1. Introduction
The increasing demand and diminishing availability of raw materials requires us to look beyond conventional resources; particularly, the importance of low-grade ores is expected to increase in the near future [
1]. An example is the depletion of accessible high-grade sulfidic ores, which makes it necessary to seek more abundant but lower grade ores, often rich in siliceous mafic minerals (olivine, pyroxene, amphibole and biotite). The processing of such ores, however, is linked to high processing costs when using traditional extraction routes (e.g., high pressure acid leaching (HPAL) and ferro-nickel smelting).
An alternative, potentially more sustainable approach is the application of biohydrometallugy, wherein microorganisms act as renewable chemical producers [
1] of substances that deteriorate and dissolve minerals, thereby liberating the immobilized metals into solution (leachate). Biogenic substances produced include not only organic acids [
2], but also chelates, mineral acids, and for certain bacteria, ammonia or amines [
3]. The three main mechanisms that can act in the solubilization of metals are: acidolysis, complexolysis and redoxolysis [
4].
Several types of microorganisms have been tested for leaching of mineral ores. These can be classified into two groups: autotrophic bacteria and archaea, and heterotrophic bacteria, archaea and fungi [
5]. Autotrophic microorganisms use carbon dioxide as their carbon source, whereas heterotrophic microorganisms use organic compounds as a carbon source. Additionally, a distinction can be made between chemoorganotrophic and chemolithotrophic microorganisms. The former obtain energy through oxidation of organic compounds, while the latter use reduced inorganic compounds as their energy resource [
6]. Here a distinction can be made between the microorganisms utilized in the present work, which do not depend on the presence of sulfur, and those typically used industrially for bioleaching of sulfidic ores, which utilize the intrinsic sulfur.
Bioleaching is influenced by a wide range of parameters including physicochemical parameters, microbiological factors of the leaching environment, and the properties of the solids to be leached. These will influence both the growth of the microorganisms as well as their leaching behavior [
1,
7,
8,
9]. Most importantly, the microorganism must be able to leach the material, and also be resistant to the metals that are leached out. In terms of types of minerals, silicates and saprolites have been found to leach more readily than limonites [
6]. In terms of the toxic effect of heavy metals, these can have an adverse impact on the microorganism’s survival due to four reasons: (i) the blocking of essential functional groups of enzymes; (ii) the displacement of essential metals; (iii) the induction of conformational changes of polymers; and (iv) the influence on the membrane integrity and transport processes [
10]. For the leaching of nickel laterite ores it has been found that
Aspergillus niger and
Penicillium funiculosum are tolerant to practical levels of nickel (0.1 g/L) concentration [
2].
In an effort to accelerate bioleaching, recent research has provided evidence for the beneficial effects of controlled sonication on the leachability of metals catalyzed by living cells. Anjum
et al. [
11] tested sonobioleaching on black shale and concluded that it enhanced metal recovery and reduced the time needed for the maximum recovery. They also found that the production of acids was higher for ultrasonically treated
Aspergillus niger. The acid yields with ultrasound treatment amounted to 6.17% citric acid, 4.68% malic acid, 2.36% oxalic acid and 0.052% tartaric acid. In comparison, not sonicated
A. niger yielded 5.25% citric acid, 3.45% malic acid, 0.94% oxalic acid and 0.09% tartaric acid.
Swamy
et al. [
12] found that for the leaching of nickel from laterites, ultrasound increased and accelerated the recovery from 92% in 20 days to 95% in 14 days. The recovery of iron, on the other hand, reduced from 12.5% to 0.16%, indicating that ultrasound also greatly increases the selectivity of nickel over iron. Swamy
et al. [
12] also investigated the influence of certain parameters of the ultrasonic treatment such as frequency, intensity and sonication time on the leaching results. The metal recovery was highest when a daily sonication time of 30 min was used. Maximum extractions were achieved at the lower frequency of 20 kHz because more citric and oxalic acids were produced at this frequency. The optimal intensity was found to be 1.5 W/cm
2. Further increasing this intensity was postulated to lead to cell disruption, causing a decrease in acid production and consequently lowering the nickel extraction.
