Flocculation of Pyrite Fines in Aqueous Suspensions with Corn Starch to Eliminate Mechanical Entrainment in Flotation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
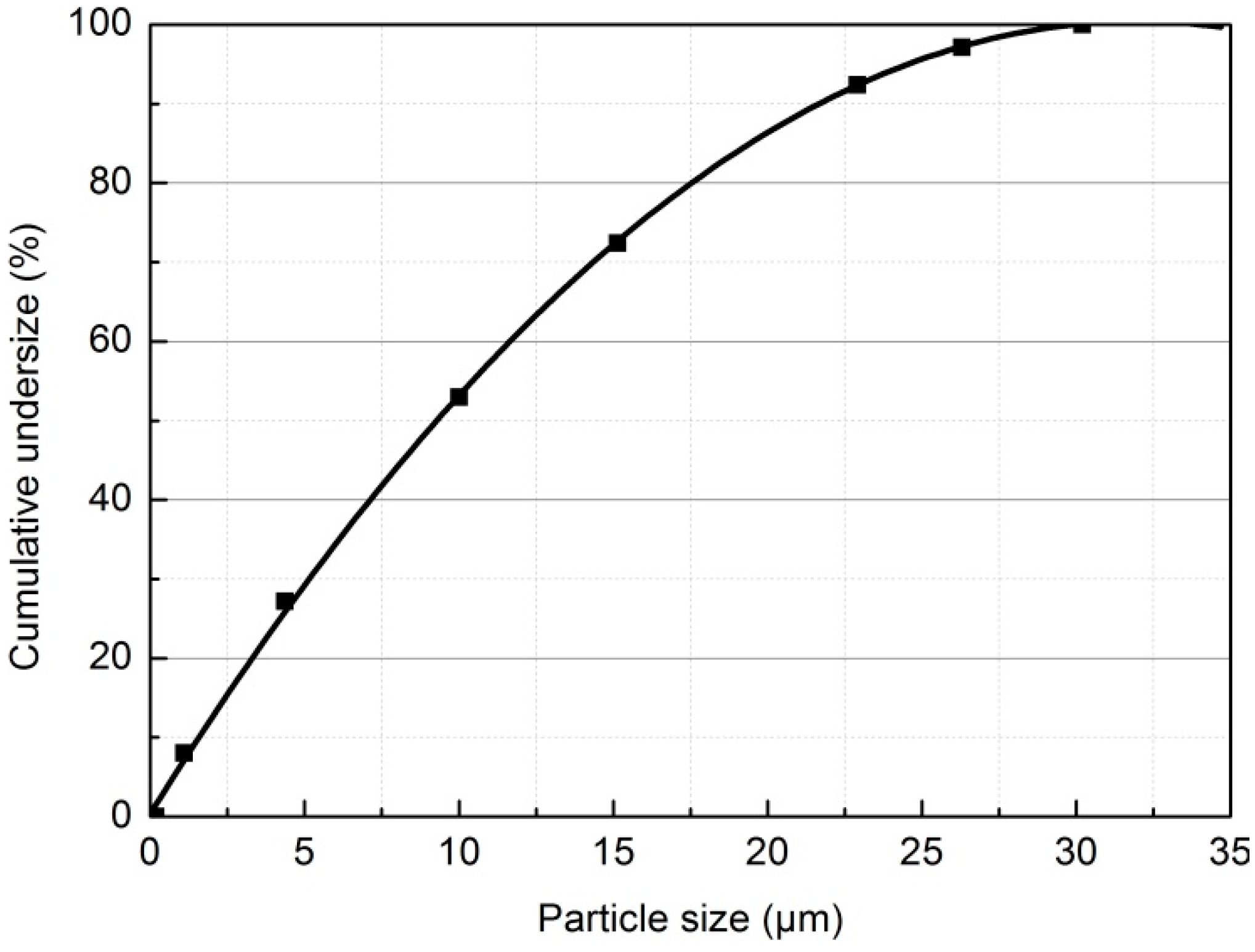
2.2. Methods
2.2.1. Hydrophilic Flocculation Test
2.2.2. Particle Size Analysis
2.2.3. Observation of Pyrite Flocs
2.2.4. Micro-Flotation Tests
2.2.5. Adsorption Density Determination
2.2.6. Zeta Potential Measurements
2.2.7. X-Ray Photoelectron Spectrometer (XPS) Studies
3. Results and Discussion
3.1. Flocculation of Pyrite Fines
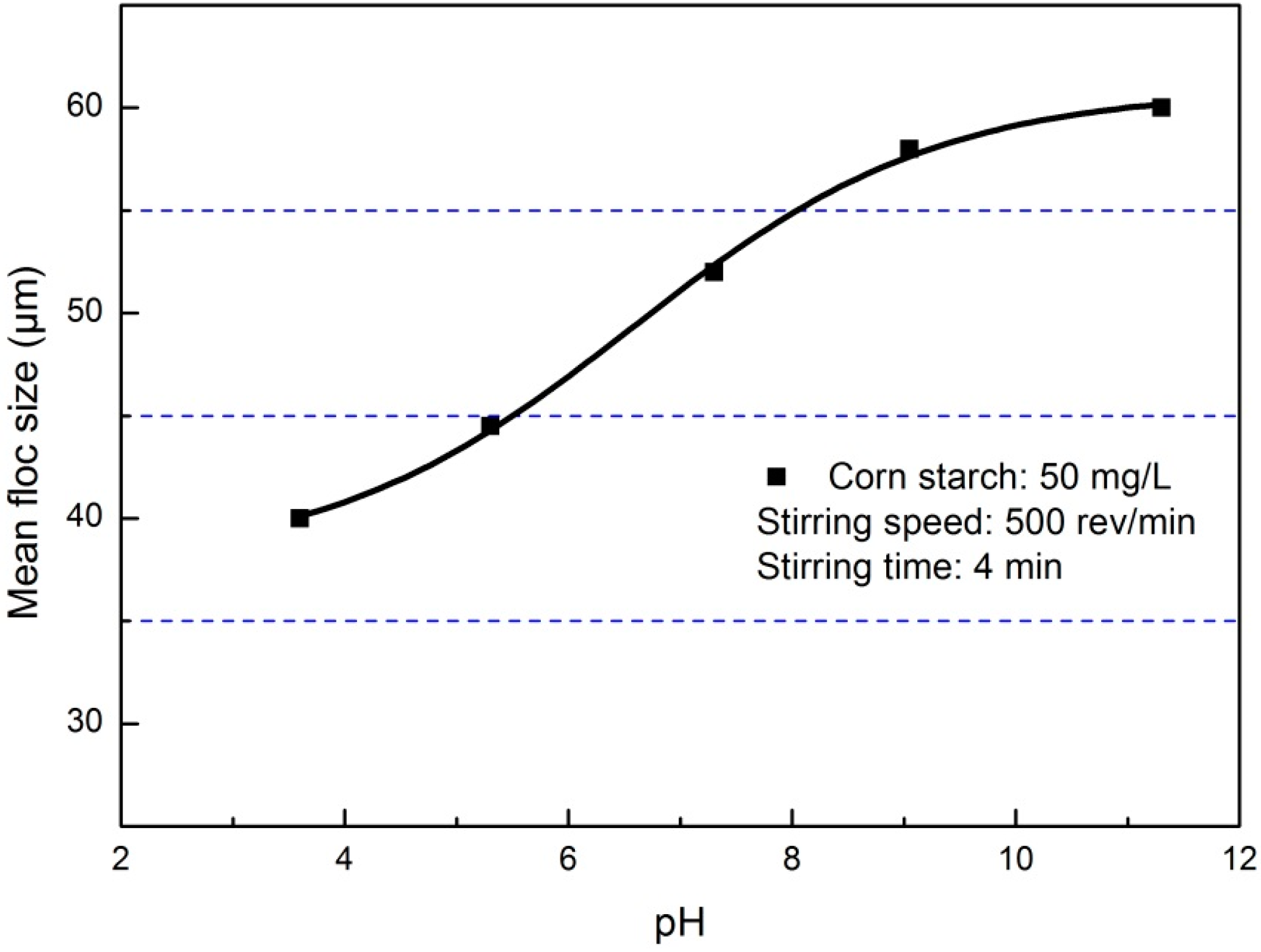


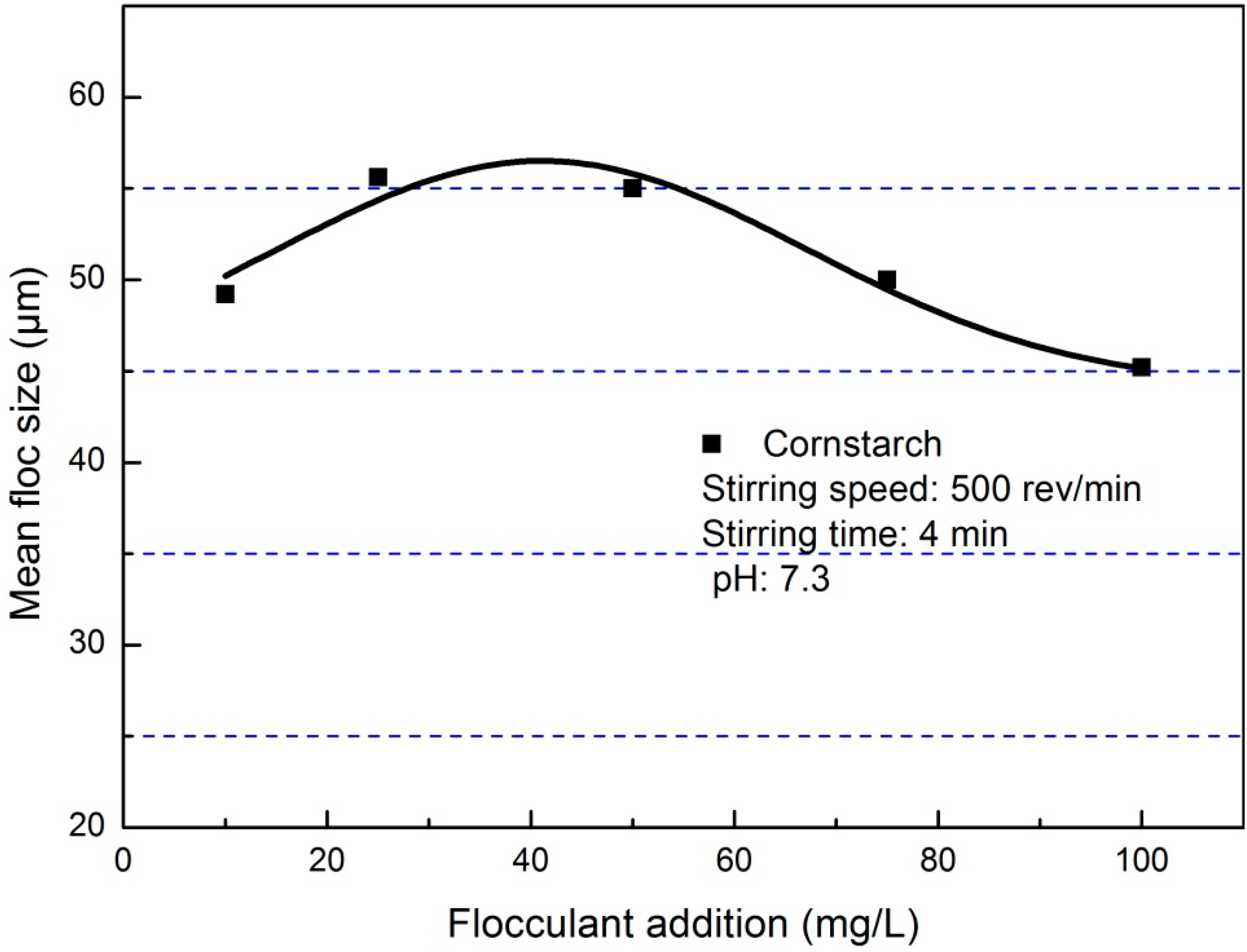
3.2. Morphology of Pyrite Flocs
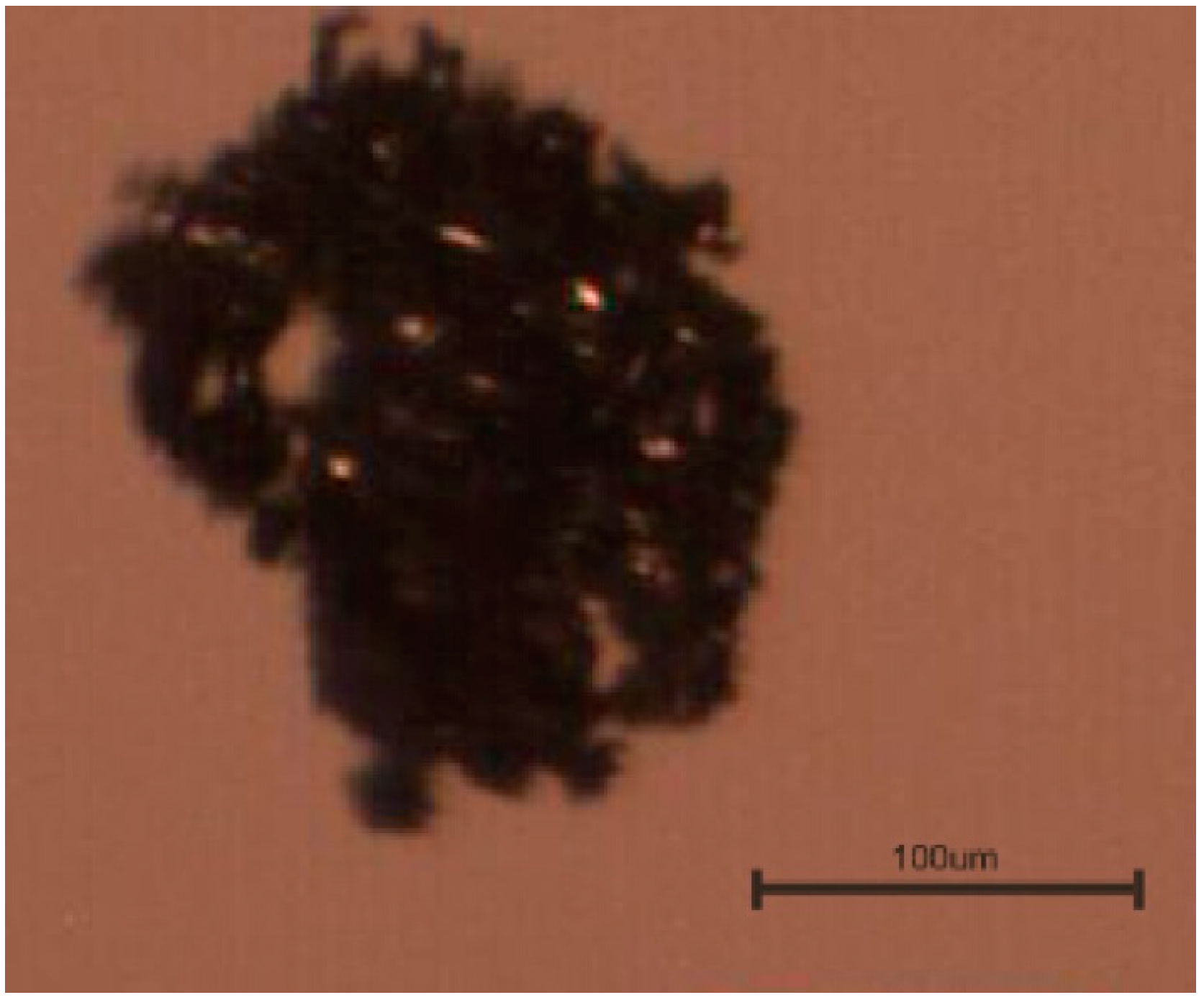
3.3. Flotation of Pyrite Flocs

3.4. Adsorption Isotherm
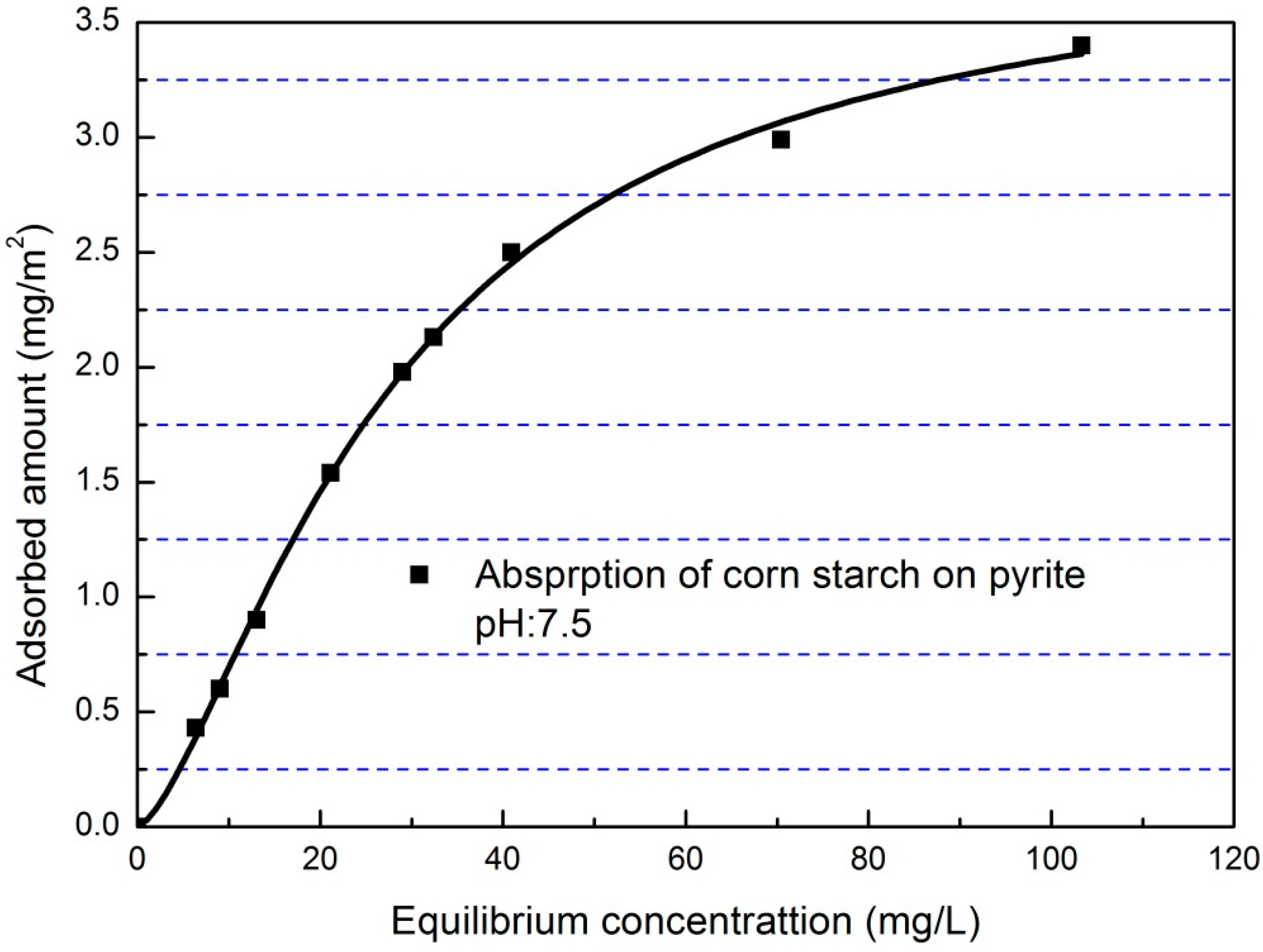
