Composition and Formation of Gabbro-Peridotite Hosted Seafloor Massive Sulfide Deposits from the Ashadze-1 Hydrothermal Field, Mid-Atlantic Ridge
Abstract
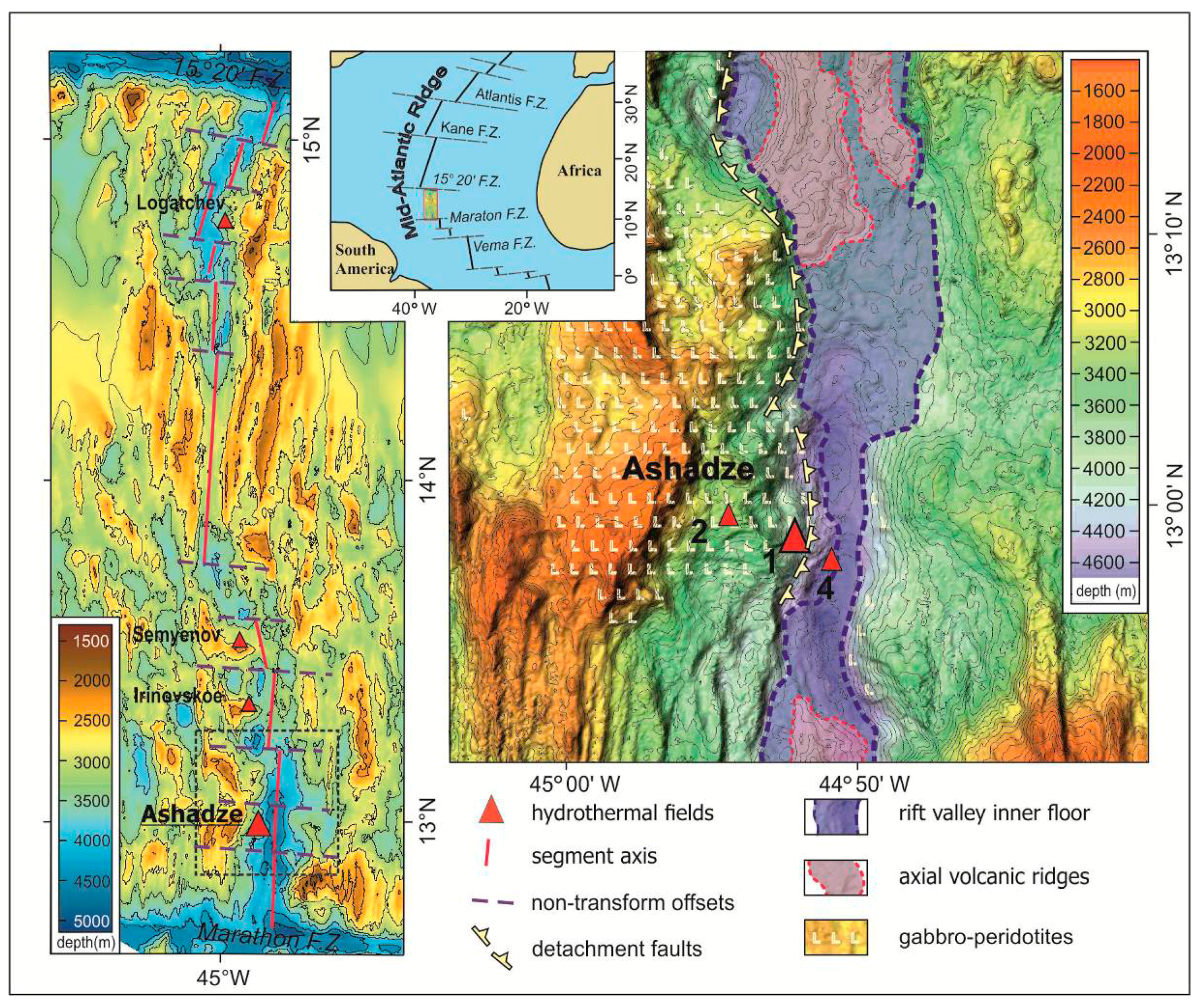
:1. Introduction