Bioleaching may also benefit from mineral processing steps that increase specific surface area, alter the mineralogy or otherwise liberate metal-rich mineral from the gangue matrix. Santos
et al. [
13] explored an approach wherein carbon dioxide was used to promote mineral alterations that led to improved extractability of nickel from olivine ((Mg,Fe)
2SiO
4). Olivine is an abundant silicate mineral within the Earth’s crust that contains minor amount of nickel and chromium, and is a precursor to lateritic nickel ores that are commercially explored. Carbonation pre-treatment was found to promote mineral liberation and concentration of metals in physically separable phases. In that study, olivine was fully carbonated at high CO
2 partial pressures (35 bar) and optimal temperature (200 °C) with the addition of pH buffering agents. The main products of the carbonation reaction included amorphous colloidal silica, chromium-rich metallic particles, and iron-substituted magnesite (Mg
1–xFe
xCO
3). The percentage of nickel extracted from carbonated olivine significantly increased compared to leaching from untreated olivine. Using HCl, 100% of nickel could be leached from carbonated olivine, while only 66% nickel was recovered from untreated olivine using the same acid concentration (2.6 N).
The present work investigates the possibility of bioleaching nickel from olivine. The microorganisms utilized were the bacterium
Paenibacillus mucilaginosus, and the fungi
Aspergillus niger,
Penicillium chrysogenum and
Humicola grisea. These microorganisms were selected based on the prior work of Chiang
et al. [
1], who screened several bacteria and fungi for bioleaching of various alkaline materials. In that study, the bioleaching performance of fungi was more comparable, hence three fungi and one bacteria were selected for the present study. Buford
et al. [
3] report that the three selected fungal species are commonly associated in the natural environment with sandstone, marble and granite rock substrata, indicating their adaptability to silicate materials. The soil-inhabiting bacterium has been commonly applied in bioleaching of silica-rich materials, e.g., Yao
et al. [
9]. The extent of leaching with these different microorganisms is compared. The effect of carbonation as a mineral alteration/liberation pre-treatment step in the recovery of nickel is investigated. The influence of ultrasonic treatment on both the growth of the microorganisms as well as on the leaching are studied.
2.1. Materials
The microorganisms were acquired from culture collections: P. mucilaginosus bacterium was obtained from the China Center of Industrial Culture Collection (CICC, Beijing, China); A. niger, P. chrysogenum and H. grisea fungi were acquired from DSMZ (Braunschweig, Germany). The fungi strains were maintained on potato dextrose agar (PDA, containing infusion from potatoes, 2% glucose and 1.5% agar). The P. mucilaginosus strain was maintained on nutrient agar (NA) with 0.001% MnSO4·H2O for sporulation enhancement. All growth media components were obtained from Sigma Aldrich (Bornem, Belgium). Each strain was incubated at 30 °C for growth and preserved at 4 °C for storage.
Two types of olivine mineral were tested to investigate the effect of pre-treatment on the leaching behavior: fresh olivine (GL30, Eurogrit B.V., Papendrecht, The Netherlands) and fully carbonated olivine. The fresh olivine was milled using a centrifugal mill (Retsch ZM100, 1400 rpm, 80 μm sieve mesh), resulting in a material with 86 vol % below 80 μm, and the average mean diameter (D{4,3}, determined by laser diffraction) of 34.8 μm. The chemical composition of the olivine, determined following chemical digestion by inductively coupled plasma mass spectrometry (ICP-MS, X Series, Thermo Electron Corporation, Waltham, MA, USA) and by wavelength dispersive X-ray fluorescence (WDXRF, PW2400, Panalytical, Almelo, The Netherlands), was: 27.5 wt % Mg, 20.7 wt % Si, 3.7 wt % Fe, 0.27 wt % Ni, 0.24 wt % Cr, and 0.17 wt % Al. The carbonated olivine consisted of a mixture of iron-substituted magnesite, amorphous colloidal silica, and chromium-rich metallic particles (more details in Santos
et al. [
13] and Van Audenaerde [
14]). It was obtained by reacting 200 grams of fresh milled olivine in 800 mL 1 M NaCl aqueous solution for 72 h, at 200 °C, with 35 bar CO
2 partial pressure. The carbonation conversion achieved, determined by thermogravimetric analysis, was 99.0%.