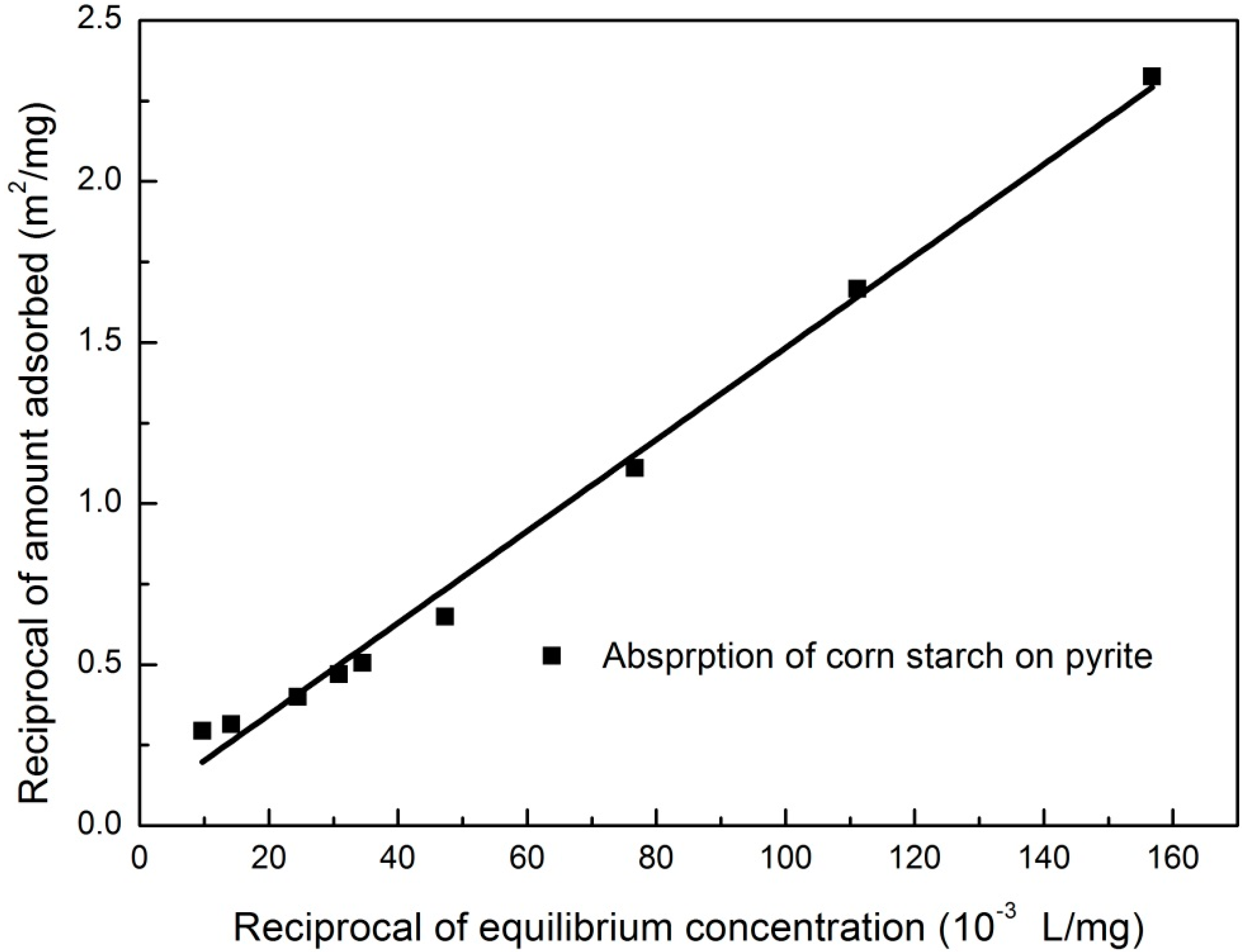
3.5. Effect of Corn Starch on the Zeta Potential of Pyrite


3.6. Interaction Mechanism between Pyrite and Digested Corn Starch
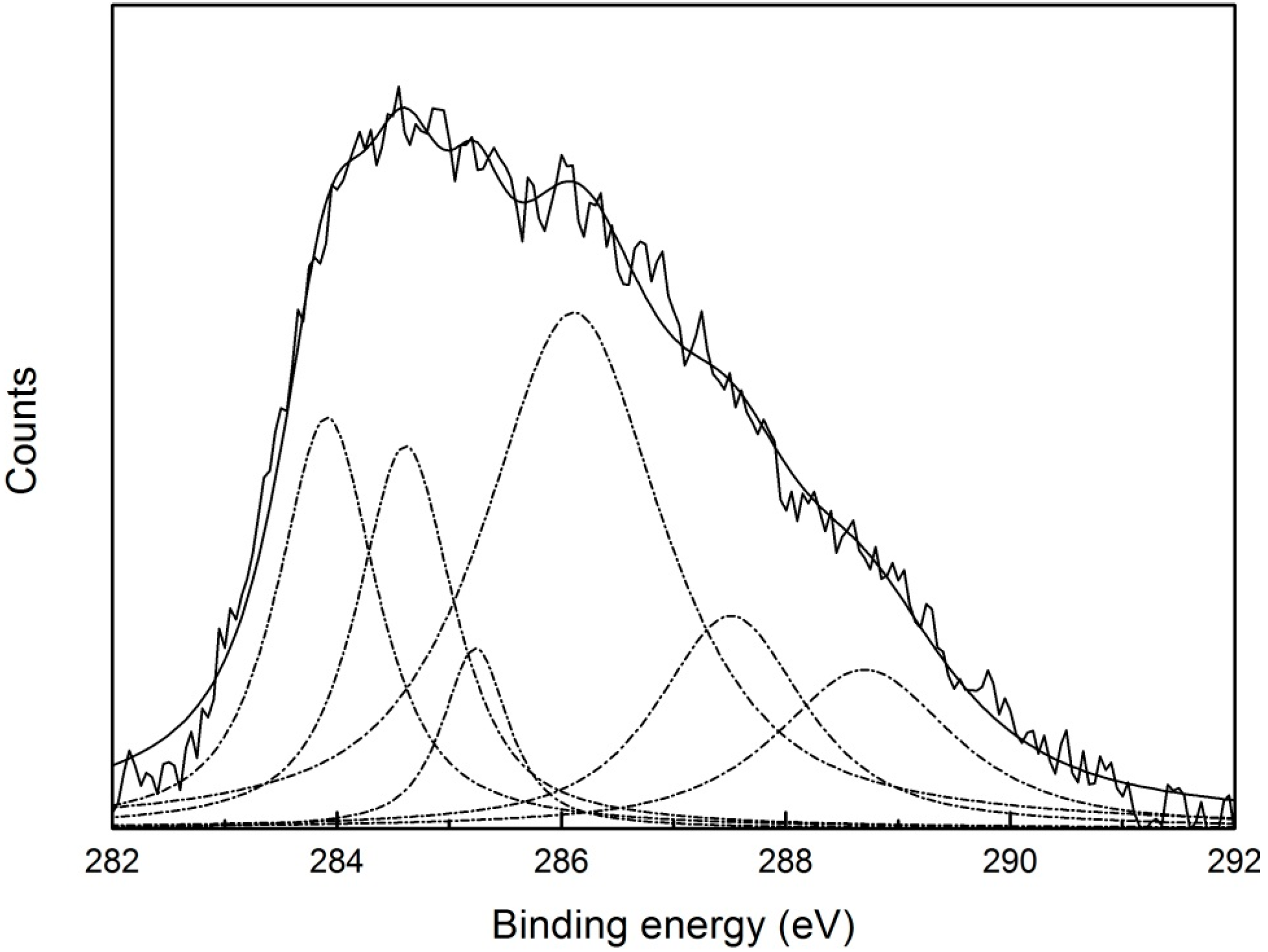
| Chemical State | C | |||||
|---|---|---|---|---|---|---|
| C–C/C–H | C–O | O=C–O | O–C–O | Unknown | Unknown | |
| BE (eV) | 284.61 | 286.11 | 288.70 | 287.51 | 293.91 | 285.23 |

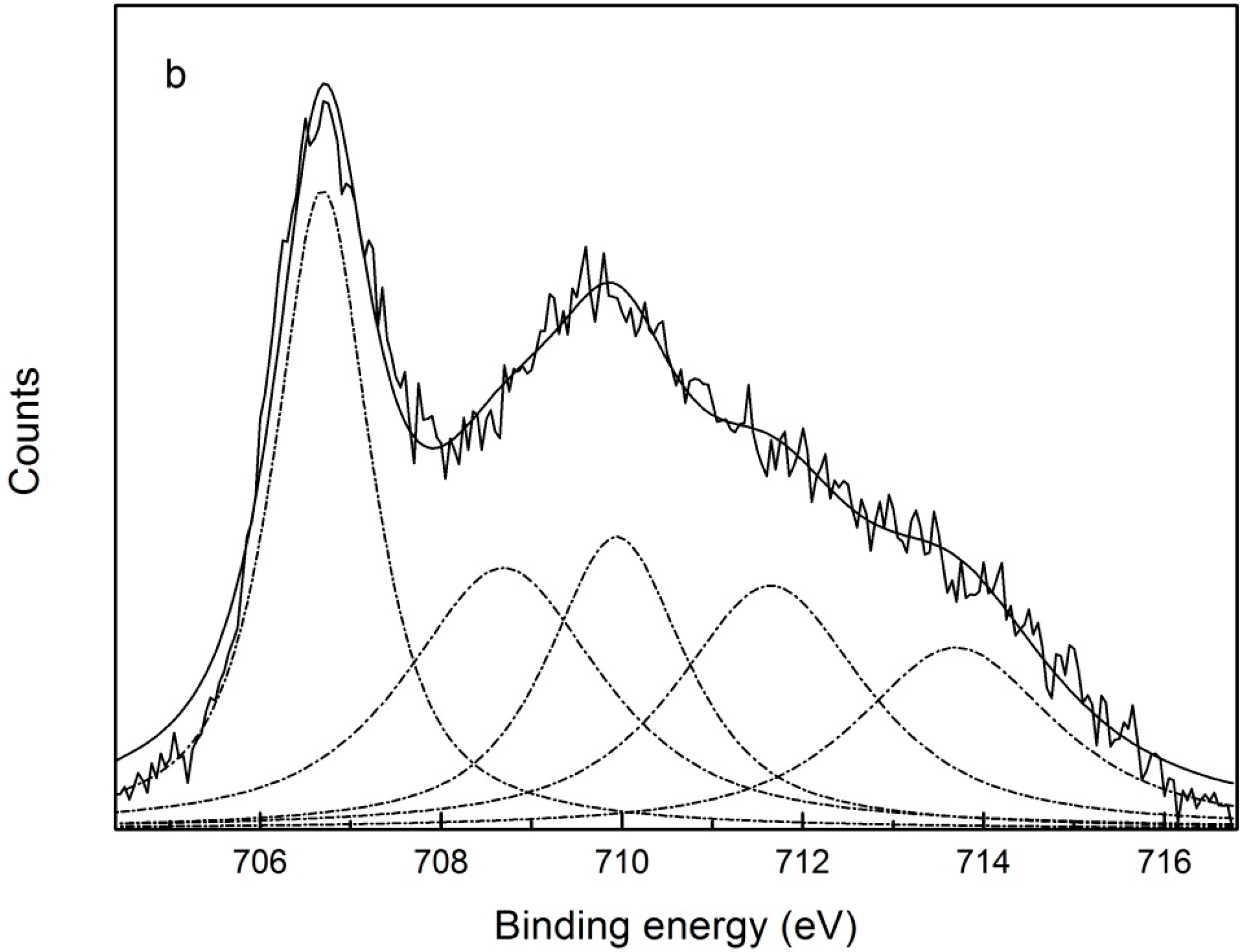
| Species | Fe | ||||
|---|---|---|---|---|---|
| FeS2 | FeO | Fe2O3 | FeOOH | Fe2(SO4)3 | |
| BE (eV) a | 707.30 | 708.73 | 710.15 | 711.8 | 713.88 |
| BE (eV) b | 706.69 | 708.69 | 710.09 | 711.6 | 713.69 |
| Shift (eV) | 0.61 | 0.04 | 0.06 | 0.2 | 0.19 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- George, P.; Nguyen, A.V.; Jameson, G.J. Assessment