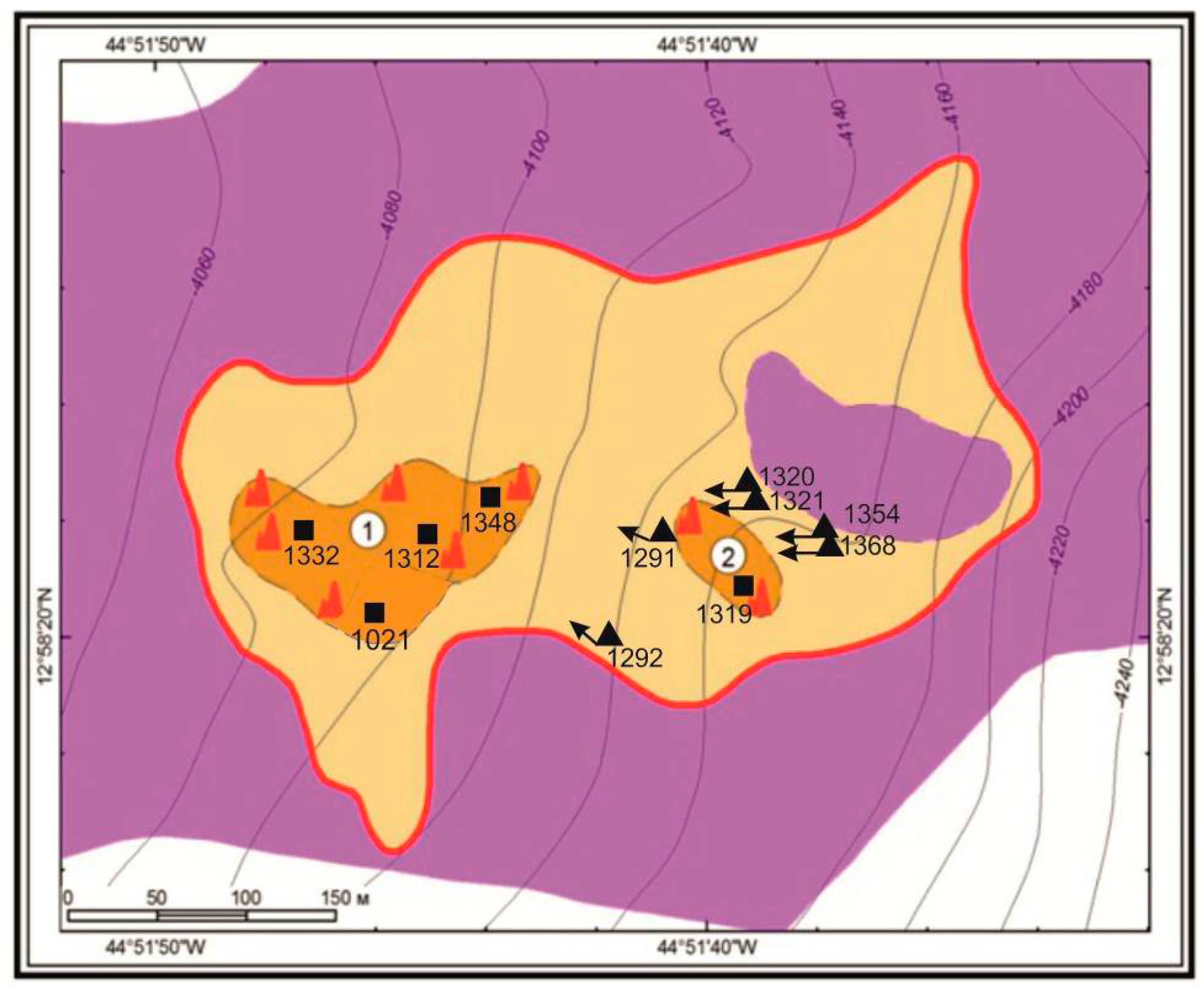
2. Materials and Methods
3. Results
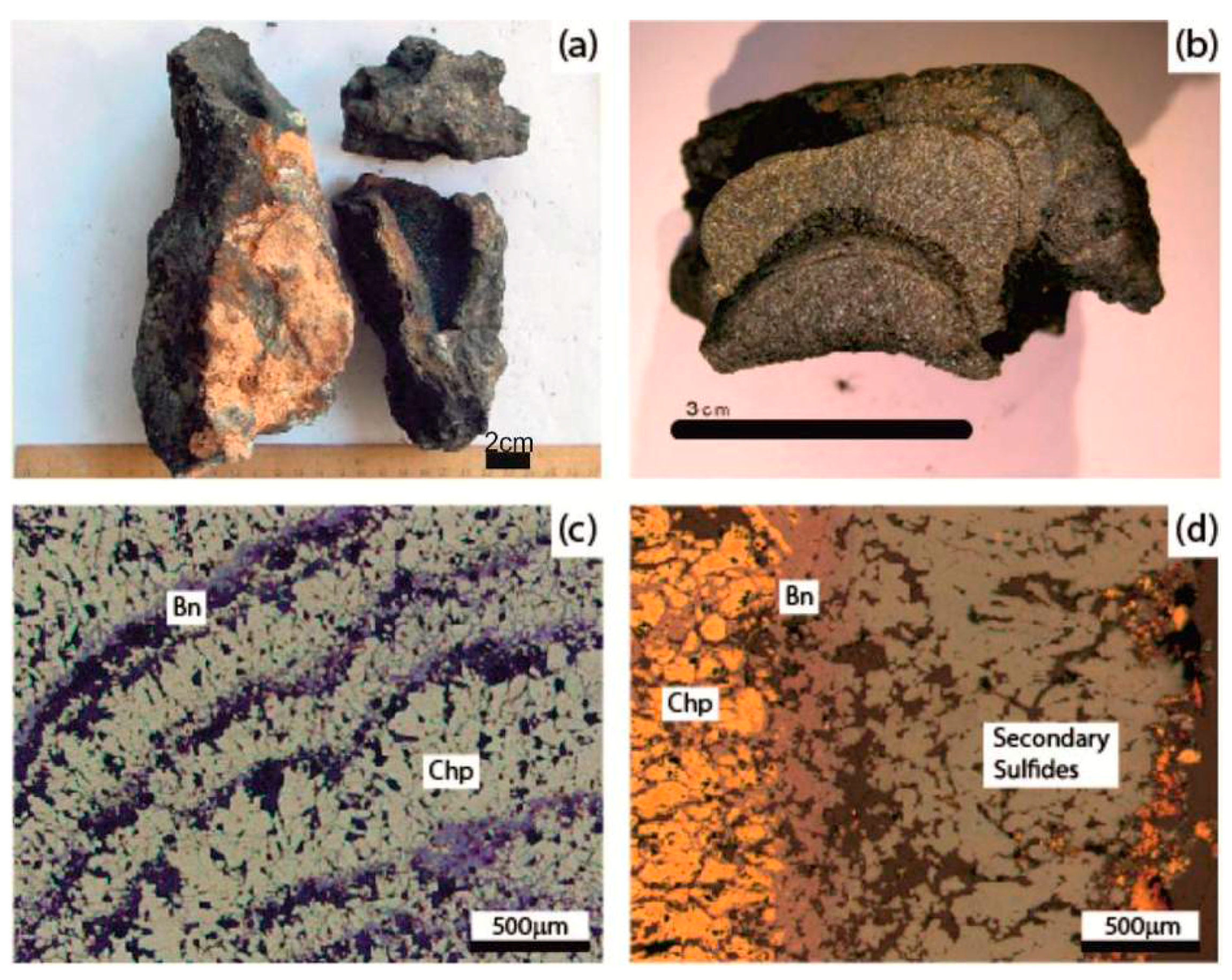
3.1. Mineralogical Types: Description and Characteristics
3.2. Geochemical Characteristics
