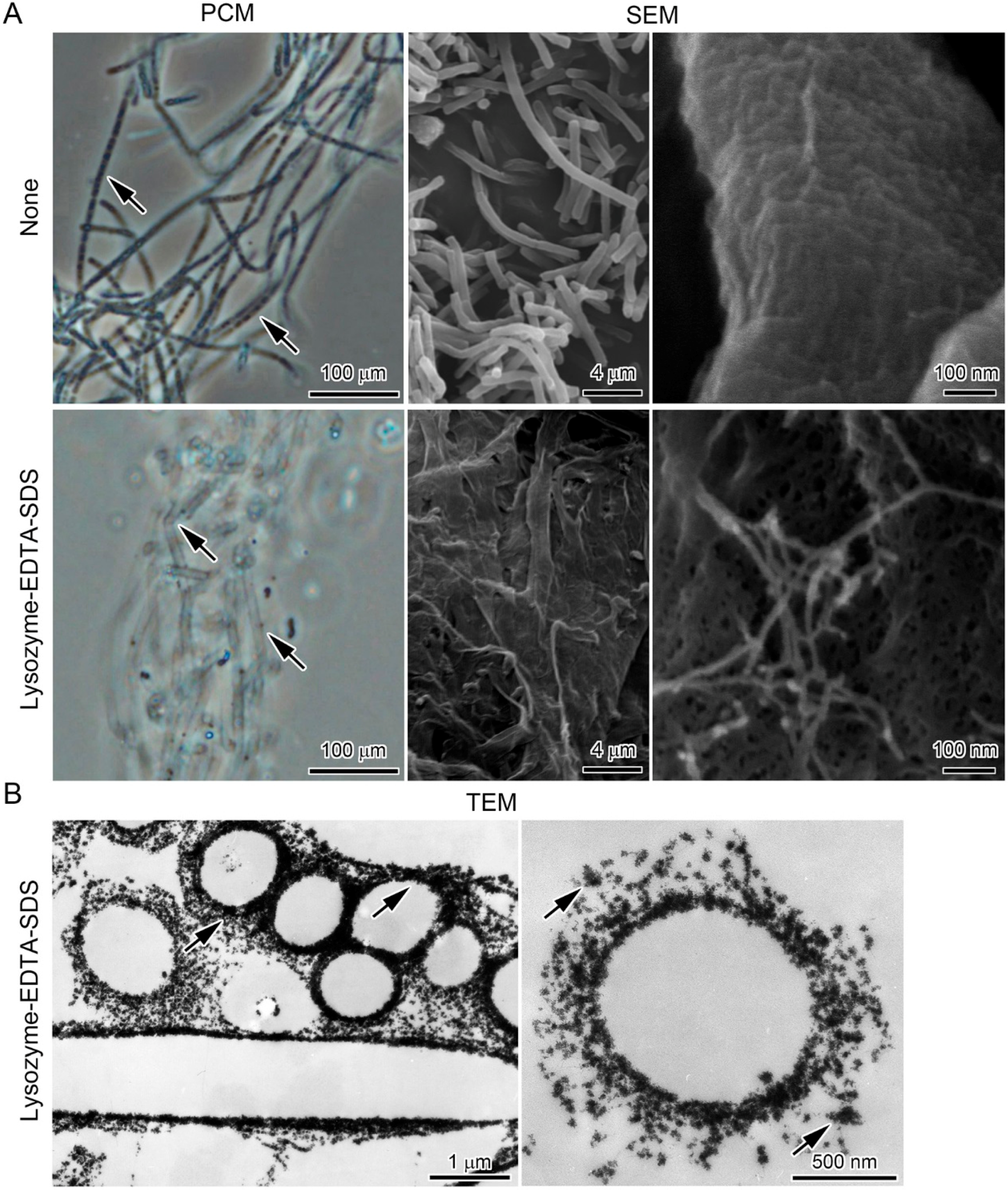
2.3. Differential Interference Contrast Microscopic Observation after Adherence of Fe Particles on Sheath Surfaces
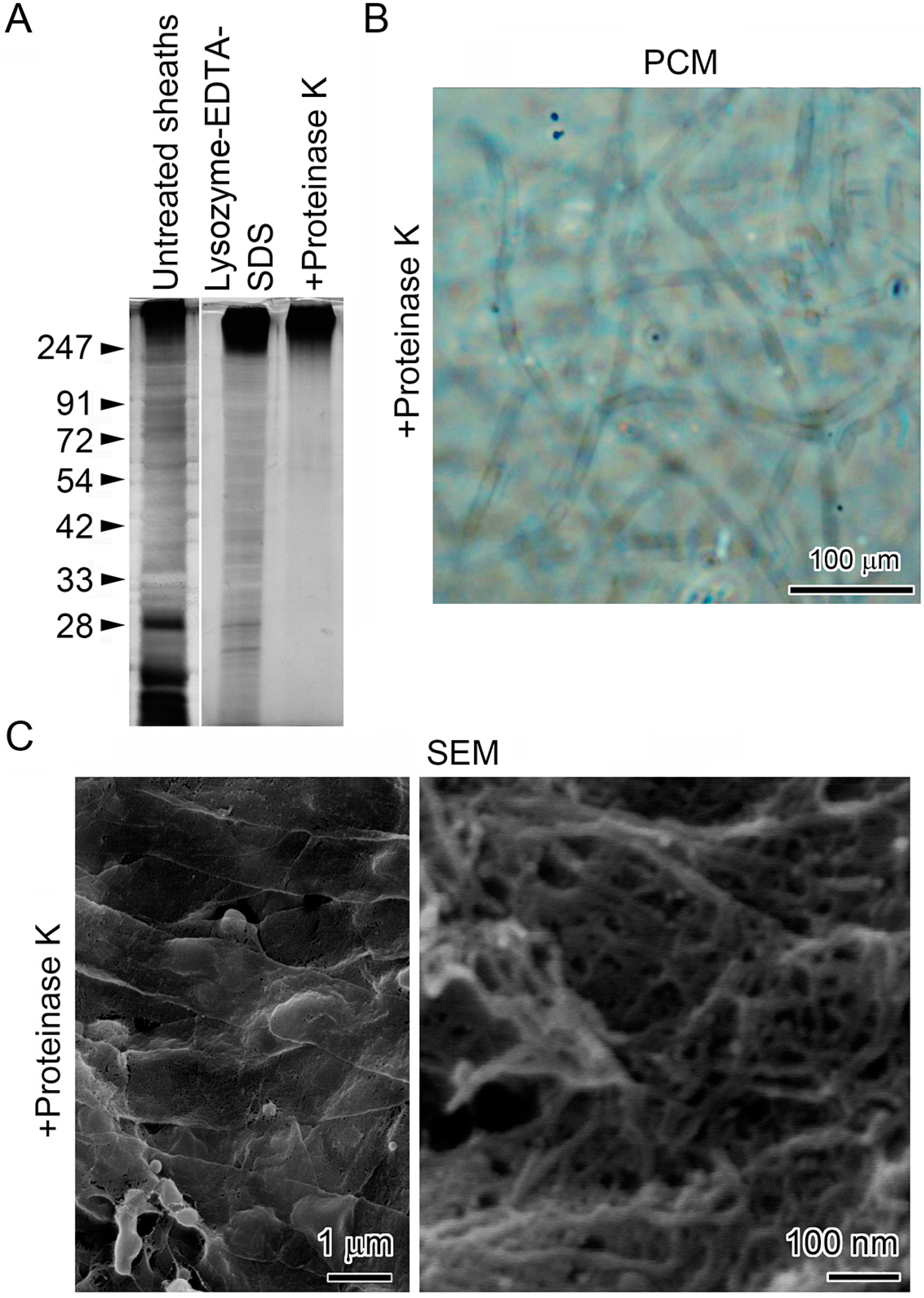
2.4. Lysozyme-EDTA-SDS (LES)-Proteinase K Treatment of the Sheaths
2.5. SDS-PAGE (Polyacrylamide Gel Electrophoresis) and Silver Staining to Confirm Removal of Protein from Sheath Remnants
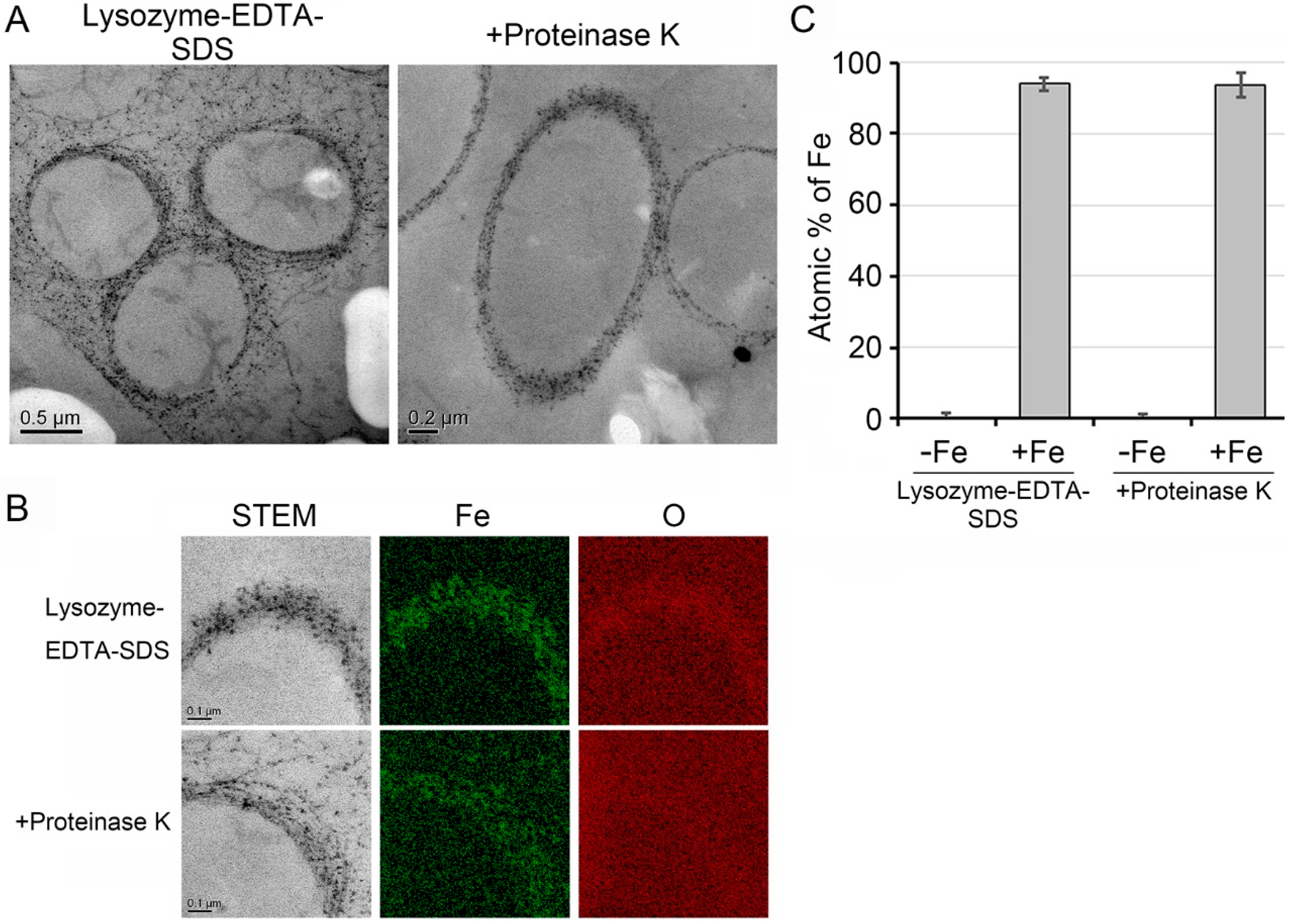
2.6. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-Ray Spectroscopy (EDX)
2.7. Transmission Electron Microscopy (TEM), High-Angle Annular Dark Field-Scanning Transmission Electron Microscopy (HAADF-STEM) and Energy Dispersive X-Ray Spectroscopy (EDX)
2.8. Determination of Fe(II) and Fe(II) + Fe(III) Concentrations in Culture Medium with O-Phenanthroline
2.9. Mössbauer Analysis of Precipitated Fe Particles Formed in Medium
3. Results and Discussion
3.1. Generation of Fe(III) Particles Containing P and Si in SGP + Fe by Abiotic Oxidation
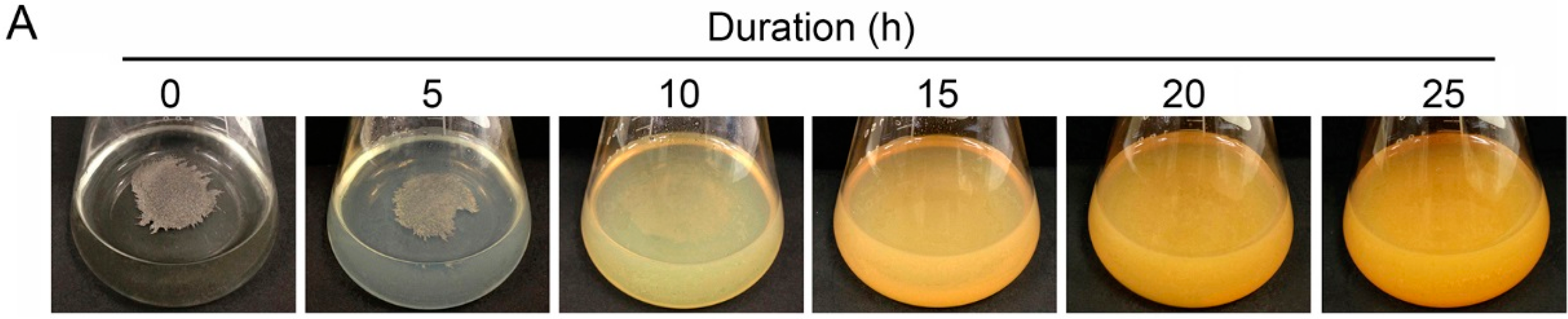
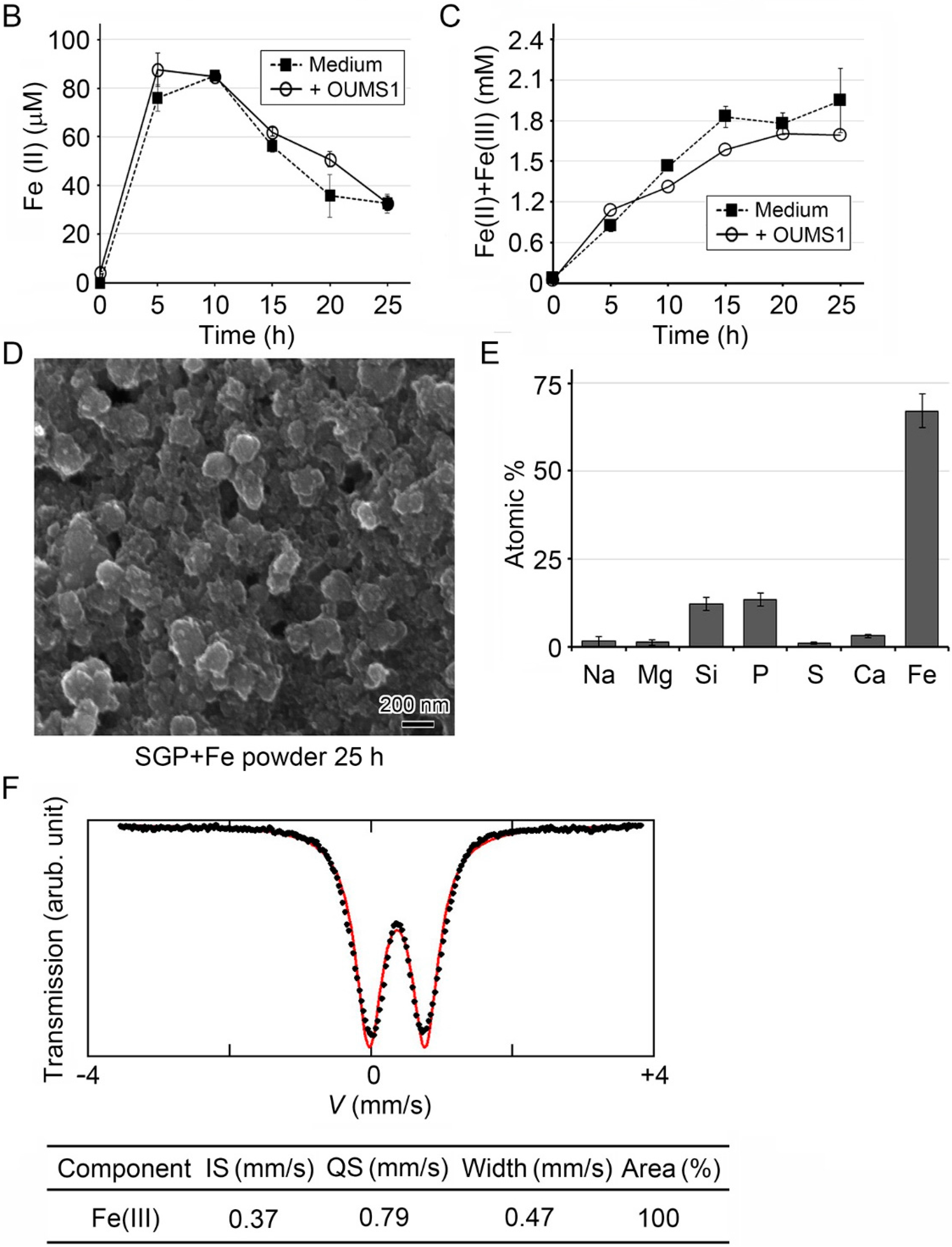
3.2. Adherence of Fe(III) Particles Preformed in SGP + Fe to Sheath Surfaces of OUMS1
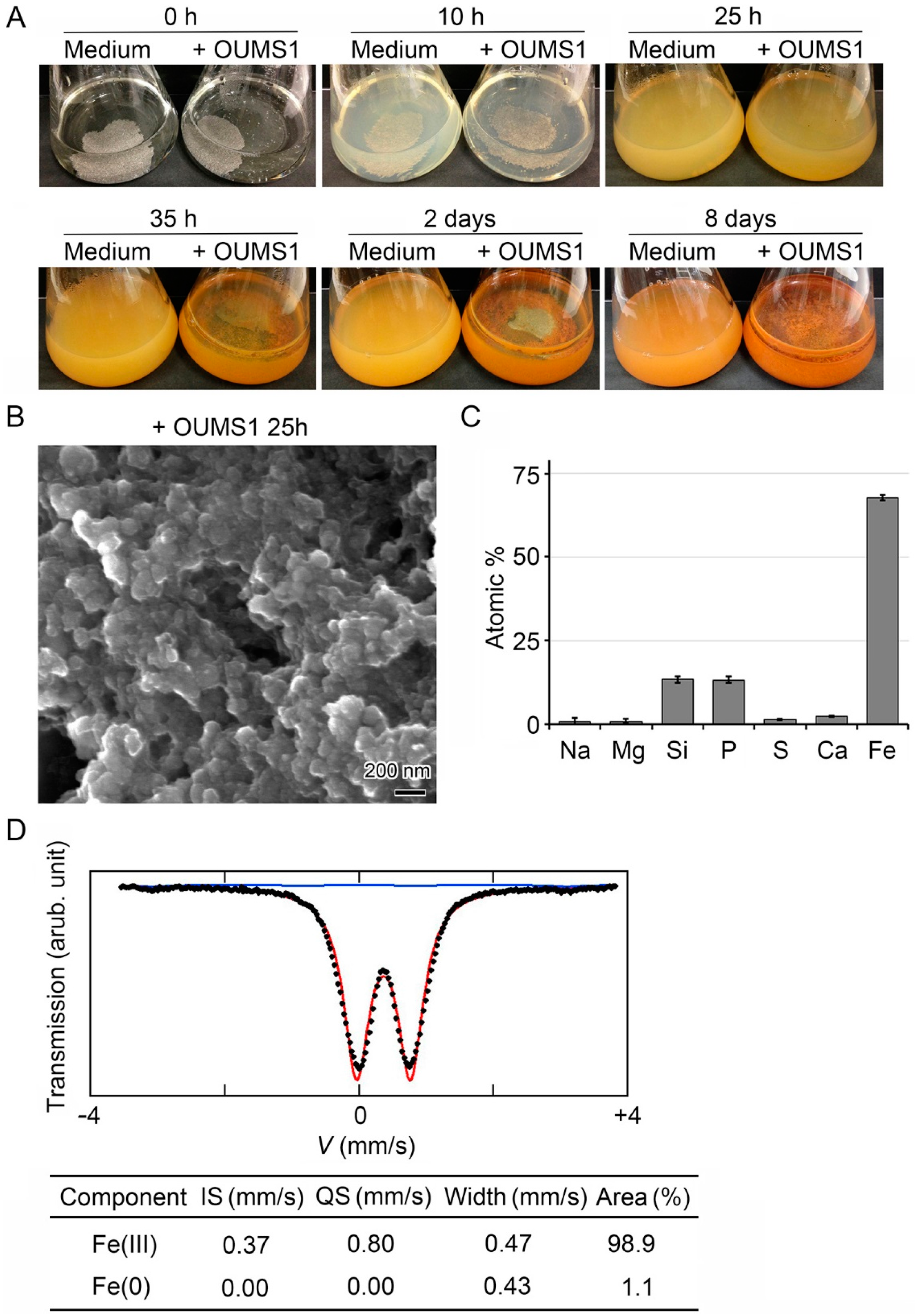
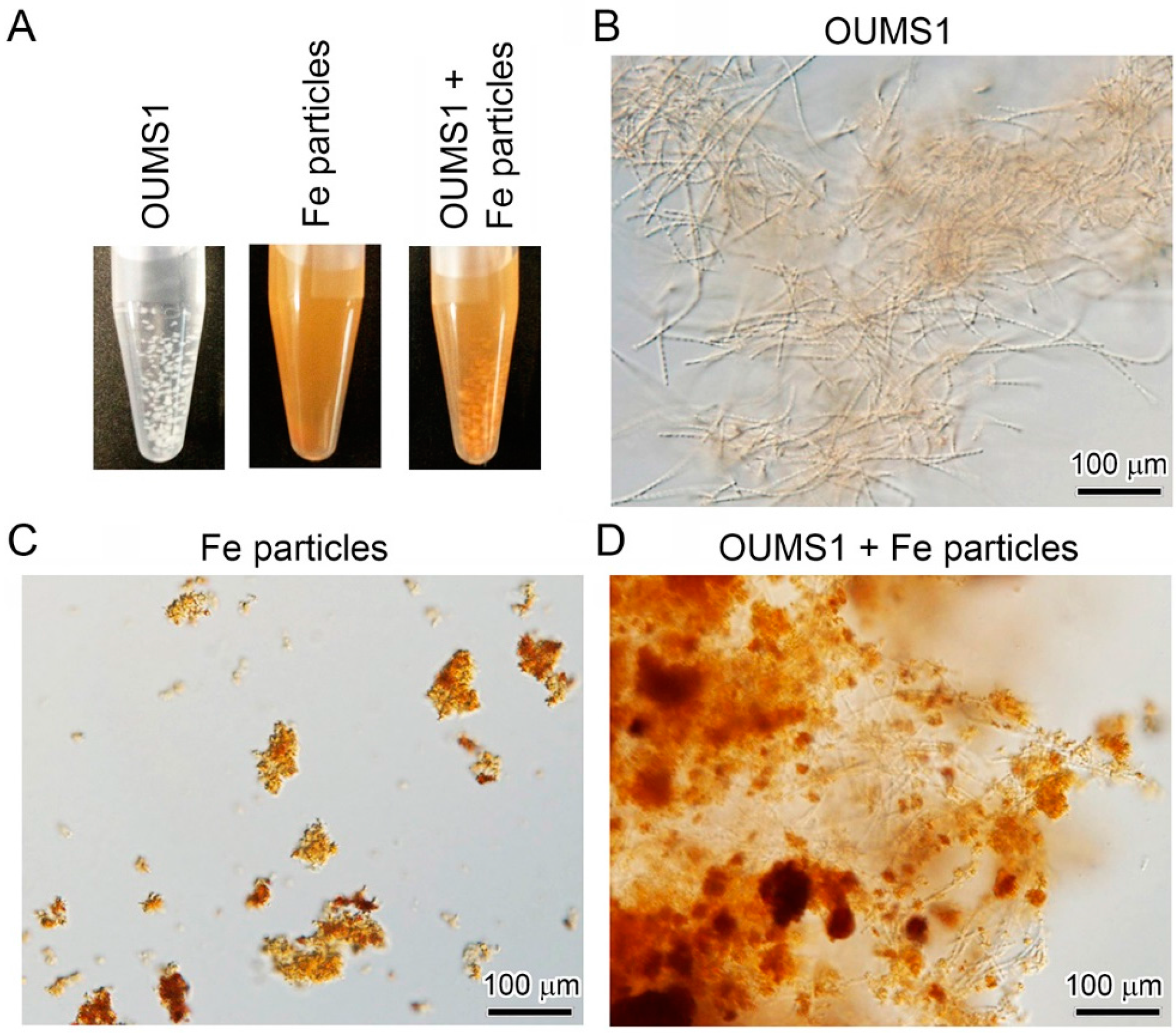
3.3. Encrustation of “Cell-Killed” and “Cell-Free” Sheaths of OUMS1 with Numerous Fe(III) Particles after Incubation in SGP + Fe
3.4. Deposition of Fe Particles onto the LES- and Proteinase K-Treatments