of true flotation and entrainment in the flotation of submicron particles by fine bubbles. Miner. Eng. 2004, 17, 847–853. [Google Scholar] [CrossRef]
- Guler, T.; Akdemir, Ü. Statistical evaluation of flotation and entrainment behavior of an artificial ore. Trans. Nonferr. Met. Soc. China 2012, 22, 199–205. [Google Scholar] [CrossRef]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Gong, J.; Peng, Y.; Bouajila, A.; Ourriban, M.; Yeung, A.; Liu, Q. Reducing quartz gangue entrainment in sulphide ore flotation by high molecular weight polyethylene oxide. Int. J. Miner. Process. 2010, 97, 44–51. [Google Scholar] [CrossRef]
- Liu, D.; Peng, Y. Reducing the entrainment of clay minerals in flotation using tap and saline water. Powder Technol. 2014, 253, 216–222. [Google Scholar] [CrossRef]
- Liu, Q.; Wannas, D.; Peng, Y. Exploiting the dual functions of polymer depressants in fine particle flotation. Int. J. Miner. Process. 2006, 80, 244–254. [Google Scholar] [CrossRef]
- Cao, M.; Liu, Q. Reexamining the functions of zinc sulfate as a selective depressant in differential sulfide flotation—The role of coagulation. J. Colloid Interface Sci. 2006, 301, 523–531. [Google Scholar] [CrossRef] [PubMed]
- Boulton, A.; Fornasiero, D.; Ralston, J. Selective depression of pyrite with polyacrylamide polymers. Int. J. Miner. Process. 2001, 61, 13–22. [Google Scholar] [CrossRef]
- Huang, P.; Cao, M.; Liu, Q. Selective depression of pyrite with chitosan in Pb-Fe sulfide flotation. Miner. Eng. 2013, 46–47, 45–51. [Google Scholar] [CrossRef]