2.2. Bioleaching Methodology
Bioleaching experiments consisted of two phases: the growth phase and the leaching phase. At the beginning of the growth phase, the microorganisms were inoculated in 400 mL nutrient broth in 1 L cotton wad-sealed Erlenmeyer flasks and placed on magnetic stirring plates inside an incubator set to 30 °C to grow. Once the growth phase was completed (after seven days), olivine solids were added (50 g/L) to the microbial broth and to a reference sterile nutrient broth, to start the leaching phase. Two methods of bioleaching were used: microbially-assisted leaching, and biogenic substance leaching. In microbially-assisted leaching, the living microorganisms were present during the leaching phase, and assisted with the extraction of metals. In biogenic substance leaching, the microbial broth was autoclaved (15 min at 120 °C) at the end of the growth phase to sterilize the broth, and filtered to remove the dead biomass; this way, only the soluble biogenic substances produced by the microorganisms were responsible for the subsequent leaching. It should be noted that autoclaving may denature some biogenic substances, such as enzymes, though it is expected that the main substances responsible for bioleaching, such as organic acids, remained unaltered. During the leaching phase the flasks were agitated on a shaking table at 160 rpm under a temperature controlled hood set to 25 °C. Liquid samples were collected and analyzed during the leaching phase to monitor the metal concentrations at various times. The pH was measured at various intervals during the growth and the leaching phases to track biogenic acid production and consumption. Viable microbial concentrations in solution were determined by spread plating serial dilutions onto nutrient agar plates using a sterile Drigalski spatula and counting colony forming units (CFU) after incubation for 24 h. Upon completion of the leaching phase (7 to 21 days duration), the liquid and solids were separated by centrifugation. The liquids were analyzed for soluble metals concentrations using ICP-MS, whereas the solids were analyzed by wet laser diffraction (LD, Mastersizer S, Malvern Instruments, Malvern, UK). Leaching experiments were carried out in duplicate and mean values are reported. Owing to occasional variability caused by uneven inoculation or biomass pelletization (particularly with fungi), and to the low number of replicates, reported values and trends should be treated qualitatively.
The experiments that tested the influence of sonication on bioleaching used an ultrasonic bath (Elmasonic S300 (H), Elma Hans Schmidbauer, Singen, Germany) operated at a non-adjustable frequency of 37 kHz, 300 W power, and 0.2 W/cm2 sonication intensity. Flasks containing microbial broth were ultrasonically treated during both the growth and leaching phases daily by placing them inside the bath for 15 min.
