1. Introduction
Bioleaching of copper sulfide ores in acid conditions, using autotrophic bacteria such as
Acidithiobacillus ferrooxidans [
1,
2,
3,
4,
5], is a mature process and has been successfully used for the recovery of copper. In recent years, due to the depletion of copper sulfides, low-grade copper ores with high contents of oxide ore and carbonate gangue gradually account for a high proportion in the copper extraction industry [
6]. However, bioleaching in the acid condition becomes ineffective and uneconomical for these copper oxide ores due to the limited energy source in oxide ores for autotrophic bacteria [
7], excessive acid consumption [
8,
9] and undesired impurity dissolution which causes some problems in the further processing of the leaching solution [
10].
Ammonia has been extensively used as an effective lixiviant in copper leaching [
11,
12]. Ammonia leaching can solve the corrosion problems encountered in the acidic systems and extract copper from ore selectively, leaving undesired components in the residue [
9]. Many studies reported the leaching of copper ore containing oxides and carbonates in ammonia medium, yet little attention has been paid to the bioleaching in alkaline conditions. Low-grade complex copper ores, dominated with oxides and carbonates, contain few energy sources for bacterial utilization. Such ores may be bioleached in an alkaline condition by heterotrophic bacteria [
13]. Avakyan [
14] demonstrated the release of silicon from nepheline or plagioclase by using
Sarcina ureae, which can grow and produce ammonia in the presence of urea. Groudeva [
15] studied the bioleaching of rich-in-carbonates copper ore in an alkaline condition, and the results showed that bioleaching using urease-possessing bacteria can achieve the highest copper extraction of 64.4% within 30 days. From the above, it is possible to cultivate a heterotrophic strain which can grow and bioleach copper ore in alkaline condition.
In this study, a heterotrophic strain, which can grow in the presence of urea and produce ammonia, was isolated and used in the bioleaching of low-grade copper ore. A series of morphological and metabolic characteristics were investigated, and then the 16S rRNA gene sequence of the strain was analyzed. After identification, bioleaching experiments were carried out and the leaching capacity of the strain on low-grade complex copper ore was revealed.
2. Methods
2.1. Sample Source and Culture Medium
The strain was isolated from alkaline soil sample, collected from a copper mine in Inner Mongolia, China. To screen the strain, the urea medium and the nutrient agar solid medium were used in this experiment. The urea medium consists of: carbon source (5–25 g/L), urea (5–30 g/L), Na2HPO4 (2.1 g/L), KH2PO4 (1.4 g/L), MgSO4·7H2O (0.02 g/L). The nutrient agar medium contains peptone (10 g/L), beef extract (3 g/L), NaCl (5 g/L) and agar (15 g/L). The medium was sterilized by steam autoclaving at 121 °C for 30 min before inoculation, while the urea was separately sterilized through a 0.2 μm filter before being added aseptically into the urea-free medium.
2.2. Isolation of Strain
Soil sample (10 g) was added into 90 mL distilled water, and then 10 mL of supernatant was transferred into 90 mL urea medium for enrichment culture. When the solution became turbid, the culture medium was diluted by the gradient from 10
−1–10
−10 and spread on the nutrient agar solid medium by spread plate method. According to the color, shape and size of the colonies formed on the solid medium, single colonies were inoculated into urea medium. After incubating at 30 °C for 48 h, the bacteria, which can grow robustly in urea medium and produce ammonia, were selected and transferred onto nutrient agar solid again. After repeating for several times, the cell morphology of isolated bacteria appeared to be homologous (observed by ZEISS AX10 light microscope, ZEISS, Oberkochen, Germany), pure culture was considered to be obtained [
16].
The isolated strain was incubated and used for Gram staining and morphological observation. Gram staining was performed with a Gram stain reagent kit (Haitai Biotech, Shanghai, China). Morphological features of cells were revealed by scanning electron microscope (SEM, JEOL JSM-6360LV, Tokyo, Japan) The sample for SEM was prepared as follows: cells solution in the logarithmic phase were obtained and dropped on the aluminum sheet, after drying, coated with carbon, then observed by SEM. The conditions used for the SEM analysis were extra high tension (EHT) 20 kV, work distance (WD) 7.5 mm and 10,000 times magnification.
2.3. Identification of Strain
The strain was enriched and used for DNA extraction by DNA extraction kit (BioBasic Inc., Markham, ON, Canada), then the 16S rRNA gene was amplified by polymerase chain reaction (PCR). Two primers, 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT-3’), were used for PCR reaction. The PCR program was as follows: initial denaturation at 94 °C for 5 min, 30 cycles of denaturation at 94 °C for 45 s, annealing at 51 °C for 45 s and extension 72 °C for 90 s, then followed by another 10 min extension at 72 °C. Subsequently, PCR products were sequenced by the Institute of Microbiology, Chinese Academy of Sciences (Beijing, China). Then, sequences were aligned using Molecular Evolutionary Genetics Analysis software (MEGA 5,
http://www.megasoftware.net/), the phylogenetic tree was created based on the neighbor-joining method by MEGA 5 [
17] according to reference sequences obtained from the National Center for Biotechnology Information (NCBI) database.
2.4. Investigation of Strain Growth
2.4.1. Effect of Carbon Source on Strain Growth
To investigate the utilization of the carbon source and effect of carbon source concentration on strain growth, different concentrations (5, 10, 15, 20, 25 g/L) of sodium citrate, glucose and carbonate were added into the urea medium, respectively. The strain at the logarithmic phase was inoculated into the urea medium with the inoculation size of 20% (v/v), and then incubated at 30 °C and 180 rpm in a rotary shaker. The growth was monitored by cell counting using Neubauer counting chamber (observed by ZEISS AX10 light microscope) and the cell density at stationary phase was recorded.
2.4.2. Effect of Nitrogen Source on Strain Growth
The strain can utilize urea as a nitrogen source and produce ammonia. To obtain the optimal urea concentration, experiments were carried out to investigate the effect of different urea concentrations (5, 10, 15, 20, 25, 30 g/L) on strain growth and ammonia production, with the experiment condition mentioned above. The growth of strain under different urea concentration was observed; the concentration of ammonia in solution was measured by DX-120 ion chromatograph (Dionex, Sunnyvale, CA, USA). Then, the urea concentration with the highest biomass concentration and ammonia concentration was selected as the best urea concentration for the strain.