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mann, S. Biomineralization: Principles and Concepts in Bioinorganic Materials Chemistry; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Ghiorse, W.C. Biology of iron- and manganese-depositing bacteria. Annul. Rev. Microbiol. 1984, 38, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Spring, S. The general Leptothrix and Sphaerotilus. Prokaryotes 1979, 5, 758–777. [Google Scholar]
- Chan, S.C.; Fakra, C.S.; Edwards, C.D.; Emerson, D.; Banfield, F.J. Iron oxyhydroxide mineralization on microbial extracellular polysaccharides. Geochim. Cosmochim. Acta 2009, 73, 3807–3818. [Google Scholar] [CrossRef]
- Ghiorse, W.C.; Hirsch, P. An ultrastructural study of iron and manganese deposition associated with extracellular polymers of pedomicrobium-like budding bacteria. Arch. Microbiol. 1979, 123, 213–226. [Google Scholar] [CrossRef]
- Furutani, M.; Suzuki, T.; Ishihara, H.; Hashimoto, H.; Kunoh, H.; Takada, J. Initial assemblage of bacterial saccharic fibrils and element deposition to form an immature sheath in cultured Leptothrix sp. strain OUMS1. Minerals 2011, 1, 157–166. [Google Scholar] [CrossRef]
- Ishihara, H.; Suzuki, T.; Hashimoto, H.; Kunoh, H.; Takada, J. Initial parallel arrangement of extracellular fibrils holds a key for sheath frame construction by Leptothrix sp. strain OUMS1. Minerals 2013, 3, 73–81. [Google Scholar] [CrossRef]