3.1. Bioleaching of Fresh Olivine by Bacterium versus Fungus
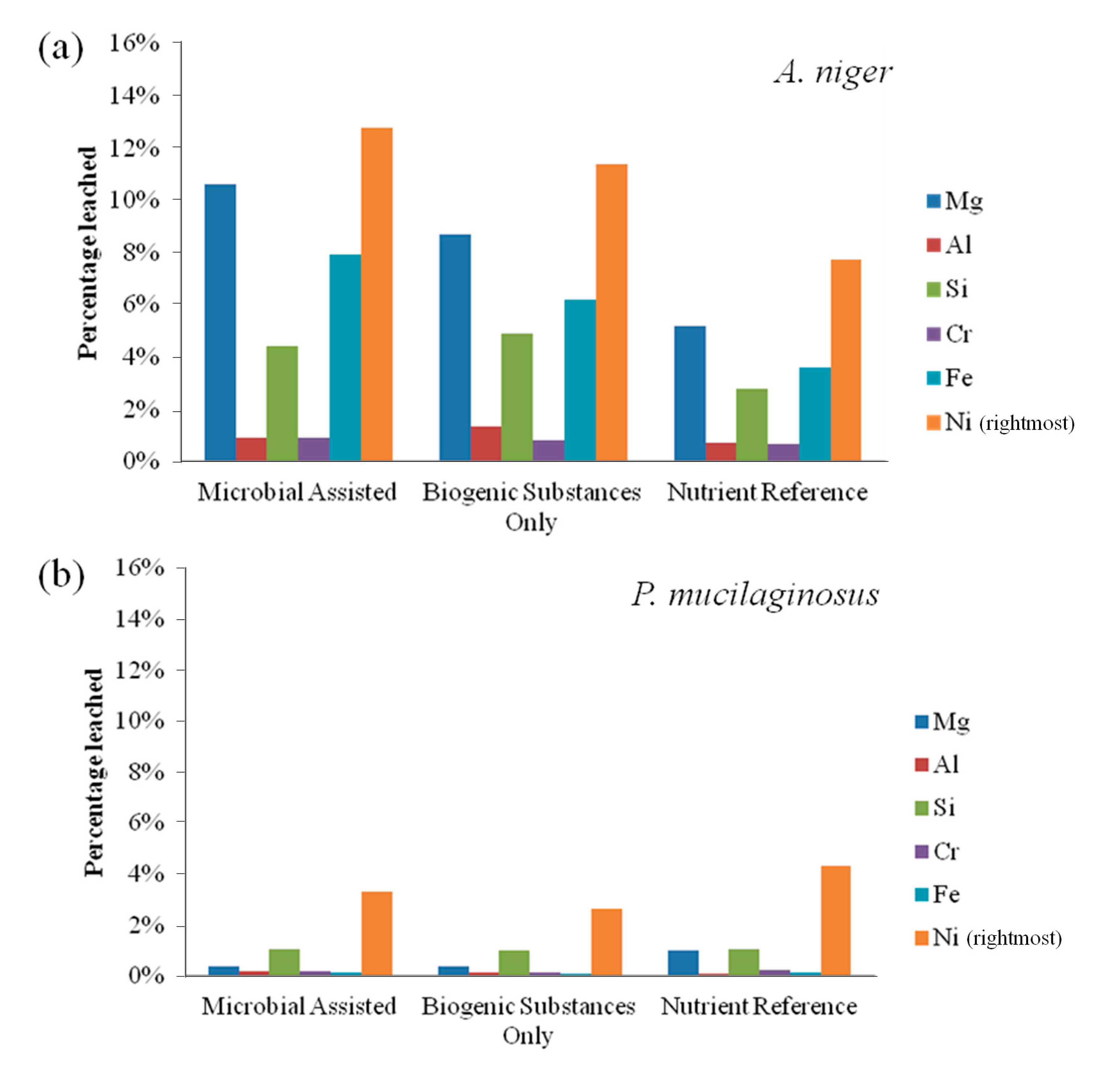
Bioleaching results for fresh olivine by
A. niger and
P. mucilaginosus are presented in
Figure 1. The leaching extent with
A. niger was substantially better than that with
P. mucilaginosus.
A. niger leached 12.8% nickel in the microbially-assisted subset after 21 days, compared to 11.4% with only the biogenic substances. For
P. mucilaginosus, these same extractions were only 3.3% and 2.7%, respectively. In fact, these extraction extents were comparable to the nutrient reference for
P. mucilaginosus, indicating that the bacterium or its biogenic products did not improve olivine leaching.
Figure 2 suggests that biogenic acids produced by
A. niger were largely responsible for its better bioleaching performance. This is consistent with the finding of Castro
et al. [
5], who compared various bacteria and fungi for the leaching of nickel and zinc from calamine and garnierite silicates, and concluded that fungi were more effective due to the production of citric and oxalic acids together with other organic metabolites (e.g., amino acids, peptides and proteins [
10]). Therefore, the subsequent set of experiments focused on the leaching behavior of various fungi towards olivine.
From
Figure 1a, it also appears that live
A. niger provides enhanced leaching compared to the experiment performed with sterile biogenic substances. Burgstaller and Schinner [
10] have noted that cell-particle contact may contribute to the enhancement of fungal bioleaching, for example by stimulating biogenic acid production. Finally, it is notable from
Figure 1 that elemental leaching was incongruent, with higher yields of nickel leaching compared to magnesium and iron, the two main constituents of olivine. Leaching of silicon was further reduced compared to these elements, as can be expected by the low solubility of silicic acid at these conditions, while chromium and aluminum remained largely unaffected, likely due to their presence primarily within metal-rich crystals [
13] and their differing geochemical characteristics.
Figure 1.
(a) Elemental leaching extent by Aspergillus niger from fresh olivine over 21 days; and (b) elemental leaching extent by Paenibacillus mucilaginosus from fresh olivine over 21 days.
Figure 1.
(a) Elemental leaching extent by Aspergillus niger from fresh olivine over 21 days; and (b) elemental leaching extent by Paenibacillus mucilaginosus from fresh olivine over 21 days.