- López-Valdivieso, A.; Celedón, C.T.; Song, S.; Robledo, C.A.; Laskowski, J.S. Dextrin as a non-toxic depressant for pyrite in flotation with xanthates as collector. Miner. Eng. 2004, 17, 1001–1006. [Google Scholar]
- Rath, R.K.; Subramanian, S.; Pradeep, T. Surface chemical studies on pyrite in the presence of polysaccharide-based flotation depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Bulut, G.; Ceylan, A.; Soylu, B.; Goktepe, F. Role of starch and metabisuphite on pure pyrite and pyritic copper ore flotation. Physicochem. Probl. Miner. Process. 2012, 48, 39–48. [Google Scholar]
- Sarquís, P.E.; Menéndez-Aguado, J.M.; Mahamud, M.M.; Dzioba, R. Tannins: The organic depressants alternative in selective flotation of sulfides. J. Clean. Prod. 2014, 84, 723–726. [Google Scholar] [CrossRef]
- Gül, A.; Baran, E.; Burat, F. The effect of non-toxic depressants in chalcopyrite flotation. In Proceedings of the XXV International Mineral Processing Congress (IMPC) 2010, Brisbane, Australia, 6–10 September 2010; Volume 3, pp. 1899–1903.
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Fred, S. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Song, S.; Lopez-Valdivieso, A.; Reyes-Bahena, J.L.; Bermejo-Perez, H.I.; Trass, O. Hydrophobic flocculation of galena fines in aqueous suspensions. J. Colloid Interface Sci. 2000, 227, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D.J. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960. [Google Scholar] [CrossRef]