- Corstjens, P.L.; de Vrind, J.P.; Westbroek, P.; de Vrind-de Jong, E.W. Enzymatic iron oxidation by Leptothrix discophora: Identification of an iron-oxidizing protein. Appl. Environ. Microbiol. 1992, 58, 450–454. [Google Scholar] [PubMed]
- De Vrind-de Jong, E.W.; Corstjens, P.L.; Kempers, E.S.; Westbroek, P.; de Vrind, J.P. Oxidation of manganese and iron by Leptothrix discophora: Use of N,N,N′,N′-tetramethyl-p-phenylenediamine as an indicator of metal oxidation. Appl. Environ. Microbiol. 1990, 56, 3458–3462. [Google Scholar] [PubMed]
- Van Veen, W.L.; Mudler, E.G.; Deinema, M.H. The Sphaerotilus-Leptothrix group of bacteria. Microbiol. Rev. 1978, 42, 329–356. [Google Scholar] [PubMed]
- Adams, L.F.; Ghiorse, W.C. Physiology and ultrastructure of Leptothrix discophora SS-1. Arch. Microbiol. 1986, 145, 126–135. [Google Scholar] [CrossRef]
- Chan, C.S.; de Stasio, G.; Welch, S.A.; Girasole, M.; Frazer, B.H.; Nesterova, M.V.; Fakra, S.; Banfield, J.F. Microbial polysaccharides template assembly of nanocyrstal fibers. Science 2004, 303, 1656–1658. [Google Scholar] [CrossRef] [PubMed]
- Rentz, J.A.; Kraiya, C.; Luther, G.W., III; Emerson, D. Control of ferrous iron oxidation within circumneutral microbial iron mats by cellular activity and autocatalysis. Environ. Sci. Technol. 2007, 41, 6084–6089. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.F.; Ghiorse, W.C. Characterization of extracellular Mn2+-oxidizing activity and isolation of an Mn2+-oxidizing protein from Leptothrix discophora SS-1. J. Bacteriol. 1987, 169, 1279–1285. [Google Scholar] [PubMed]