Figure 2.
Variation of pH, measured weekly, during bioleaching of fresh olivine over 21 days by Aspergillus niger and Paenibacillus mucilaginosus and their respective nutrient broth references.
Figure 2.
Variation of pH, measured weekly, during bioleaching of fresh olivine over 21 days by Aspergillus niger and Paenibacillus mucilaginosus and their respective nutrient broth references.
3.2. Bioleaching of Fresh and Carbonated Olivine by Various Fungi
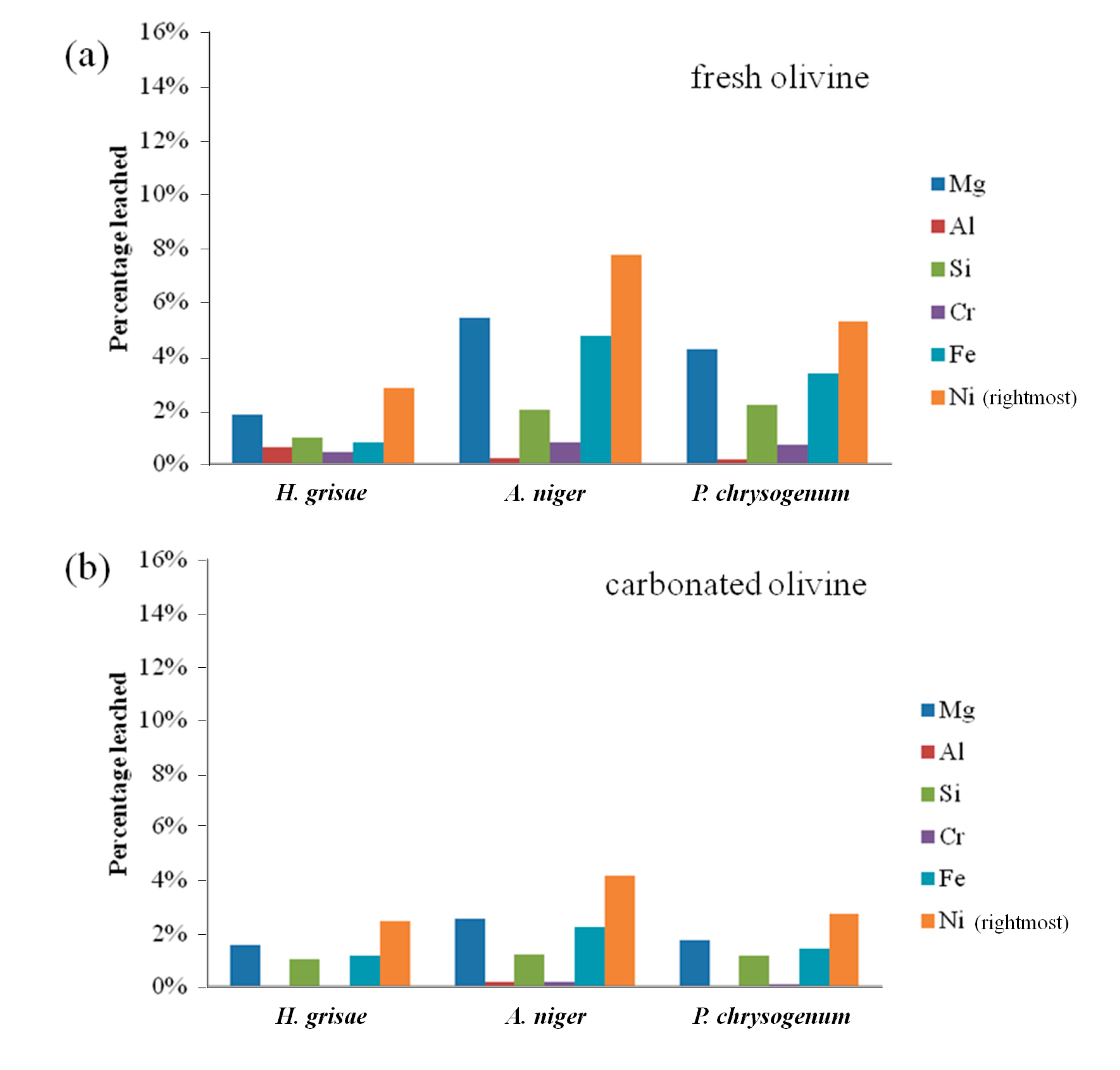
Bioleaching results from fresh and carbonated olivine using various fungi are presented in
Figure 3.