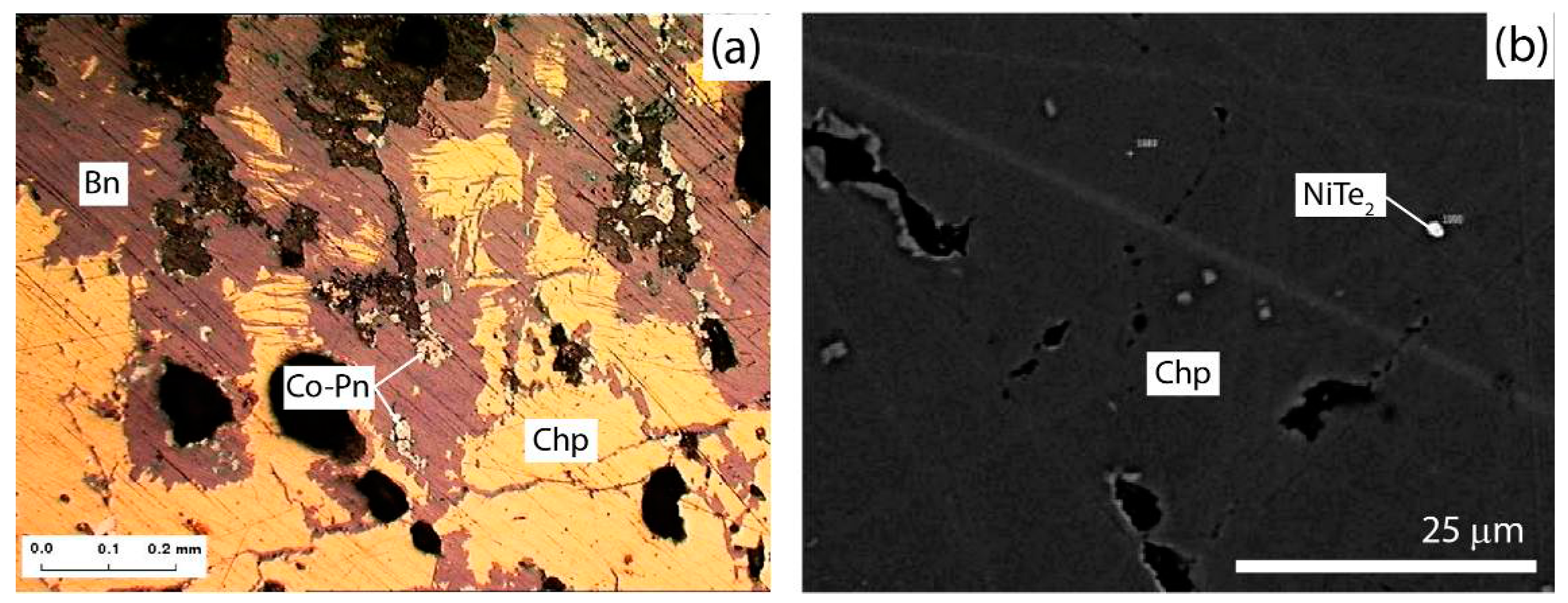
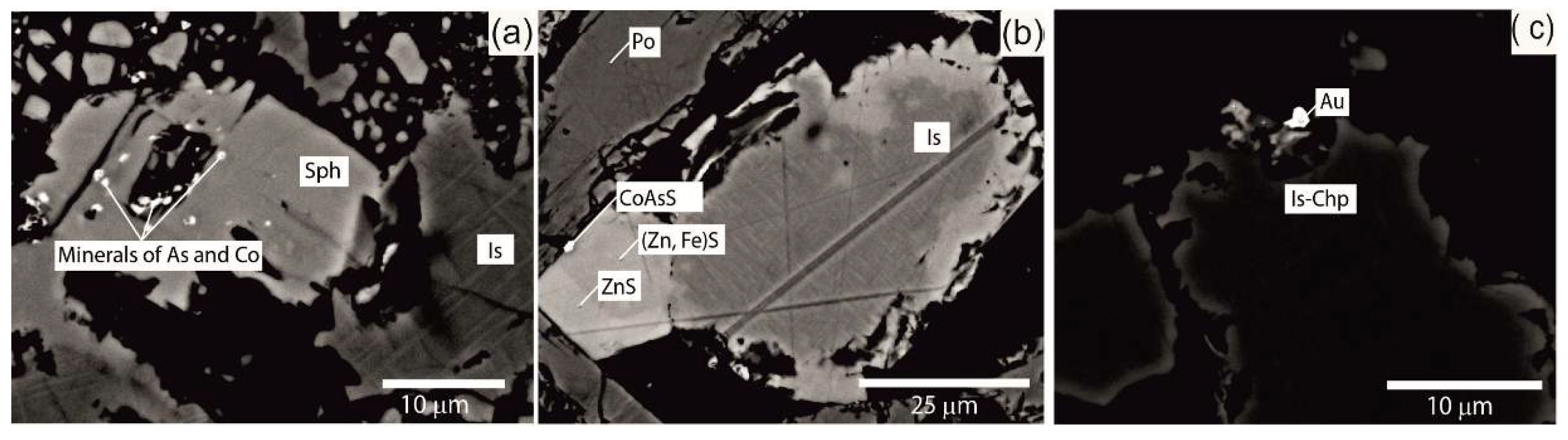
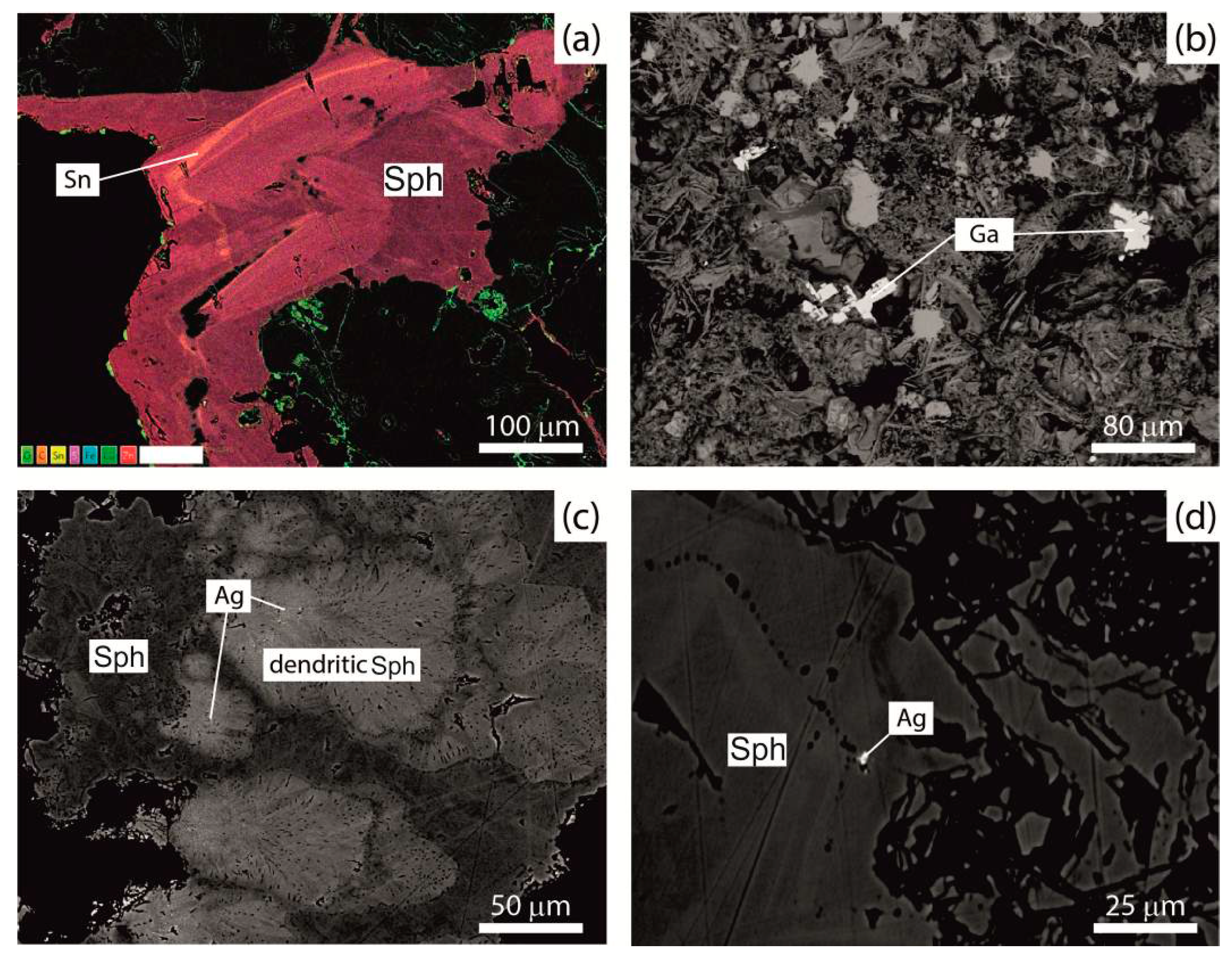
3.3. Accessory Mineralization
- (1)
- Co, Ni, Sb, Pb and Ag form minerals with high concentrations of these elements.