- Boogerd, F.C.; de Vrind, J.P. Manganese oxidation by Leptothrix discophora. J. Bacteriol. 1987, 169, 489–494. [Google Scholar] [PubMed]
- Adams, L.F.; Ghiorse, W.C. Oxidation state of Mn in the Mn oxide produced by Leptothrix discophora SS-1. Geochim. Cosmochim. Acta 1988, 52, 2073–2076. [Google Scholar] [CrossRef]
- Emerson, D.; Ghiorse, W.C. Isolation, cultural maintenance, and taxonomy of a sheath-forming strain of Leptothrix discophora and characterization of manganese-oxidizing activity associated with the sheath. Appl. Environ. Microbiol. 1992, 58, 4001–4010. [Google Scholar] [PubMed]
- Takeda, M.; Makita, H.; Ohno, K.; Nakahara, Y.; Koizumi, J. Structural analysis of the sheath of a sheathed bacterium, Leptothrix cholodnii. Int. J. Biol. Macromol. 2005, 37, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Ghiorse, W.C. Ultrastructure and chemical composition of the sheath of Leptothrix discophora SP-6. J. Bacteriol. 1993, 175, 7808–7818. [Google Scholar] [PubMed]
- Suzuki, T.; Hashimoto, H.; Ishihara, H.; Kasai, T.; Kunoh, H.; Takada, J. Structural and spatial associations between Fe, O, and C in the network structure of the Leptothrix ochracea sheath surface. Appl. Environ. Microbiol. 2011, 77, 7873–7875. [Google Scholar] [CrossRef] [PubMed]