2.4.3. Optimization of Temperature and Initial pH for Strain Growth
The optimal temperature for strain was obtained by determining growth of strain in urea medium at 21, 24, 27, 30, 33 and 36 °C, respectively. The optimal pH was obtained by determining growth of strain in urea medium at pH values ranging from 5 to 11. The growth was monitored by cell counting using Neubauer counting chamber and the highest cell density at stationary phase was recorded. The pH was measured using a digital pH meter (SX-620, Sanxin, Shanghai, China).
2.5. Bioleaching Procedure
2.5.1. Ore Sample
The copper ores were collected from Yangla copper mine in Yunnan province, China. The sample was ground and sieved to less than 74 μm. The elemental composition of the copper ore is shown in
Table 1, which was measured by X-Ray Fluorescence (XRF) for major elements and Inductively Coupled Plasma-Atomic Emission Spectrometry (ICP-AES) for Cu. The phases of copper in the ore were identified by the polarizing microscope method to be malachite, chrysocolla, chalcopyrite and chalcocite; the Cu content in above four copper phases were 0.36%, 0.29%, 0.29% and 0.07%, respectively. The main gangue minerals were quartz, limonite, calcite and dolomite.
Table 1.
Elemental composition of copper ore used in this study.
Table 1.
Elemental composition of copper ore used in this study.
| Element | Cu | Fe2O3 | MgO | CaO | SiO2 | Al2O3 | ZnO | WO3 |
| Content (wt %) | 1.01 | 27.26 | 1.35 | 10.68 | 47.78 | 7.62 | 0.2 | 0.16 |
2.5.2. Acclimatization of Strain with Copper Ore
Prior to bioleaching, the strain was acclimatized over a prolonged adaptation period to increase the tolerance of cells to the ores. The bacterial cells at the logarithmic phase were centrifuged for 20 min (5000 r/min), and then solid cells were adjusted to a concentration of 1 × 108 cells/mL using sterilized physiologic saline (9 g/L NaCl). Ten milliliters of adjusted cells suspension was added to a 250 mL Erlenmeyer flask containing 1% (w/v) of copper ore and 100 mL of urea medium. The flasks were incubated at 30 °C and 180 rpm in a rotary shaker. Acclimatization of strain to copper ore was achieved by increasing the concentration of copper ore, in steps of 1% (w/v), to a concentration at which strain growth was obviously inhibited. For each step, a 10% (v/v) inoculation was performed using a sample obtained from the previous step.
2.5.3. Bioleaching Experiment
The bioleaching experiments were carried out in 250 mL Erlenmeyer flasks at different pulp densities. The acclimatized strain at the logarithmic phase was inoculated into urea medium, the cell density was 2 × 107 cells/mL after inoculation. All flasks were incubated at 30 °C and 180 rpm in a rotary shaker. During the experiments, samples were withdrawn at regular intervals to monitor the strain growth, pH, concentration of ammonia and concentration of copper ions in the leaching solution. The concentration of copper ions was determined by atomic absorption spectrometry (Z-8000, Hatichi, Suzhou, China).
2.6. Chemical Leaching
Ammonia produced by strain plays an important role in copper extraction. The purpose of performing the chemical leaching was to investigate the importance of ammonia produced by strain in bioleaching. Chemical leaching was conducted in a solution containing ammonia at the same concentration as those produced by strain in the absence of copper ore. Control experiment in urea medium without bacterial inoculation was also conducted. The experiments were carried out at 30 °C and 180 rpm in a rotary shaker.
3. Results and Discussion
3.1. Isolation and Morphological Observation
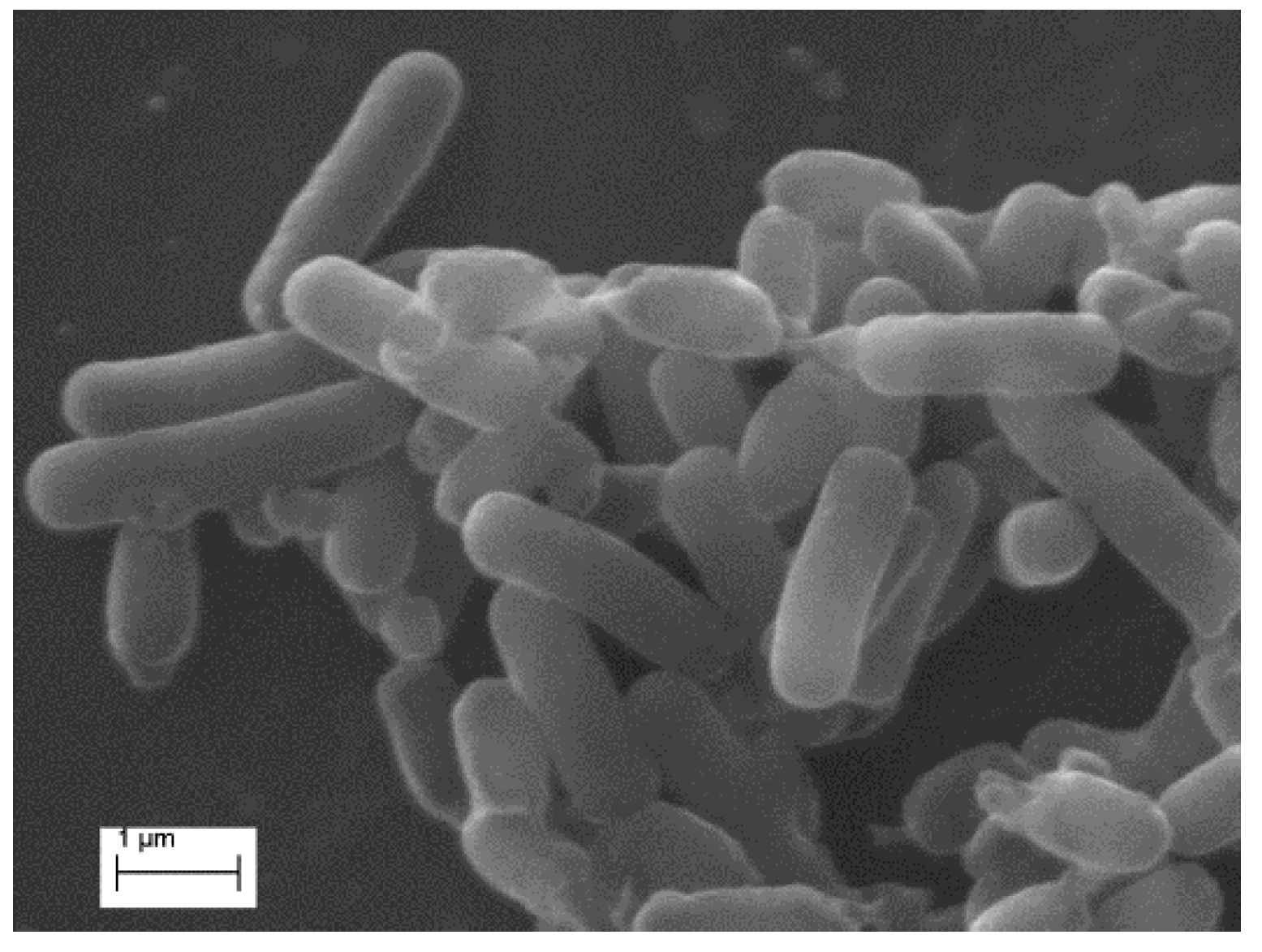
A heterotrophic strain named JAT-1 was isolated. After incubation for about three to five days, colonies appeared on the solid medium plate, with morphological characteristics as follows: 1–2 mm in diameter, circular, smooth and ivory-white. The Gram-staining result showed that the strain was Gram-negative. The morphological characteristics of cells are shown in
Figure 1. The cells were rod-shaped, with a diameter of 0.3–0.6 μm and a length of 1–3 μm.