- (2)
- Au forms in minerals despite its low concentrations.
- (3)
- Sn, Cd, Se and Ge are detected in major and minor minerals, interpreted as having replaced other elements in the crystal lattice of the analyzed mineral and do not form minerals.
3.4. Isotopic Studies of Sulfur
4. Discussion
4.1. Source of Metals and Ore-Forming Factors
4.2. Sulfur Isotope
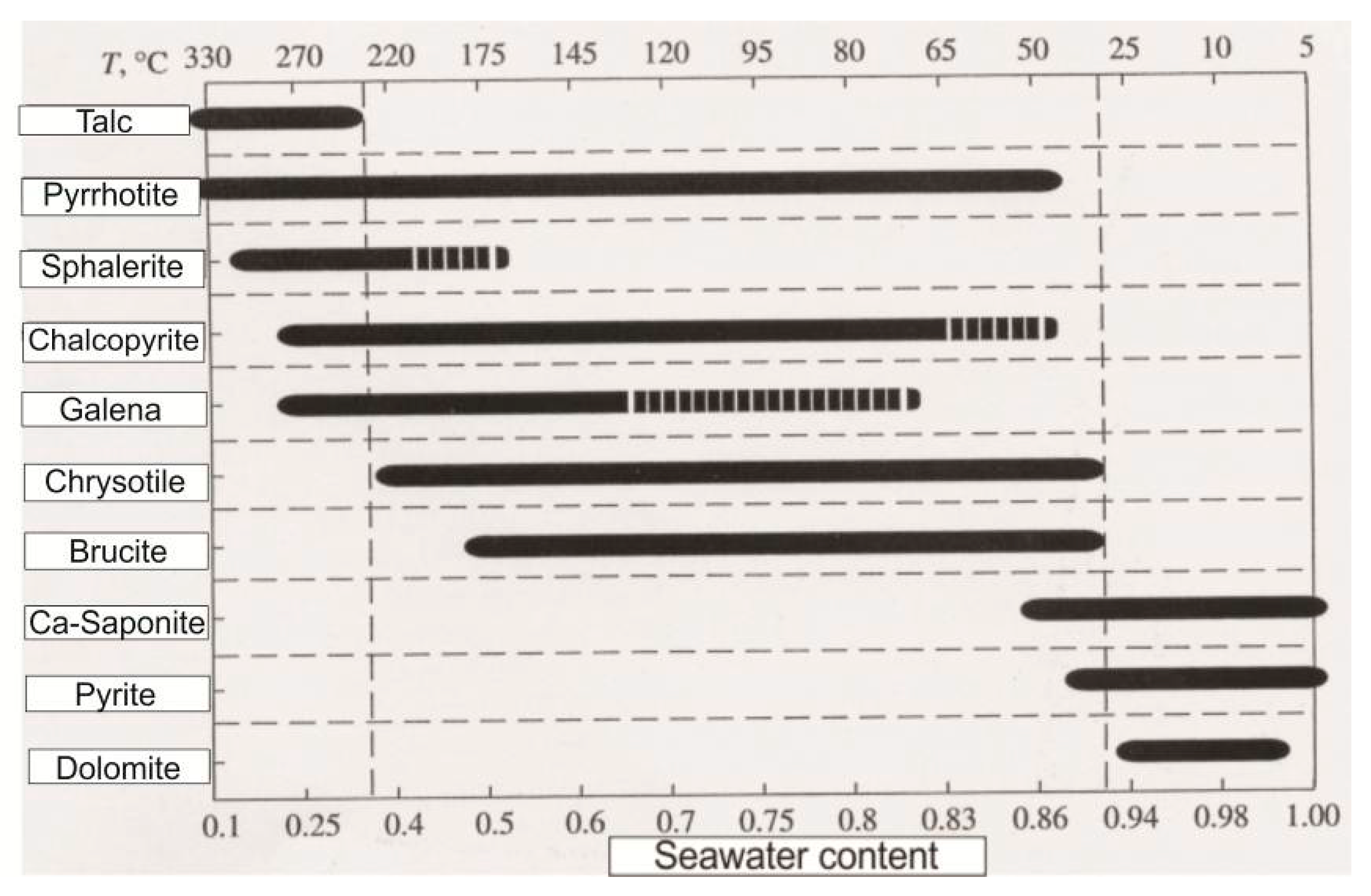
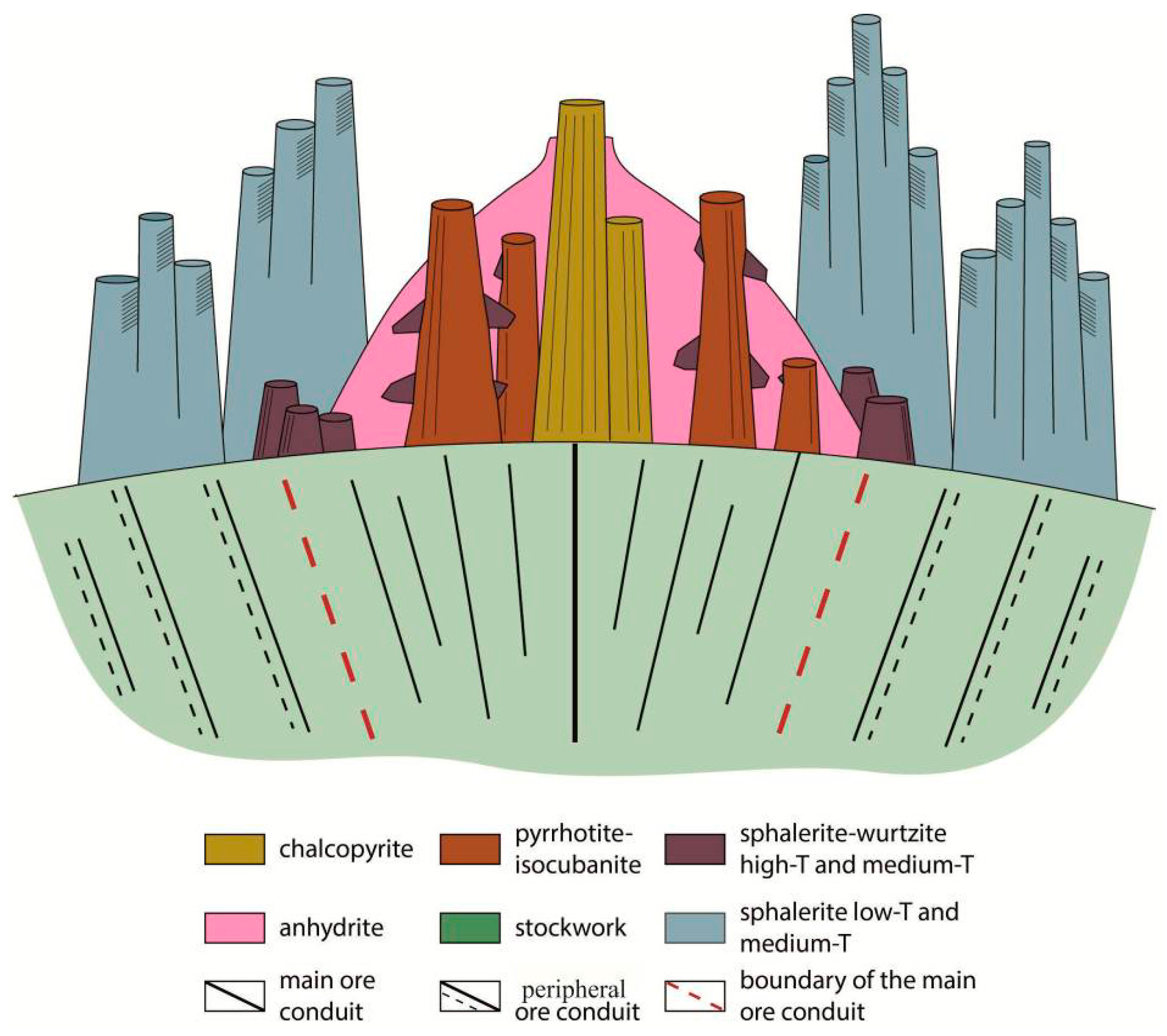
4.3. Formation
- -
- The initial stage (1A) of hydrothermal activity is characterized by focused pulsing venting along with precipitation of the highest temperature; layered chalcopyrite chimneys marked centre of main conduit.
- -
- The next stage of venting (2A) is characterized by "stable intensity" discharge and formation of pyrrhotite-isocubanite chimneys. During this stage the chalcopyrite chimneys are destroyed.
- -
- During the next stages (2B and 3A), a lower temperature portion of fluids transforms minerals and forms a new generation of pyrrhotite and isocubanite along with Fe-wurtzite and sphalerite. Zn-sulfide chimneys enriched in Sn and Ge are formed close to the centre of the discharge zone and main conduit.
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MAR | Mid-Atlantic Ridge |
| OCC | Oceanic core complex |
| SMS | Seafloor massive sulfides |
References
- Rona, P.A.; Klinkhammer, G.; Nelson, T.; Trefry, J.; Elderfield, H. Black smokers, massive sulfides and vent biota at the Mid-Atlantic Ridge. Nature 1986, 321, 33–37. [Google Scholar] [CrossRef]
- Fouquet, Y.; Cambon, P.; Etoubleau, J.; Charlou, J.; Ondréas, H.; Barriga, F.; Cherkashov, G.; Semkova, T.; Poroshina, I.; Bohn, M.; et al. Geodiversity of hydrothermal processes along the Mid-Atlantic Ridge and ultramafic-hosted mineralization: A new type of oceanic Cu-Zn-Co-Au volcanogenic massive sulfide deposit. In Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, Geophysical Monograph Series; Rona, P., Devey, C., Dyment, J., Murton, B., Eds.; American Geophysical Union: Washington, D.C., USA, 2010; Volume 188, pp. 321–367. [Google Scholar]
- Beaulieu, S.E.; Baker, E.T.; German, C.R.; Maffei, A. An authoritative global database for active submarine hydrothermal vent fields. Geochem. Geophys. Geosyst. 2013, 14, 4892–4905. [Google Scholar] [CrossRef]
- Cherkashov, G.; Poroshina, I.; Stepanova, T.; Ivanov, V.; Bel’tenev, V.; Lazareva, L.; Rozhdestvenskaya, I.; Samovarov, M.; Shilov, V.; Glasby, G.; et al. Seafloor massive sulfides from the northern equatorial Mid-Atlantic Ridge: New discoveries and perspectives. Mar. Georesour. Geotechnol. 2010, 28, 222–239. [Google Scholar] [CrossRef]
- Escartın, J.; Smith, D.K.; Cann, J.; Schouten, H.; Langmuir, C.H.; Escrig, S. Central role of detachment faults in accretion of slow-spreading oceanic lithosphere. Nature 2008, 455, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Bel’tenev, V.; Nescheretov, A.; Shilov, V.; Shagin, A.; Stepanova, T.; Cherkashev, G.; Batuev, M.; Samovarov, M.; Rozhdestvenskaya, I.; Andreeva, I.; et al. New discoveries at 12°58′ N, 44°52′ W, MAR: Professor Logatchev-22 cruise, initial results. InterRidge News 2003, 12, 13–14. [Google Scholar]
- Cherkashov, G.; Bel’tenev, V.; Ivanov, V.; Lazareva, L.; Samovarov, M.; Shilov, V.; Stepanova, T.; Glasby, G.P.; Kuznetsov, V. Two new hydrothermal fields at the Mid-Atlantic Ridge. Mar. Georesour. Geotechnol. 2008, 26, 308–316. [Google Scholar] [CrossRef]