- Sawayama, M.; Suzuki, T.; Hashimoto, H.; Kasai, T.; Furutani, M.; Miyata, N.; Kunoh, H.; Takada, J. Isolation of a Leptothrix strain, OUMS1, from ocherous deposits in groundwater. Curr. Microbiol. 2011, 63, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Fleming, E.J.; McBeth, J.M. Iron-oxidizing bacteria: An environmental and genomic perspective. Annu. Rev. Microbiol. 2010, 64, 561–583. [Google Scholar] [CrossRef] [PubMed]
- Kunoh, T.; Hashimoto, H.; McFarlane, R.I.; Hayashi, N.; Suzuki, T.; Taketa, E.; Tamura, K.; Takano, M.; El-Naggar, Y.M.; Kunoh, H.; et al. Abiotic deposition of Fe complexes onto Leptothrix sheaths. SpringerPlus 2016. under review. [Google Scholar]
- Hashimoto, H.; Kobayashi, G.; Sakuma, R.; Fujii, T.; Hayashi, N.; Suzuki, T.; Kanno, R.; Takano, M.; Takada, J. Bacterial nanometric amorphous Fe-based oxide: A potential lithium-ion battery anode material. ACS Appl. Mater. Interfaces 2014, 6, 5374–5378. [Google Scholar] [CrossRef] [PubMed]
- Sakuma, R.; Hashimoto, H.; Kobayashi, G.; Fujii, T.; Nakanishi, M.; Kanno, R.; Takano, M.; Takada, J. High-rate performance of a bacterial iron-oxide electrode material for lithium-ion battery. Mater. Lett. 2015, 139, 414–417. [Google Scholar] [CrossRef]
- Ema, T.; Miyazaki, Y.; Kozuki, I.; Sakai, T.; Hashimoto, H.; Takada, J. Highly active lipase immobilized on biogenous iron oxide via an organic bridging group: The dramatic effect of the immobilization support on enzymatic function. Green Chem. 2011, 13, 3187–3195. [Google Scholar] [CrossRef]