Figure 1.
Scanning electron microscope image of strain JAT-1.
Figure 1.
Scanning electron microscope image of strain JAT-1.
3.2. Identification of Strain
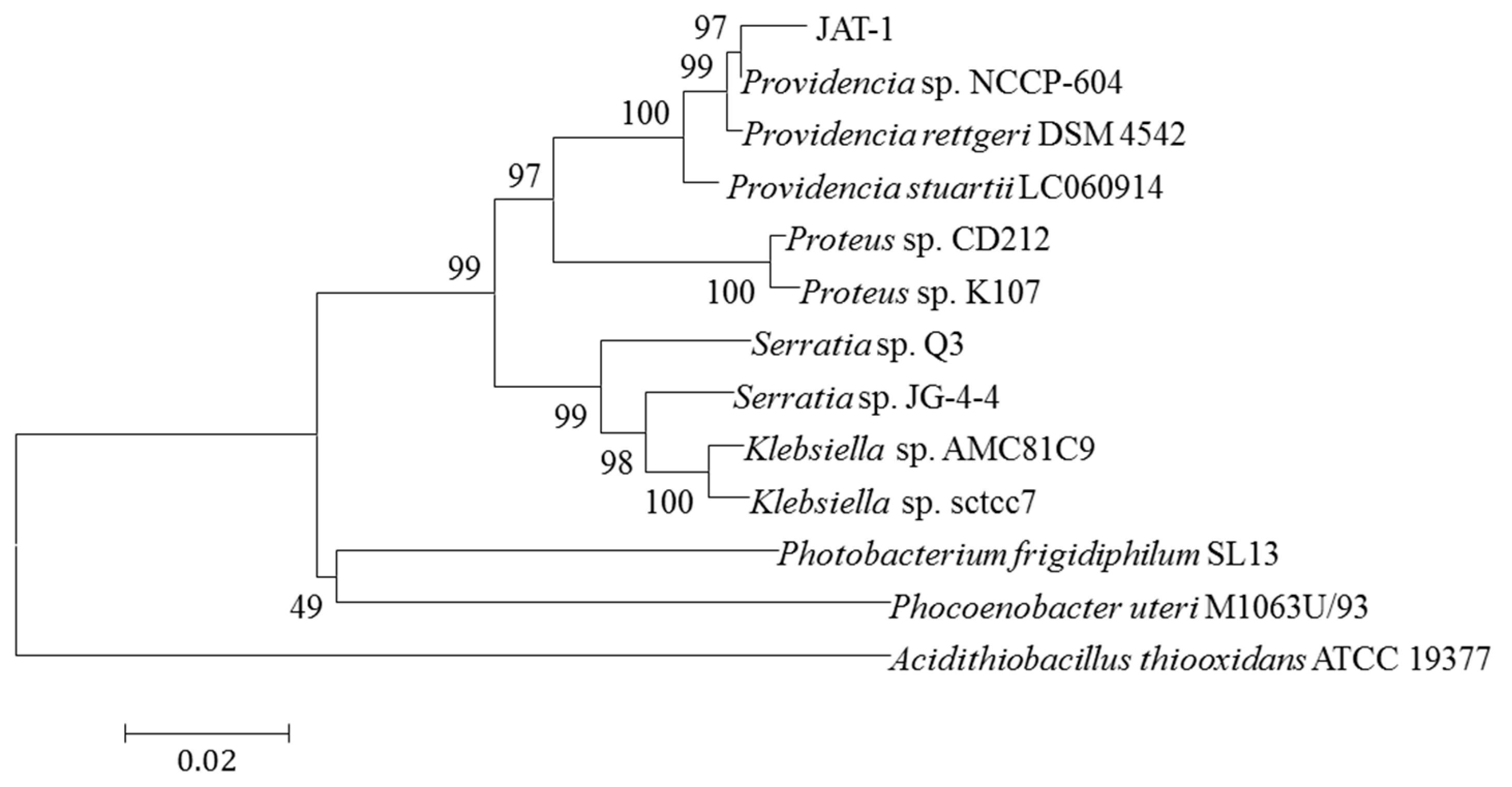
The 16S rRNA gene of strain was amplified by PCR and a total of 1422 nucleotides were sequenced. The sequence was blasted into the database of GenBank and the similar Basic local alignment search tool (BLAST) hits of 16S rRNA indicated that the strain belonged to the genus
Providencia. Identification of the strain was also supported by the phylogenetic analysis as shown in
Figure 2. The result indicates that the strain belonged to
Providencia sp. with a bootstrap support of 100% and it was most closely related to
Providencia sp. NCCP-604 with a similarity of 99% and
Providencia rettgeri DSM 4542 with a similarity of 99%. Thus, the strain is designated as
Providencia sp. JAT-1.