- Ondreas, H.; Cannat, M.; Fouquet, Y.; Normand, A. Geological context and vents morphology of the ultramafic-hosted Ashadze hydrothermal areas (Mid-Atlantic Ridge 13° N). Geochem. Geophys. Geosys 2013, 13. [Google Scholar] [CrossRef]
- Fouquet, Y.; Cherkashov, G.; Charlou, J.L.; Ondreas, H.; Birot, D.; Cannat, M.; Bortnikov, N.; Silantyev, S.; Sudarikov, S.; Cambon-Bonavita, M.A.; et al. Serpentine cruise—Ultramafic hosted hydrothermal deposits on the Mid-Atlantic Ridge: First submersible studies on Ashadze 1 and 2, Logatchev 2 and Krasnov vent fields. InterRidge News 2008, 17, 15–19. [Google Scholar]
- Cannat, M.; Mangeney, A.; Ondreas, H.; Fouquet, Y.; Normand, A. High-resolution bathymetry reveals contrasting landslide activity shaping the walls of the Mid-Atlantic Ridge axial valley. Geochem. Geophys. Geosyst. 2013, 14, 4892–4905. [Google Scholar]
- Mozgova, N.N.; Trubkin, N.V.; Borodaev, Y.S.; Cherkashev, G.A.; Stepanova, T.V.; Semkova, T.A.; Uspenskaya, T.Y. Mineralogy of massive sulfides from the Ashadze hydrothermal field, 13 N, Mid-Atlantic Ridge. Can. Miner. 2008, 46, 545–567. [Google Scholar] [CrossRef]
- Lein, A.U.; Cherkashov, G.A.; Ulyanov, A.A.; Stepanova, T.V.; Sagalevich, A.M.; Bogdanov, Y.A.; Gurvich, E.G.; Torokhov, M.P. Mineralogy and geochemistry of sulfide fields Logachev-2 and Rainbow: Similarities and differences. Geochemistry 2003, 3, 304–328. (In Russian) [Google Scholar]
- Hannington, M.D.; Jonasson, I.R.; Herzig, P.M.; Petersen, S. Physical and chemical processes of seafloor mineralization at Mid-Ocean Ridges. In Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interaction; Monograph Series; Humphris, S.E., Zierenberg, R.A., Mullineaux, L.S., Thomson, R.E., Eds.; American Geophysical AGU: Washington, D.C., USA, 1995; Volume 91, pp. 115–157. [Google Scholar]
- Solomon, M.; Tornos, F.; Large, R.R.; Badham, J.N.P.; Both, R. Zn-Pb-Cu volcanic-hosted massive sulfide deposits criteria for distinguishing brine pooltype from black smoker-type sulfide deposition. Ore Geol. Rev. 2004, 25, 259–283. [Google Scholar] [CrossRef]
- Charlou, J.; Donval, J.; Konn, C.; Ondreas, H.; Fouquet, Y. High production and fluxes of H2 and CH4 and evidence of abiotic hydrocarbon synthesis by serpentinization in ultramafic-hosted hydrothermal systems on the Mid-Atlantic Ridge. In Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, Geophysical Monograph Series; Rona, P., Devey, C., Dyment, J., Murton, B., Eds.; American Geophysical Union: Washington, D.C., USA, 2010; Volume 188, pp. 265–296. [Google Scholar]
- Silant’ev, S.A.; Mironenko, M.V.; Novoselov, A.A. Hydrothermal systems in peridotite substrate in slow-spreading ridge. Modeling of phase transformation and substance balance: Ascending branch. Petrology 2009, 17, 563–577. (In Russian) [Google Scholar]
- Silant’ev, S.A.; Mironenko, M.V.; Novoselov, A.A. Serpentinite hosted hydrothermal systems of Mid-Ocean Ridges: Kinetic and thermodynamic modeling of downwelling limb of a hydrothermal circulation cell. Geochim. Cosmochim. Acta Suppl. 2009, 73, 1222. [Google Scholar]
- Sakai, H.; Desmarais, D.J.; Ueda, A.; Moore, J.G. Concentrations and isotope ratios of carbon, nitrogen, and sulfur in ocean-floor basalts. Geochim. Cosmochim. Acta 1984, 48, 2433–2441. [Google Scholar] [CrossRef]