- Ema, T.; Miyazaki, Y.; Taniguchi, T.; Takada, J. Robust porphyrin catalysts immobilized on biogenous iron oxide for the repetitive conversions of epoxides and CO2 into cyclic carbonates. Green Chem. 2013, 15, 2485–2492. [Google Scholar] [CrossRef]
- Mandai, K.; Korenaga, T.; Ema, T.; Sakai, T.; Furutani, M.; Hashimoto, H.; Takada, J. Biogenous iron oxide-immobilized palladium catalyst for the solvent-free Suzuki–Miyaura coupling reaction. Tetrahedron Lett. 2012, 53, 329–332. [Google Scholar] [CrossRef]
- Hashimoto, H.; Asaoka, H.; Nakano, T.; Kusano, Y.; Ishihara, H.; Ikeda, Y.; Nakanishi, M.; Fujii, T.; Yokoyama, T.; Horiishi, N.; et al. Preparation, microstructure, and color tone of microtubule material composed of hematite/amorphous-silicate nonocomposite from iron oxide of bacterial origin. Dye. Pigment. 2012, 95, 639–643. [Google Scholar]
- Ishihara, H.; Hashimoto, H.; Taketa, E.; Suzuki, T.; Mandai, K.; Kunoh, H.; Takada, J. Silicon-rich, iron oxide microtubular sheath produced by an iron-oxidizing bacterium, Leptothrix sp. strain OUMS1, in culture. Minerals 2014, 4, 565–577. [Google Scholar]
- Suzuki, T.; Kunoh, T.; Nakatsuka, D.; Hashimoto, H.; Tamura, K.; Kunoh, H.; Takada, J. Use of iron powder to obtain high yields of Leptothrix sheaths in culture. Minerals 2015, 5, 335–345. [Google Scholar] [CrossRef]
- Luman, C.R.; Castellano, F. Comprehensive Coordination Chemistry II; McCleverty, J.A., Meyer, T.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 25–39. [Google Scholar]