Figure 2.
Phylogenetic tree of JAT-1 based on 16S rRNA sequences with the most similar sequences retrieved from National Center for Biotechnology Information (NCBI) database. The scale bar indicates 0.02 changes per nucleotide. Numbers at nodes represent bootstrap values in 1000 times resampling analysis.
Figure 2.
Phylogenetic tree of JAT-1 based on 16S rRNA sequences with the most similar sequences retrieved from National Center for Biotechnology Information (NCBI) database. The scale bar indicates 0.02 changes per nucleotide. Numbers at nodes represent bootstrap values in 1000 times resampling analysis.
3.3. Effect of Carbon and Nitrogen Source on Strain Growth
3.3.1. Effect of Carbon Source on Strain Growth
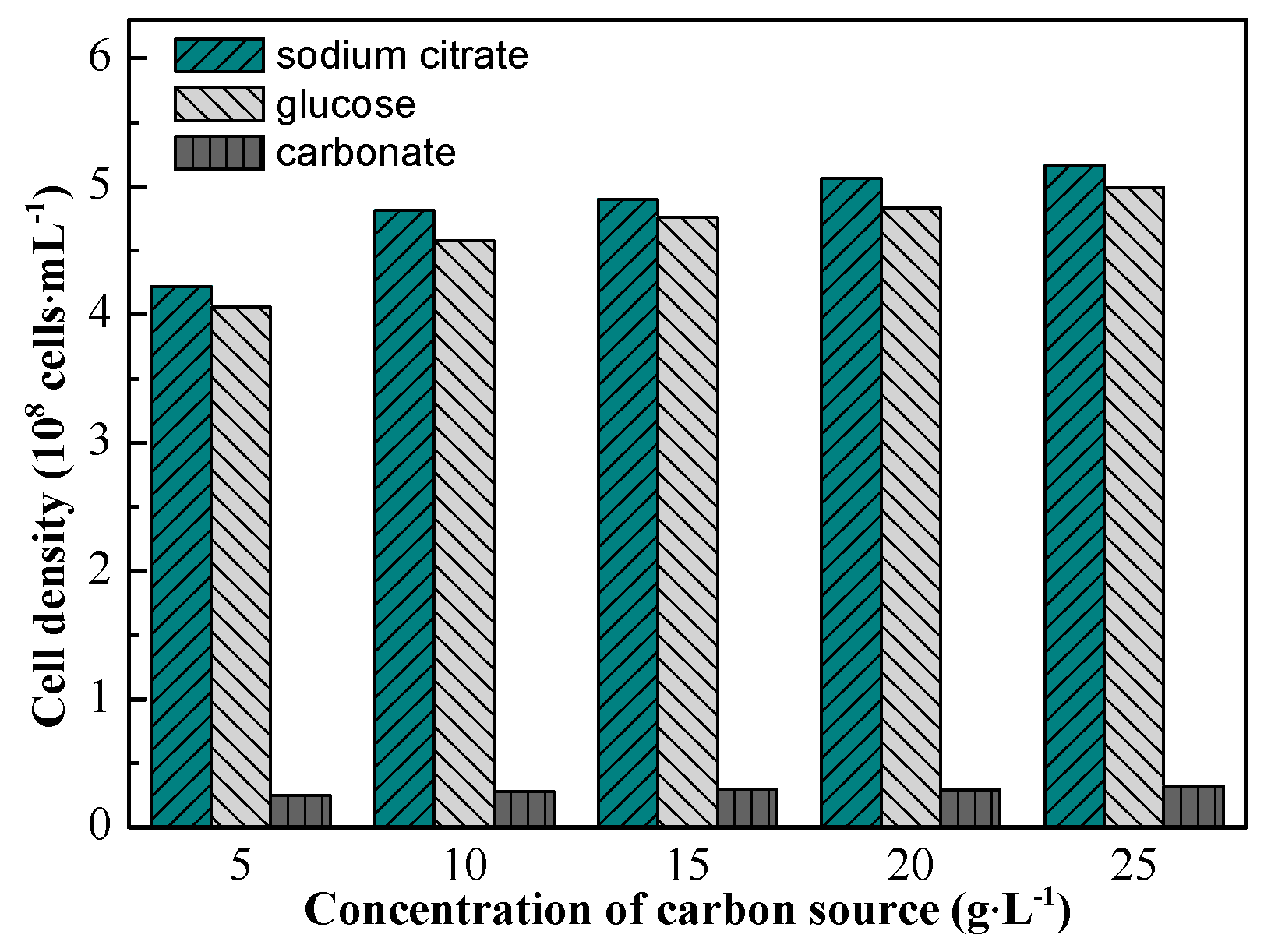
The effect of the carbon source on strain growth is shown in
Figure 3. Almost no growth was observed with the addition of carbonate, indicating that the strain cannot utilize carbonate as a carbon source. Therefore, an organic carbon source is necessary for strain growth even when bioleaching copper ore containing carbonate gangue. The addition of sodium citrate or glucose could favor its growth. However, when the concentration of the carbon source was higher than 10 g/L, the cell density almost no longer increased; consequently, 10 g/L was considered as the optimal concentration of the carbon source. Moreover, with addition of 10 g/L sodium citrate and glucose, cell density reached the highest value of 4.81 × 10
8 and 4.58 × 10
8 cells/mL, respectively. So, the sodium citrate with 10 g/L was used in the following experiments as an energy and carbon source for the strain.
Figure 3.
Effect of different kinds of carbon source and different concentrations of carbon source on strain growth. The cell densities were measured at the stationary phase of strain.
Figure 3.
Effect of different kinds of carbon source and different concentrations of carbon source on strain growth. The cell densities were measured at the stationary phase of strain.
3.3.2. Effect of Nitrogen Source on Strain Growth
Urea is utilized as a nitrogen source for the growth of the strain. The strain JAT-1 can grow in the presence of urea and produce ammonia. The effects of the urea concentration on the growth of strain and ammonia production were shown in
Figure 4. The cell density and ammonia production increased with the increase of the urea concentration up to 20 g/L. However, when the urea concentration was higher than 20 g/L, the increasing of the urea concentration led to a decrease in cell density and decline in ammonia production. It seems that the high urea concentration is adverse to bacterial activity. Hence, 20 g/L was considered as the optimal urea concentration.