- Styrt, M.; Brackman, A.J.; Holland, H.D.; Clark, B.C.; Pisutharnond, V.; Eldridge, C.S.; Ohmoto, H. The mineralogy and the isotopic composition of sulfur in hydrothermal sulfide/sulfate deposits on the East Pacific Rise, 21° N latitude. Earth Planet. Sci. Lett. 1981, 53, 382–390. [Google Scholar] [CrossRef]
- Bogdanov, Y.; Bortnikov, N.; Vikent’ev, I. Mineralogical and geochemical features of Rainbow hydrothermal deposits and fluid related to serpentinites, Mid-Atlantic Ridge (36°14′ N). Geol. Ore Depos. 2002, 44, 510–542. (In Russian) [Google Scholar]
- Shanks, W.C. Stable isotopes in seafloor hydrothermal systems: Vent fluids, hydrothermal deposits, hydrothermal alteration, and microbial processes. Rev. Miner. Geochem. 2001, 43, 469–525. [Google Scholar] [CrossRef]
- Shanks, W.C., III; Seyfried, W.E., Jr. Stable isotope studies of vent fluids and chimney minerals. Southern Juan de Fuca Ridge: Sodium metasomatism and seawater sulfate reduction. J. Geophys. Res. 1987, 92, 11387–11399. [Google Scholar] [CrossRef]
- Woodruff, L.G.; Shanks, W.C., III. Sulfur isotope studies of chimney minerals and vent fluids from 21° N, East Pacific rise: Hydrothermal sulfur sources and disequilibrium sulfate reduction. J. Geophys. Res. 1988, 93, 4562–4572. [Google Scholar] [CrossRef]
- Janecky, D.R.; Shanks, W.C., III. Computational modelling of chemical and sulfur isotopic reaction processes in seafloor hydrothermal systems: Chimneys. Massive sulfides and subjacent alteration zones. Can. Miner. 1988, 26, 603–625. [Google Scholar]
- Hannington, M.D.; Galley, A.G.; Herzig, P.M.; Petersen, S. Comparison of the TAG mound and stockwork complex with cyprus-type massive sulfide deposits. Proc. Ocean Drill. Program Sci. Results 1998, 158, 389–414. [Google Scholar]
- Kuznetsov, V.; Tabuns, E.; Kuksa, K.; Cherkashov, G.; Maksimov, F.; Zherebtsov, I.; Grigoriev, V.; Baranova, N.; Bel’Tenev, V.; Lazareva, L. The oldest seafloor massive sulfide deposits at the Mid-Atlantic Ridge: 230Th/U chronology and composition. Geochronometria 2005, 42, 100–106. [Google Scholar] [CrossRef]



| Sulfides | Chimney Type | Chalcopyrite | Pyrrhotite-Isocubanite | Sphalerite | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Station | 1292 | 1368 | 1319 | 1354 | 1087 | 1319 | 1320 | 1321 | 1354 | 1368 | 1332 | 1319 | 1348 | 1321 | 1312 | 1291 | 1292 | 1021 | ||
| Mineral | ||||||||||||||||||||
| Fe | Pyrite FeS2 | □ | ∆ | ∆ | □ | ∆ | ∆ | ∆ | ||||||||||||
| Pyrrhotite Fe1-xS | ▪ | ▪ | □ | □ | ∆ | □ | □ | |||||||||||||
| Marcasite FeS2 | □ | ∆ | □ | |||||||||||||||||
| Greigite FeFe2S4 | ∆ | |||||||||||||||||||
| Zn | Sphalerite (Zn,Fe)S | □ | □ | ▪ | □ | □ | □ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | |||||
| Wurtzite (Zn,Fe)S | ▪ | ∆ | ∆ | ∆ | ||||||||||||||||
| Rudashevs Kyite-2H(Fe,Zn)S | □ | |||||||||||||||||||
| Cu | Chalcopyrite CuFeS2 | ▪ | ▪ | ▪ | ▪ | ▪ | ▪ | □ | □ | ▪ | □ | □ | □ | □ | ∆ | □ | ||||
| Isocubanite CuFe2S3 | □ | □ | ▪ | ▪ | ▪ | ▪ | ▪ | □ | ▪ | □ | □ | ∆ | ||||||||
| Y-phase Cu2Fe3S5 | ∆ | ∆ | ∆ | ∆ | ∆ | |||||||||||||||
| Bornite Cu5FeS4 | □ | □ | ∆ | ▪ | ||||||||||||||||
| Chalcocite Cu2S | ∆ | |||||||||||||||||||
| Roxbyite Cu9S5 | □ | |||||||||||||||||||
| Digenite Cu9S5 | □ | |||||||||||||||||||
| Geerite Cu8 S5 | □ | ∆ | ∆ | |||||||||||||||||
| Spionkopite Cu1.4S | ∆ | □ | ||||||||||||||||||
| Yarrowite Cu9S8 | □ | ∆ | ||||||||||||||||||
| Covellite CuS | ∆ | |||||||||||||||||||