Aspergillus niger was found to be the preferential type of fungi for leaching of both types of olivine, followed by
Penicillium chrysogenum.
A. niger leached 7.8% of nickel from fresh olivine after 7 days, compared to 5.3% for
P. chrysogenum and only 2.8% for
H. grisae. These results are in agreement with findings of Valix
et al. [
2], who concluded that the strain of
Aspergillus niger is more effective than
Penicillium funiculosum for the bioleaching of nickel from silicate-rich saprolite ores. The leaching selectivity did not vary between the fungal species, with nickel being preferentially leached in all cases.
From
Figure 3 it is clear that more metals were leached from fresh olivine compared to carbonated olivine. For
A. niger, the leaching extent of nickel over 7 days decreased from 7.8% to 4.2%. The decreases for the other elements and fungi were similar. This decrease could, however, be due to the mild process conditions of the bioleaching process. In our related work (Santos
et al. [
13]), it was also noticed that at low absolute leaching (namely low acid concentrations), olivine carbonation brings little benefit, while at higher acidity carbonation acts as a mineral liberation, and thus leaching enhancing treatment. Improving the absolute percentages of the bioleaching, by optimizing the process conditions further than done in the present study, might help carbonated olivine to leach better than fresh olivine.
Figure 3.
(a) Elemental leaching extent from fresh olivine by sterile biogenic substances derived from different fungi over 7 days; and (b) elemental leaching extent from carbonated olivine by sterile biogenic substances derived from different fungi over 7 days.
Figure 3.
(a) Elemental leaching extent from fresh olivine by sterile biogenic substances derived from different fungi over 7 days; and (b) elemental leaching extent from carbonated olivine by sterile biogenic substances derived from different fungi over 7 days.
3.3. Ultrasound-Assisted Fungal Bioleaching
The influence of sonication on the leaching of fresh olivine by
Aspergillus niger is shown in
Figure 4. Treating the fungus both during the growth phase as well as during the leaching phase clearly enhanced the leaching. Nickel leaching after 17 days increased from 9.9% without ultrasonic conditioning to 15.7% with ultrasonic conditioning. This enhancement can be due to a wide combination of effects. One of the most important being that sonication increases the microbial growth, which will in turn lead to an increased production of biogenic substances [
11]. Ultrasound can disrupt fungal pellets and cause the biomass to grow mainly as dispersed hyphae [
15]; this affects the broth rheology, since solution viscosity depends on the morphology of the suspended biomass, and can promote the production of fungal metabolites that require freely dispersed fungal morphology. The latter effect was confirmed by pH measurements of the fungal broth at the end of the growth phase. The pH of the broth under standard conditions was 2.8, whereas the pH of ultrasonically treated broth was 2.3. Plating indicated a viable fungal concentration above 10
5 CFU/mL in both cases. The reduction in pH may be explained by an increase in the production of biogenic organic acids, as measured by Anjum
et al. [
11]. Yao
et al. [
9] pointed out, however, that pH effect is not the only driving force for the accelerated dissolution of minerals. Organic compounds other than acids can act as ligands to form surface or aqueous complexes; the formation of surface complexes can weaken the chemical bonds in the bulk material, leading to an accelerated elemental release.