Figure 4.
Effect of urea concentration on strain growth and ammonia production. Ammonia production was determined by the concentration of ammonia in culture solution. The data were measured at the stationary phase of strain.
Figure 4.
Effect of urea concentration on strain growth and ammonia production. Ammonia production was determined by the concentration of ammonia in culture solution. The data were measured at the stationary phase of strain.
3.4. Investigation of Pure Culture in Urea Medium
3.4.1. Optimal Temperature and Initial pH for Strain Growth
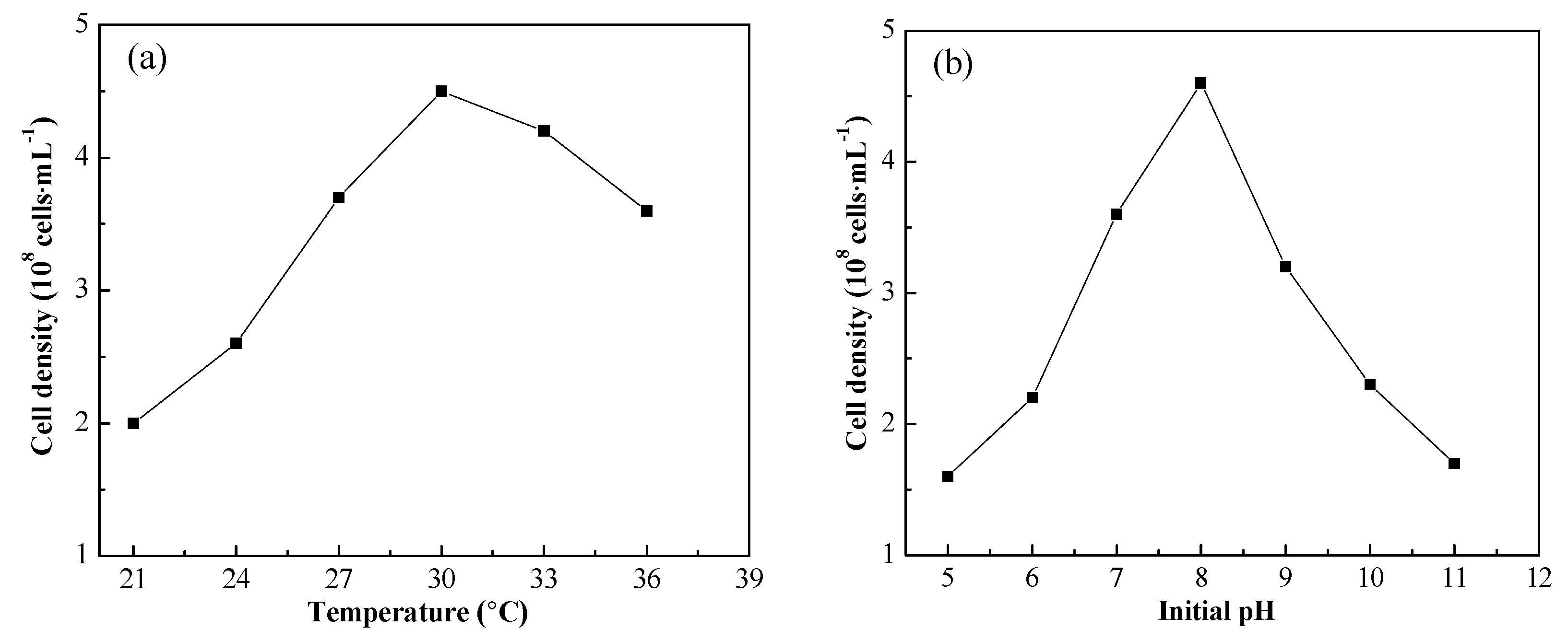
The effect of temperature and initial pH on strain growth is shown in
Figure 5. According to
Figure 5a, the strain grew well between 22 and 36 °C, with the optimal temperature of 30 °C. The strain growth was inhibited obviously when the initial pH was either higher than 10.0 or lower than 6.0, as shown in
Figure 5b, so the optimal initial pH was 8. Thus, the optimal growth condition of the strain is 30 °C and initial pH 8, respectively.
Figure 5.
Effect of temperature (a) and initial pH (b) on strain growth. The cell densities were measured at the stationary phase of strain.
Figure 5.
Effect of temperature (a) and initial pH (b) on strain growth. The cell densities were measured at the stationary phase of strain.
3.4.2. Pure Culture Investigation
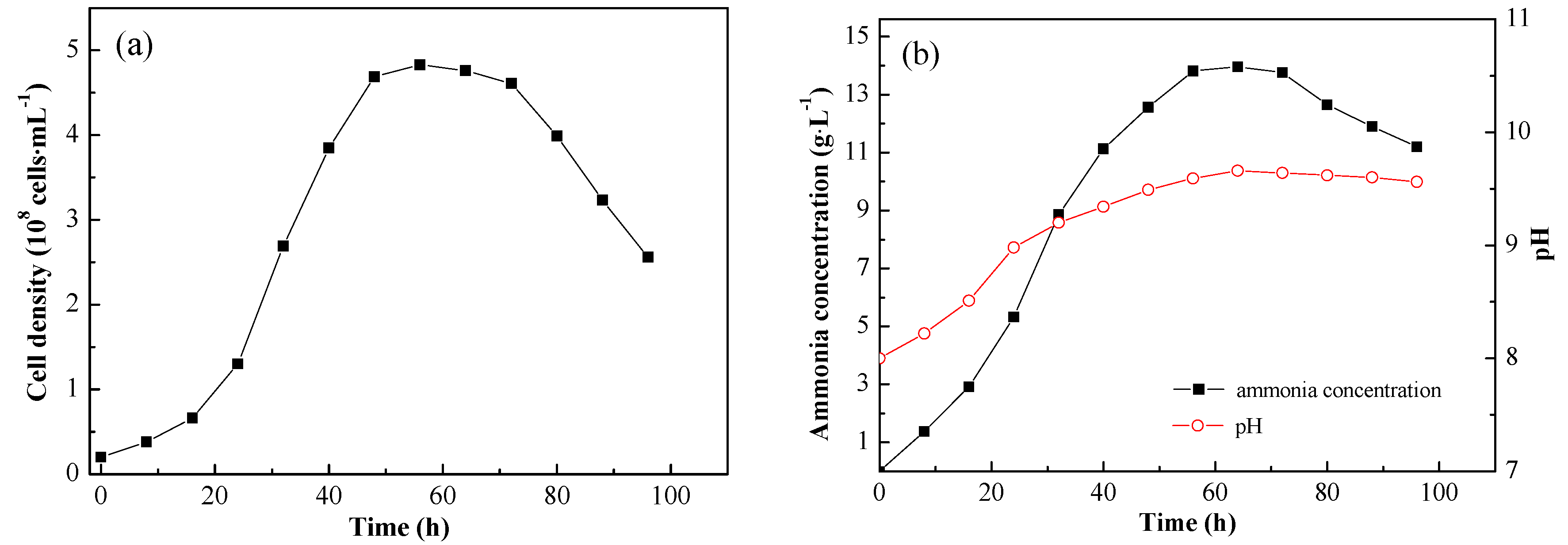
The strain JAT-1 was cultured under the optimal growth condition obtained above. The cells at the logarithmic phase were inoculated into the urea medium, and the cell density was 2 × 10
7 cells/mL after inoculation. The growth curve of strain JAT-1 is shown in
Figure 6a. The strain stayed in lag phase for the first 16 h. The logarithmic phase occurred at 16–48 h and then the cell density reached a maximum of 4.83 × 10
8 cells/mL. After culturing for 72 h, the strain reached the decline phase and the cell density began to decrease, and the cell density was 2.56 × 10
8 cells/mL at the 96th hour.