| Number of samples | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 4 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 3 | ||
| Mineral (Number of Analyses) | Element (wt %) | |||||
| Fe | Cu | Zn | Co | Ni | S | |
| Average | Average | Average | - | Average | Average | |
| Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | ||
| Chalcopyrite (8) | 31.85 | 33.92 | 0.17 | - | - | 34.04 |
| 31.35–32.15 | 31.35–32.15 | 0–1.12 | 33.5–34.96 | |||
| Isocubanite (3) | 41.2 | 23.92 | 0.15 | - | 0.003 | 34.68 |
| 39.56–43.52 | 21.16–25.86 | 0–0.37 | 0–0.01 | 34.55–34.95 | ||
| Bornite (6) | 13.91 | 60.93 | 0.002 | - | - | 25.15 |
| 11.51–19.21 | 55.09–63.91 | - | 24.11–26.55 | |||
| Mineral (Number of Analyses) | Element (wt %) | |||||
| Fe | Cu | Zn | Co | Ni | S | |
| Average | Average | Average | Average | Average | Average | |
| Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | |
| Pyrrhotite (8) | 61.83 | - | - | 0.42 | 0.04 | 37.7 |
| 61.35–62.15 | 0.14–0.65 | 0–0.13 | 37.24–38 | |||
| Pyrite-marcasite (5) | 42.77 | 0.11 | 0.8 | 1.14 | 0.15 | 55.07 |
| 33.04–48.48 | 0–0.37 | 0–2.41 | 0.47–1.96 | 0–0.24 | 49.52–63.35 | |
| Chalcopyrite (7) | 31.68 | 32.15 | 1.63 | 0.3 | - | 34.22 |
| 27.76–35.41 | 29.66–34.06 | 0–4.75 | 0–0.64 | 30.57–34.78 | ||
| Isocubanite (12) | 42.74 | 22.09 | 0.23 | 0.38 | 0.01 | 34.49 |
| 40.54–43.91 | 20.89–24.46 | 0–0.82 | 0–0.75 | 0–0.19 | 33.17–35.18 | |
| Y-phase (4) | 37.67 | 26.79 | 0.32 | 0.08 | - | 35.12 |
| 36.53–39.21 | 25.14–28.48 | 0.09–0.7 | 0–0.31 | 33.93–37.66 | ||
| Sphalerite (6) | 15.06 | 0.37 | 51.26 | 0.24 | 0.07 | 33.0 |
| 5.82–21.67 | 0–0.76 | 46.15–60.8 | 0.02–0.41 | 0–0.2 | 31.9–33.61 | |
| Mineral (Number of Analyses) | Element (wt %) | |||||
| Fe | Cu | Zn | Co | Ni | S | |
| Average | Average | Average | Average | Average | Average | |
| Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | Min.–Max. | |
| Pyrrhotite (5) | 60.07 | 0.04 | 0.24 | - | - | 39.65 |
| 58.16–62.24 | 0–0.18 | 0–1.19 | 36.49–41.86 | |||
| Pyrite (2) | 35.91 | 1.35 | 12.79 | - | - | 49.95 |
| 35.52–36.3 | 0.99–1.71 | 11.84–13.74 | 48.98–50.93 | |||
| Chalcopyrite (2) | 27.95 | 30.52 | 6.41 | - | - | 35.07 |
| 27.21–28.69 | 28.81–32.23 | 3.01–9.81 | 34.17–35.98 | |||
| Isocubanite (4) | 42.87 | 21.41 | 1.07 | 0.05 | 0.02 | 34.57 |
| 40.29–44.26 | 20.51–22.56 | 0–2.73 | 0–0.2 | 0.08 | 33.9–35.24 | |
| Sphalerite (30) | 7.7 | 1.27 | 57.87 | - | 0.01 | 33.11 |
| 1.16–20.17 | 0.–4.34 | 42.46–65.36 | 0–0.12 | 31.86–34.41 | ||
| Elements | Sulfides | ||||||
|---|---|---|---|---|---|---|---|
| Type of Chimneys | MAR * (Our Data) | Basalt-Hosted [12] | |||||
| Chalcopyrite | Pyrrhotite-Isocubanite | Sphalerite | All Types of Chimneys (Our Data) | ||||
| Fe | wt % | 27.51 | 35.20 | 21.81 | 25.73 | 32.43 | 30.75 |
| S | 29.52 | 31.55 | 25.86 | 27.86 | 36.44 | 34.06 | |
| Cu | 31.00 | 15.02 | 5.06 | 12.14 | 9.56 | 9.64 | |
| Zn | 0.33 | 7.57 | 33.41 | 21.51 | 4.39 | 5.21 | |
| Si | 0.31 | 0.50 | 0.52 | 0.48 | 3.2 | 12.1 | |
| Ca | 0.80 | 0.23 | 0.61 | 0.53 | 0.42 | 1.51 | |
| Mg | 0.14 | 0.17 | 0.19 | 0.17 | 0.14 | 0.4 | |
| Al | 0.23 | 0.08 | 0.16 | 0.14 | 0.16 | 0.25 | |
| Ti | ppm | 300 | 697 | 381 | 468 | 450 | 220 |
| Co | 3200 | 5362 | 941 | 2373 | 619 | 200 | |
| Ni | 1262 | 16.5 | 5.9 | 250 | 55.4 | 29 | |
| Bi | 2.1 | 5.7 | 2.6 | 3.5 | 4.5 | 2.2 | |
| Se | 1237 | 33 | 12 | 229 | 280 | 11 | |
| Pb | 5.0 | 73.5 | 523 | 319 | 157 | 33 | |