During the leaching phase, when solids are present, an additional effect of sonication that can promote leaching is the abrasion of the surface of olivine particles by the collapsing cavitations and micro-jets formed by ultrasound, which can lead to particle/agglomerate breakage or the removal of passivating depleted layers that cover the unreacted particle core [
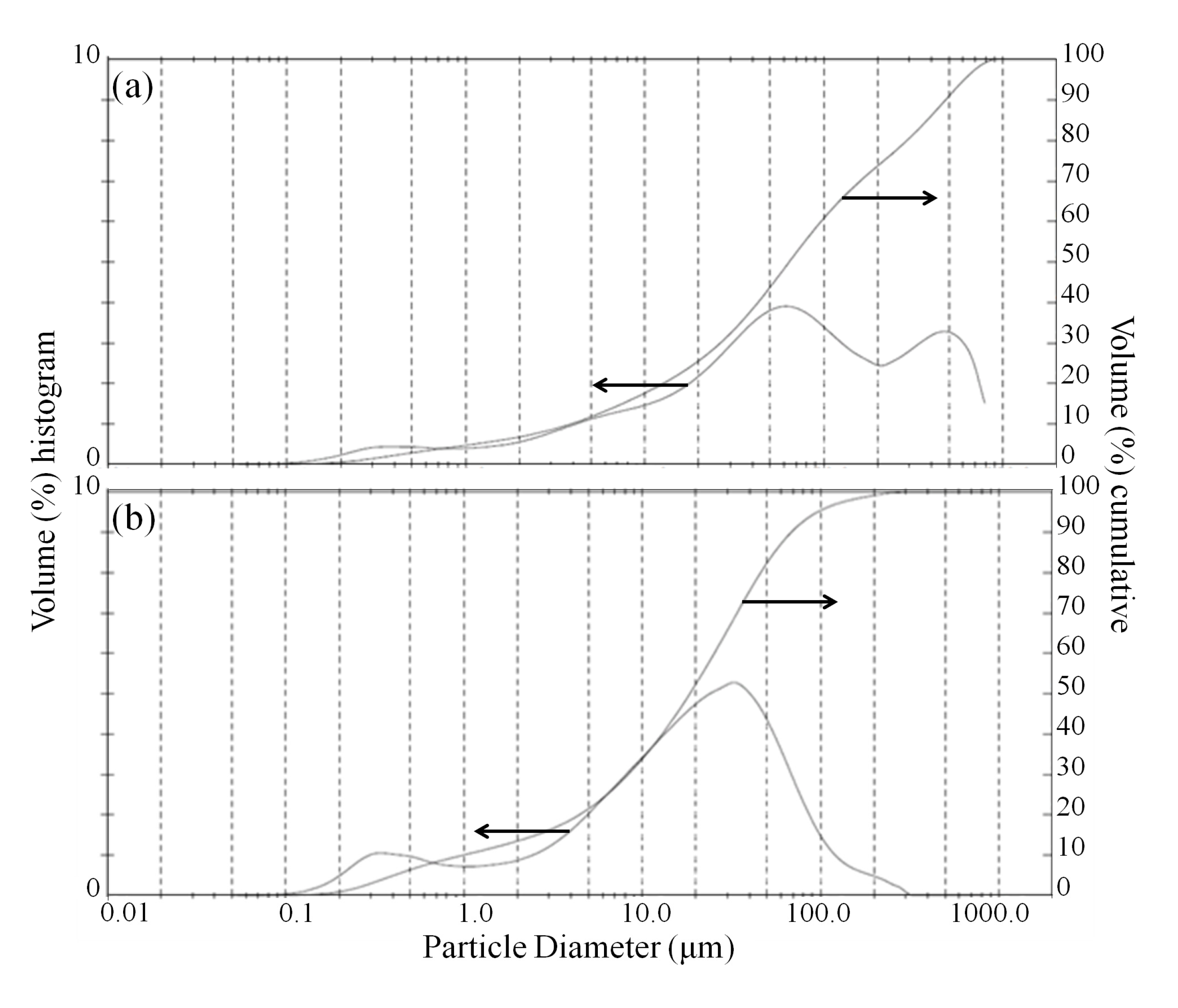
16]. The size reduction of olivine particles was confirmed by laser diffraction analysis after bioleaching, as presented in
Figure 5. The volume-moment mean diameter (D{4,3}) of the bioleached olivine under standard conditions was 158.2 μm, whereas using ultrasonic conditioning this value essentially halved to 79.2 μm. Based on the shape of the particle size distribution, it appears that the dispersion of large (~200–1000 μm) agglomerates, likely bonded by biofilm, was the dominant sonication effect on morphology.
Figure 4.
Elemental leaching extent from fresh olivine in untreated and ultrasonically conditioned Aspergillus niger broth over 17 days; error bars indicate range of duplicates.
Figure 4.
Elemental leaching extent from fresh olivine in untreated and ultrasonically conditioned Aspergillus niger broth over 17 days; error bars indicate range of duplicates.