- Tang, M.; Liu, Q. The acidity of caustic digested starch and its role in starch adsorption on mineral surfaces. Int. J. Miner. Process. 2012, 112–113, 94–100. [Google Scholar] [CrossRef]
- Uliniuc, A.; Popa, M.; Drockenmuller, E.; Boisson, F.; Leonard, D.; Hamaide, T. Toward tunable amphiphilic copolymers via CuAAC click chemistry of oligocaprolactones onto starch backbone. Carbohydr. Polym. 2013, 96, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Pan, Y.; Xue, J.; Sun, Q.; Su, G.; Li, X. Comparative XPS study between experimentally and naturally weathered pyrites. Appl. Surface Sci. 2009, 255, 8750–8760. [Google Scholar] [CrossRef]
- Laskowski, J.S.; Liu, Q.; O’Connor, C.T. Current understanding of the mechanism of polysaccharide adsorption at the mineral/aqueous solution interface. Int. J. Miner. Process. 2007, 84, 59–68. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, W.; Li, H.; Ren, Y.; Zhao, F.; Song, S. Flocculation of Pyrite Fines in Aqueous Suspensions with Corn Starch to Eliminate Mechanical Entrainment in Flotation. Minerals 2015, 5, 654-664. https://doi.org/10.3390/min5040515
Ge W, Li H, Ren Y, Zhao F, Song S. Flocculation of Pyrite Fines in Aqueous Suspensions with Corn Starch to Eliminate Mechanical Entrainment in Flotation. Minerals. 2015; 5(4):654-664. https://doi.org/10.3390/min5040515
Chicago/Turabian StyleGe, Wei, Hongqiang Li, Yanzeng Ren, Feiyu Zhao, and Shaoxian Song. 2015. "Flocculation of Pyrite Fines in Aqueous Suspensions with Corn Starch to Eliminate Mechanical Entrainment in Flotation" Minerals 5, no. 4: 654-664. https://doi.org/10.3390/min5040515