| Ga | 4.9 | 10.2 | 12.2 | 10.2 | 19.1 | 41,2 | |
| Ge | 2.0 | 15.5 | 78.7 | 43,9 | 36.1 | 39.2 | |
| Sn | 32.6 | 164.2 | 713 | 419 | 64.5 | 20.9 | |
| Cd | 4.3 | 126.2 | 516 | 329 | 110 | 190 | |
| Sb | 25.0 | 25.0 | 70.6 | 50.6 | 32.9 | n.d. | |
| As | 57.1 | 94.6 | 186 | 130 | 318 | 270 | |
| Mn | 23.9 | 39.1 | 271 | 158 | 159 | 483 | |
| Ba | 105.0 | 126.9 | 1171 | 664 | 2900 | 790 | |
| Ag | 0.5 | 23.5 | 155 | 99.7 | 3.2 | 77.2 | |
| Au | 1.9 | 6.4 | 1.9 | 2.9 | 48.5 | 3 | |
| n | 10 | 15 | 30 | 55 | 1051 * | 427 | |
| Age, kyr [4] | 6.2 ± 1.5 | 7.2 ± 1.8 | 7.1 ± 0.6; 5 ± 0.9; 3.3 ± 0.3; 2.1 ± 0.3 | - | - | - | |
| Chimney Type | Chalcopyrite | Pyrrhotite-Isocubanite | Sphalerite | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Station | 1292 | 1368 | 1319 | 1354 | 1087 | 1319 | 1320 | 1321 | 1354 | 1368 | 1332 | 1319 | 1348 | 1321 | 1312 | 1291 | 1292 | 1021 | |
| Mineral | |||||||||||||||||||
| Co-pentladite (Co,Fe,Ni)9S8 | + | + | + | + | + | ||||||||||||||
| Millerite NiS | + | + | + | ||||||||||||||||
| Cobaltite CoAsS | + | + | + | + | + | + | + | + | + | + | |||||||||
| Glaucodite (Co,Fe)AsS | + | + | + | + | + | + | |||||||||||||
| Carrollite CuCo2S4 | + | + | + | + | |||||||||||||||
| Skutterudite (Co,Ni)As3(CoAs3) | + | + | |||||||||||||||||
| Melonite NiTe2 | + | ||||||||||||||||||
| Native gold Au | + | + | + | + | |||||||||||||||
| Electrum AuAg | + | + | + | + | + | + | + | + | |||||||||||
| Native silver Ag | + | + | + | + | + | + | |||||||||||||
| Acanthine Ag2S | + | ||||||||||||||||||
| Ag-pyrite AgFe2S3 | + | ||||||||||||||||||
| Stibnite Sb2S3 | + | + | |||||||||||||||||
| Galena PbS | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| Boulangerite Pb5Sb4S11 | + | ||||||||||||||||||
| Number of samples | 1 | 1 | 2 | 2 | 1 | 2 | 4 | 1 | 4 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | |
| Type of Chimney | Sample | δ34S (‰) | |||
|---|---|---|---|---|---|
| Chalcopyrite | Isocubanite | Sphalerite | Pyrrhotite | ||
| Chalcopyrite | 1319-T-10 | +4.3 | |||
| 1319-T-10 (I) | +3.7 | ||||
| 1319-T-10 (II) | +3.7 | ||||
| 1354-T-1/1 | +3.9 | ||||
| 1368-T-1 | +4.5 | ||||
| Pyrrhotite-Isocubanite | 1319-T-2 | +4.5 | |||
| 1319-T-2/1 | +3.8 | ||||
| 1354-T-3 | +4.4 | ||||
| 1354-T-3/2 | +6.5 | ||||
| 1354-T-3/3 | +7.3 | ||||
| 1354-T-4 | +7.1 | ||||
| Sphalerite | 1354-T-5/1 | +4.1 | |||
| 1321-TК-2/1 | +4.4 | ||||
| 1321-TК-1 | +4.4 | ||||
| 1319-T-3/2 | +6.4 | ||||
| 1312-T-2 | +7.8 | ||||
| 1021-T-1 | +11.9 | +14.1 | |||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firstova, A.; Stepanova, T.; Cherkashov, G.; Goncharov, A.; Babaeva, S. Composition and Formation of Gabbro-Peridotite Hosted Seafloor Massive Sulfide Deposits from the Ashadze-1 Hydrothermal Field, Mid-Atlantic Ridge. Minerals 2016, 6, 19. https://doi.org/10.3390/min6010019
Firstova A, Stepanova T, Cherkashov G, Goncharov A, Babaeva S. Composition and Formation of Gabbro-Peridotite Hosted Seafloor Massive Sulfide Deposits from the Ashadze-1 Hydrothermal Field, Mid-Atlantic Ridge. Minerals. 2016; 6(1):19. https://doi.org/10.3390/min6010019
Chicago/Turabian StyleFirstova, Anna, Tamara Stepanova, Georgy Cherkashov, Alexey Goncharov, and Svetlana Babaeva. 2016. "Composition and Formation of Gabbro-Peridotite Hosted Seafloor Massive Sulfide Deposits from the Ashadze-1 Hydrothermal Field, Mid-Atlantic Ridge" Minerals 6, no. 1: 19. https://doi.org/10.3390/min6010019