Figure 5.
(a) Particle size distribution of fresh olivine after bioleaching with Aspergillus niger without ultrasonic conditioning; and (b) with ultrasonic conditioning.
Figure 5.
(a) Particle size distribution of fresh olivine after bioleaching with Aspergillus niger without ultrasonic conditioning; and (b) with ultrasonic conditioning.
3.4. Leaching Selectivity
In all bioleaching experiments conducted, nickel leached preferentially over iron and magnesium. In conventional acid leaching, however, there is no preferential leaching of nickel, as noted by Sanemasa
et al. [
17], and also confirmed in our related study on the leaching of fresh and carbonated olivine [
13]. Preferential leaching of nickel over iron and other metals, observed here in batch bioleaching and reported by McDonald and Whittington [
18] to occur during heap leaching of certain laterite ores, makes further processing of the leach liquor easier and could potentially reduce the recovery cost. The preferential leaching is also clear in the case when only sterile biogenic substances are used for the leaching. It thus shows that both the microorganisms as well as the biogenic substances that they produce contribute to the selectivity. Likewise, sonication was seen to improve the Ni/Fe leaching ratio, wherein the leaching of fresh olivine by
A. niger increased from 2.2 without ultrasonic conditioning to 3.5 under intermittent sonication. Sukla
et al. [
19] have reported similar effect for bioleaching of iron-rich (and nickel richer) lateritic ore, although the geochemistry of that ore differs significantly from that of silicate-based olivine, which explains the negligible iron dissolution observed in that study. The selectivity mechanism may follow two routes: (i) nickel may be preferentially chelated by the biogenic substances, and or (ii) some elements, for example magnesium, may re-precipitate in the form of inorganic or organic compounds such as (hydr)oxides, carbonates and oxalates [
3].
4. Conclusions
In conclusion, it was found that the tested fungus
Aspergillus niger leached substantially more nickel from olivine than the tested bacterium,
Paenibacillus mucilaginosus, and also outperformed the fungi
Penicillium chrysogenum and
Humicola grisea. The production of greater quantities of organic acids by the fungi, and the resulting lower pH, can be attributed to this distinction. Contrary to conventional acid leaching, the microorganisms and their biogenic substances were found to leach nickel preferentially over magnesium and iron, main components of olivine. On average, a selectivity factor of 2.2 was achieved for nickel compared to iron. This suggests that nickel was chelated preferentially by soluble biogenic substances. Carbonating the olivine, however, did not improve the bioleaching performance. It appears that at low acidity levels found during bioleaching, the mobility of nickel became reduced in carbonated olivine, but this effect reverses once the pH is lowered (as reported by Santos
et al. [
13] and Van Audenaerde [
14]), likely as a result of carbonate decomposition.
The impact of ultrasonic conditioning on bioleaching was tested and showed to substantially increase the bioleaching extent by fungi by over 50%, and to further contribute to nickel selectivity over iron, reaching a factor of 3.5. Ultrasound appeared to control fungal flocculation and reduce olivine particle agglomeration, thus improving fungal growth during the growth stage and nickel leaching during the leaching stage. The very low application rate of ultrasound (15 min every 24 h) required to achieve these benefits contributes to maintaining low processing costs of bioleaching. The assistance of ultrasonic conditioning can be further optimized by adjusting the energy intensity (e.g., 100–2000 W/L), the frequency (e.g., 16–100 kHz) of the ultrasonic waves, and the duration and the frequency of the treatment. Leaching efficiency can also likely be improved by reducing the particle size of the olivine ore, performing bioleaching for longer durations, and in continuous rather than batch mode. Further study is required to more rigorously assert the qualitative trends herein reported. In particular, care should be exercised to avoid conditions that stimulate fungal pelletization, which contributes to experimental variability. Pelletization can occur due to the presence of filamentous mycelium in the inoculum and shearing forces during mixing [
20].
The findings of this study represent a first step in verifying that olivine can potentially become a commercially exploitable source of nickel in the future. It has also been demonstrated that chemoorganotrophic bioleaching can represent an alternative approach to more conventional chemolithotrophic bioleaching, especially when considering bioleaching of non-sulfidic ores or when looking to avoid the formation of environmentally hazardous sulfuric acid.