Figure 6.
Pure culture of strain JAT-1 in urea medium: (a) Strain growth curve at optimal growth condition; (b) Variation of ammonia concentration and pH during growth.
Figure 6.
Pure culture of strain JAT-1 in urea medium: (a) Strain growth curve at optimal growth condition; (b) Variation of ammonia concentration and pH during growth.
The strain can catalyze hydrolysis of urea into ammonia, which will lead to the change of pH. Ammonia production and pH change during pure culture are shown in
Figure 6b. With the increasing of the cell density, the ammonia concentration in the culture solution improved rapidly. The ammonia concentration reached a maximum of 14.0 g/L at the 64th hour and tended to decrease at the decline phase of the strain. The pH increased with the increase of the ammonia concentration; at the stationary and decline phase of the strain, the pH change was not significant and pH value was maintained higher than 9.5.
3.5. Bioleaching of Low-Grade Copper Ore Using JAT-1
3.5.1. Adaptation of JAT-1 with Copper Ore
Adaptation of the culture was necessary because the elements Cu and Fe in copper ore have an inhibitory effect on strain growth. Additionally, adaptation with copper ore is helpful in increasing the efficiency of bioleaching [
18]. The adaptation was continued up to a pulp density of 11%. Pulp concentrations above 11% decreased cell density dramatically, indicating that the highest tolerable concentration of copper ore was 11%. Accordingly, bioleaching experiments were conducted at pulp densities up to 11%.
3.5.2. Bioleaching Using JAT-1
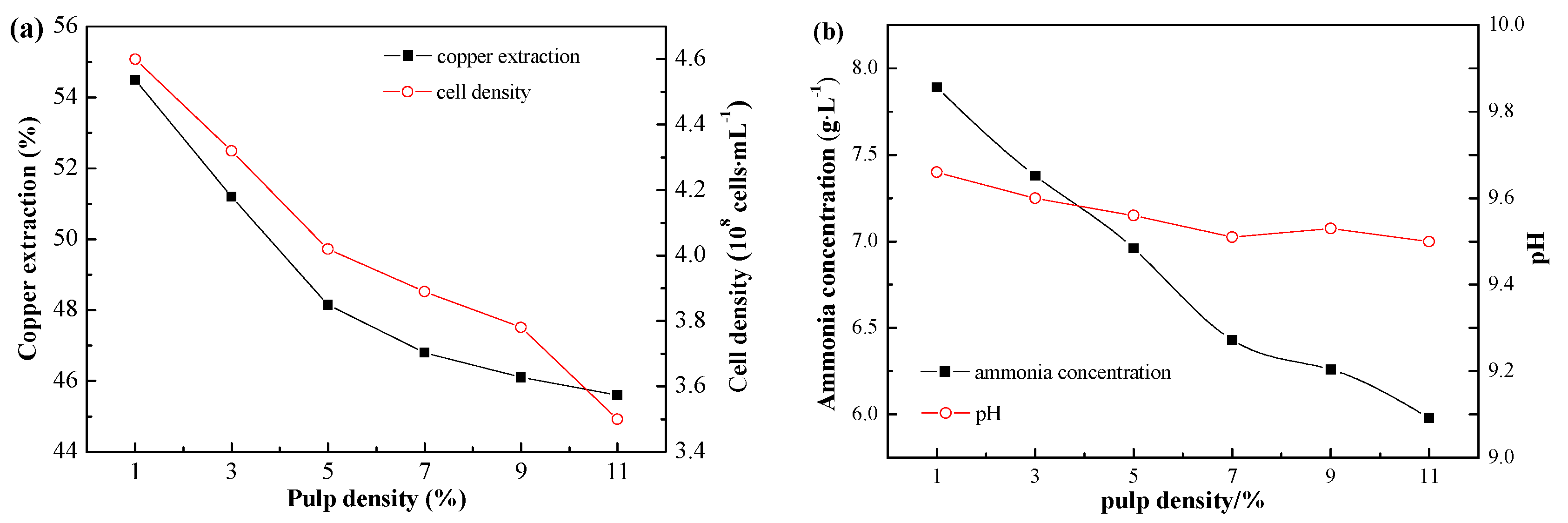
After adaptation, the bioleaching experiments were carried out at different pulp densities (1%, 3%, 5% 7%, 9%, 11%). After bioleaching for 156 h, the copper leaching rates at different pulp densities are shown in
Figure 7. The effect of pulp density on copper extraction was significant. Copper extraction decreased with the increase of pulp density. There was a direct relationship between copper extraction and strain growth. The cell density at the stationary phase shows the decreasing trend with the increase of pulp density. Higher strain growth led to a higher metabolite production and, thus, resulted in a higher copper recovery. The copper extraction reached the highest value of 54.5% at the pulp density of 1%.