- Hashimoto, H.; Fujii, T.; Nakanishi, M.; Kusano, Y.; Ikeda, Y.; Takada, J. Synthesis and magnetic properties of magnetite-silicate nanocomposites derived from iron oxide of bacterial origin. Mater. Chem. Phys. 2012, 136, 1156–1161. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate-determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Emerson, D.; Garen, E.R.; Ghiorse, C.W. Formation of metallogenium-like structures by a manganese-oxiding fungus. Arch. Microbiol. 1989, 151, 223–231. [Google Scholar] [CrossRef]
- Vollrath, S.; Behrends, T.; Koch, C.B.; van Cappellen, P. Effects of temperature on rates and mineral products of microbial Fe(II) oxidation by Leptothrix cholodnii at microaerobic conditions. Geochim. Cosmochim. Acta 2013, 108, 107–124. [Google Scholar] [CrossRef]
- Vollrath, S.; Behrends, T.; van Cappellen, P. Oxygen dependency of neutorphilic Fe(III) oxidation by Leptothrix differs from abiotic reaction. Geomicrobiol. J. 2012, 29, 550–560. [Google Scholar] [CrossRef]
- Takeda, M.; Kondo, K.; Yamada, M.; Koizumi, J.; Mashima, T.; Matsugami, A.; Katahira, M. Solubilization and structural determination of a glycoconjugate which is assembled into the sheath of Leptothrix cholodnii. Int. J. Biol. Macromol. 