Figure 8 shows the ammonia concentration and pH value of leaching solution at different pulp densities. The ammonia concentration decreased with the increase of pulp density, which is consistent with the change of cell density. Solution pH remained between 9.5 and 9.7 at all pulp densities.
Figure 7.
(a) Copper extraction at different pulp densities after bioleaching for 156 h. Cell densities were measured after bioleaching for 64 h; (b) Ammonia concentration and pH change in bioleaching system at different pulp densities. The data in the figure were measured after bioleaching for 64 h.
Figure 7.
(a) Copper extraction at different pulp densities after bioleaching for 156 h. Cell densities were measured after bioleaching for 64 h; (b) Ammonia concentration and pH change in bioleaching system at different pulp densities. The data in the figure were measured after bioleaching for 64 h.
Figure 8.
Comparison of bioleaching and chemical leaching at the pulp density of 1%. The initial ammonia concentration in chemical leaching was same with the concentration as those produced by strain in the absence of copper ore. Control experiment was carried out in urea medium without bacteria inoculation.
Figure 8.
Comparison of bioleaching and chemical leaching at the pulp density of 1%. The initial ammonia concentration in chemical leaching was same with the concentration as those produced by strain in the absence of copper ore. Control experiment was carried out in urea medium without bacteria inoculation.
3.5.3. Phase Transformation of Copper Ore after Bioleaching
The dissolution of different phases of copper ore after bioleaching is shown in
Table 2. Malachite is easy to leach out, and the dissolution rate of malachite reached 69.4%. Chrysocolla is very difficult to leach out in ammonia medium owing to the dispersion of copper atoms in the crystal lattice of gangue ores [
6]. However, 58.6% of chrysocolla was leached out in this bioleaching system. Jain [
7] has reported that the silicon-oxygen bond is disrupted in the high alkalization condition, resulting in the release of silicon from quartz, and this may explain why copper atoms could be released from the chrysocolla. Most of the chalcocite was leached out. However, chalcopyrite was leached out partly, with the oxygen dissolved in the solution as an oxidant [
6]. Liu [
11] has reported that the copper sulfides can be oxidized and dissolved effectively in ammonia solution with sodium persulfate as an oxidant, so we can infer that a more efficient oxidant will promote the dissolution of copper sulfides during bioleaching.
Table 2.
Phase composition of copper ore before and after bioleaching at the pulp density of 1% and copper extraction (%) after 156 h.
Table 2.
Phase composition of copper ore before and after bioleaching at the pulp density of 1% and copper extraction (%) after 156 h.
| Phase | Cu (wt %) | Copper Extraction (%) |
|---|
| Before Bioleaching | After Bioleaching |
|---|
| Malachite | 0.36 | 0.11 | 69.4 |
| Chrysocolla | 0.29 | 0.12 | 58.6 |
| Chalcopyrite | 0.29 | 0.22 | 24.1 |
| Chalcocite | 0.07 | 0.01 | 85.7 |
| Total Cu | 1.01 | 0.46 | 54.5 |
3.6. Comparison of Bioleaching and Chemical Leaching
Figure 8 shows the results of the comparison of bioleaching and chemical leaching at the pulp density of 1%. According to
Figure 6b, the ammonia concentration in urea medium could reach a maximum of 14 g/L in the absence of copper ore. Thus, the initial ammonia concentration of 14 g/L was used for chemical leaching.
After 156 h, the copper extraction of ammonia leaching was 36.5%, which was 18% lower than bioleaching. Results proved that bioleaching using JAT-1 is superior to ammonia leaching at the same condition. In the first 80 h, the copper extraction of bioleaching and ammonia leaching were both increased rapidly. However, after 80 h, the copper extraction of ammonia leaching increased slowly compared to bioleaching. Some of the copper ore, which is difficult to leach out in ammonia medium, such as chrysocolla, could be leached out during bioleaching. We can also infer that metabolites other than ammonia must be involved in bioleaching. A heterotrophic strain can produce exopolysaccharides, amino acids and proteins, which may interact with minerals and promote the dissolution of metals [
7,
19]. Without bacterial inoculation, almost no copper was leached out in the control experiment, indicating that the strain JAT-1 played an important role in bioleaching.
Many studies reported the leaching of copper ore containing oxides and carbonates in acid and ammonia solution. Huang [
20] studied leaching of this kind of copper ore in acid condition and the results showed that 53% of copper was leached out under the conditions of a particle size of 74–106 μm, H
2SO
4 concentration of 40 g/L and shaking rate of 150 r/min in the shake flask at room temperature in 3 h. Compared with bioleaching, the leaching efficiency was higher in the acid condition in Huang’s study. However, excessive acid consumption in acid leaching was repeatedly mentioned [
8]. Dissolution of undesired impurities will also cause some problems in the further processing of acid leaching solution [
10]. Besides, Wu [
21] reported that a large amount of the precipitate, such as CaSO
4, will enrich on the surface of copper ore and prevent the metal dissolution in acid conditions. Baba [
22] studied ammonia leaching of complex copper ore and the results revealed that under the leaching conditions of 125–212 μm, 120 °C, 1.29 mol/L and 202 kPa of oxygen partial pressure, about 83% Cu could be extracted in 2.5 h. Higher temperature, ammonia concentration and oxygen partial pressure could lead to a higher copper extraction; however, it may be not economical for low-grade ores. Being more selective and using lower reagent consumption, bioleaching using JAT-1 demonstrates a new and economical way for processing low-grade complex copper ore containing oxides and carbonates.
4. Conclusions
In this study, a heterotrophic strain, identified as Providencia sp. JAT-1, was successfully isolated. The strain can grow in the presence of urea and produce ammonia. Pure culture and bioleaching experiments were conducted in urea medium. At the optimal condition, the cell density of pure culture and ammonia concentration reached a maximum of 4.83 × 108 cells/mL and 13.96 g/L, respectively. Higher strain growth led to a higher metabolite production and, thus, resulted in a higher copper recovery. However, some elements in copper ore had an inhibitory effect on strain growth and ammonia production. With the increase of pulp density, the cell density and ammonia concentration in the leaching solution decreased at the stationary phase of the strain, leading to a decrease of copper extraction. Copper extraction reached the highest value of 54.5% at the pulp density of 1%. Most of the malachite, chrysocolla and chalcocite leached out in this system while chalcopyrite leached out partially. After leaching for 156 h, the copper extraction of chemical leaching was 18% lower than bioleaching at the same experimental condition, indicating that metabolites produced by the strain other than ammonia were also involved in bioleaching. Bioleaching using JAT-1 demonstrates a new way for processing low-grade complex copper ore containing oxides and carbonates. The factors influencing the kinetics of bioleaching using JAT-1 will be studied in the future.
Acknowledgments
The authors would like to acknowledge the financial support for this work provided by the National Key Technologies R&D Program for the 12th Five-year Plan (2012BAB08B02), National Natural Science Foundation of China (51304011 and 51374035) and Specialized Research Fund for the Doctoral Program of Higher Education (20110006130003).
Author Contributions
All of the authors conceived the research idea and designed the experiments; Kaijian Hu performed experiments, analyzed the data and co-wrote the paper. Aixiang Wu analyzed the data and provided funding for this work; Hongjiang Wang assisted with experiments and contributed analysis tools; Shaoyong Wang analyzed the data and co-wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Watling, H. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Stott, M.; Watling, H.; Franzmann, P.; Sutton, D. The role of iron-hydroxy precipitates in the passivation of chalcopyrite during bioleaching. Miner. Eng. 2000, 13, 1117–1127. [Google Scholar] [CrossRef]
- Panda, S.; Akcil, A.; Pradhan, N.; Deveci, H. Current scenario of chalcopyrite bioleaching: A review on the recent advances to its heap-leach technology. Bioresour. Technol. 2015, 196, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Devasia, P.; Natarajan, K.; Sathyanarayana, D.; Rao, G.R. Surface chemistry of Thiobacillus ferrooxidans relevant to adhesion on mineral surfaces. Appl. Environ. Microbiol. 1993, 59, 4051–4055. [Google Scholar] [PubMed]
- Hallberg, K.B.; González-Toril, E.; Johnson, D.B. Acidithiobacillus ferrivorans, sp. nov.; facultatively anaerobic, psychrotolerant iron-, and sulfur-oxidizing acidophiles isolated from metal mine-impacted environments. Extremophiles 2010, 14, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Tang, M.; Tang, C.; Jing, H.; Yang, S.H.; Yang, J.G. Dissolution kinetics of low grade complex copper ore in ammonia-ammonium chloride solution. Trans. Nonferr. Met. Soc. China 2010, 20, 910–917. [Google Scholar] [CrossRef]
- Jain, N.; Sharma, D. Biohydrometallurgy for nonsulfidic minerals—A review. Geomicrobiol. J. 2004, 21, 135–144. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.; Hu, H.; Chen, Q. Leaching kinetics of low-grade copper ore containing calcium-magnesium carbonate in ammonia-ammonium sulfate solution with persulfate. Trans. Nonferr. Met. Soc. China 2012, 22, 2822–2830. [Google Scholar] [CrossRef]
- Bingöl, D.; Canbazoğlu, M.; Aydoğan, S. Dissolution kinetics of malachite in ammonia/ammonium carbonate leaching. Hydrometallurgy 2005, 76, 55–62. [Google Scholar] [CrossRef]
- Ekmekyapar, A.; Aktaş, E.; Künkül, A.; Demirkiran, N. Investigation of leaching kinetics of copper from malachite ore in ammonium nitrate solutions. Metall. Mater. Trans. B 2012, 43, 764–772. [Google Scholar] [CrossRef]
- Liu, Z.X.; Yin, Z.L.; Xiong, S.F.; Chen, Y.G.; Chen, Q.Y. Leaching and kinetic modeling of calcareous bornite in ammonia ammonium sulfate solution with sodium persulfate. Hydrometallurgy 2014, 144, 86–90. [Google Scholar] [CrossRef]
- Arzutug, M.E.; Kocakerim, M.M.; Çopur, M. Leaching of malachite ore in NH3-saturated water. Ind. Eng. Chem. Res. 2004, 43, 4118–4123. [Google Scholar] [CrossRef]
- Willscher, S.; Bosecker, K. Studies on the leaching behaviour of heterotrophic microorganisms isolated from an alkaline slag dump. Hydrometallurgy 2003, 71, 257–264. [Google Scholar] [CrossRef]
- Avakyan, Z. Microflora of Rock and Its Role in the Leaching of Silicate Minerals. In Biogeotechnology of Metals: Proceedings of International Seminar and International Training Course; Karavaiko, G.I., Groudev, S.N., Eds.; Centre of International Projects GKNT: Moscow, Russia, 1985; pp. 175–194. [Google Scholar]
- Groudeva, V.; Krumova, K.; Groudev, S.N. Bioleaching of a Rich-in-Carbonates Copper Ore at Alkaline pH. Adv. Mater. Res. 2007, 20, 103–106. [Google Scholar] [CrossRef]
- Zeng, W.; Wu, C.; Zhang, R.; Hu, P.; Qiu, G.; Gu, G.; Zhou, H. Isolation and identification of moderately thermophilic acidophilic iron-oxidizing bacterium and its bioleaching characterization. Trans. Nonferr. Met. Soc. China 2009, 19, 222–227. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Yaghmaei, S.; Mousavi, S.; Sheibani, S. Recovery of metals from spent refinery hydrocracking catalyst using adapted Aspergillus niger. Hydrometallurgy 2011, 109, 65–71. [Google Scholar] [CrossRef]
- Welch, S.; Barker, W.; Banfield, J. Microbial extracellular polysaccharides and plagioclase dissolution. Geochim. Cosmochim. Acta 1999, 63, 1405–1419. [Google Scholar] [CrossRef]
- Huang, M.; Wu, A.; Xiao, Y. Dynamic study on acid leaching of complex alkaline copper oxide ore. Gold 2010, 12, 38–42. [Google Scholar]
- Wu, A.X.; Wang, S.Y.; Yin, S.H.; Yan, J.L. Chemical precipitation and control in process of heap leaching of high-alkali oxide copper ore. J. Cent. South. Univ. Sci. Technol. 2012, 5, 1851–1857. [Google Scholar]
- Baba, A.A.; Ghosh, M.K.; Pradhan, S.R.; Rao, D.S.; Baral, A.; Adekola, F.A. Characterization and kinetic study on ammonia leaching of complex copper ore. Trans. Nonferr. Met. Soc. 2014, 24, 1587–1595. [Google Scholar] [CrossRef]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).