2010, 46, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Fleming, E.J.; Langdon, A.E.; Martinez-Garcia, M.; Stepanauskas, R.; Poulton, N.J.; Masland, E.D.; Emerson, D. What’s new is old: Resolving the identity of Leptothrix ochracea using single cell genomics, pyrosequencing and fish. PLoS ONE 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Schultze, S.; Witten, T.C.; Fyfe, W.S.; Beveridge, T.J. Metal interactions with microbial biofilms in acidic and neutral pH environments. Appl. Environ. Microbiol. 1989, 55, 1249–1257. [Google Scholar] [PubMed]
- Toner, B.M.; Santelli, C.M.; Marcus, M.A.; Wirth, R.; Chan, C.S.; McCollom, T.; Bach, W.; Edwards, J.K. Biogenic iron oxyhydroxide formation at mid-ocean ridge hydrothermal vents: Juan de Fuca Ridge. Geochim. Cosmochim. Acta 2009, 73, 388–403. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunoh, T.; Hashimoto, H.; Suzuki, T.; Hayashi, N.; Tamura, K.; Takano, M.; Kunoh, H.; Takada, J. Direct Adherence of Fe(III) Particles onto Sheaths of Leptothrix sp. Strain OUMS1 in Culture. Minerals 2016, 6, 4. https://doi.org/10.3390/min6010004
Kunoh T, Hashimoto H, Suzuki T, Hayashi N, Tamura K, Takano M, Kunoh H, Takada J. Direct Adherence of Fe(III) Particles onto Sheaths of Leptothrix sp. Strain OUMS1 in Culture. Minerals. 2016; 6(1):4. https://doi.org/10.3390/min6010004
Chicago/Turabian StyleKunoh, Tatsuki, Hideki Hashimoto, Tomoko Suzuki, Naoyuki Hayashi, Katsunori Tamura, Mikio Takano, Hitoshi Kunoh, and Jun Takada. 2016. "Direct Adherence of Fe(III) Particles onto Sheaths of Leptothrix sp. Strain OUMS1 in Culture" Minerals 6, no. 1: 4. https://doi.org/10.3390/min6010004




